Abstract
The effect of 24-epibrassinolide and fennel essential oil on postharvest life and quality of orange fruits (Citrus sinensis var. ‘Valencia’) during storage was studied. Fruits were treated with 24-epibrassinolide (0, 4 and 8 µlL−1) and fennel essential oil (0, 300 and 600 µlL−1), as well as various combinations thereof and stored for 45 and 90 days. The results showed that the highest amount of total acidity was associated with 600 µlL−1 essential oil and 4 µlL−1 24-epibrassinolide on day 45. The highest level of phenylalanine ammonia-lyase was observed during the storage period of 90 days in the treatment of 300 μlL−1 of essential oil and 24-epibrassinolide at a concentration of 4 μlL−1. The results indicate that the combination treatment of 24-epibrassinolide and fennel essential oil increases the health promoting potential, maintains fruit quality, and extends storage life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 80% of total citrus production comes from 10 countries: Brazil, the USA, China, Mexico, Nigeria, Spain, India, Iran, and Argentina. In 2018, Iran was ranked seventh among the top 10 citrus-producing countries with 4 million tonnes produced, accounting for 3% of world citrus production (FAO Statistics 2020). In Iran, the income of a number of people is directly or indirectly dependent on citrus, and this number is increasing every year. Citrus fruits have high nutritional value and play an essential role in health due to their compounds such as ascorbic acid, phenols, flavonoids, carotenoids, pectin, etc. (Kahramanoglu et al. 2021). The main components of orange (C. sinensis) essential oil were limonene (71.80%), beta-myrcene (4.55%), sabinene (1.39%), linalool (3.89%), octanal (1.64%), and cc-pinene (1.17%) (Duman et al. 2016). The major fatty acid compositions of citrus seed oil are oleic, linoleic and palmitic acids (Matthaus and Özcan 2012).
Citrus fruits are non-climacteric and have a low rate of respiration and ethylene production. Chemical changes are minimal and do not continue after harvest (Fujioka and Yokota 2003; Tadeo et al. 2008). ‘Valencia’ orange is the latest commercial citrus cultivar in Iran. This cultivar is in the group of seedless or low seed varieties. Today, due to the increasing demand for organic products, as well as concerns about residual toxins and the adverse effects they have on the environment and human health, postharvest management techniques using healthy and environmentally friendly methods are being developed to increase the shelf life of fruits (Munhuweyi et al. 2020). Today, the increasing use of growth regulators in postharvest technology of unclimacteric fruits such as oranges promises to increase the methods in postharvest technology and thus prolong the shelf life of fruits (Ziv and Fallik 2021). In non-climacteric fruits such as grapes, brassinosteroid treatment significantly increased soluble solids and total acidity and antioxidant content, phenols, and ascorbic acid (Asghari and Rezaei-Rad 2018). Treatment of 40 μlL−1 of brassinosteroids in bananas reduced ion leakage and increased chlorophyll fluorescence (Li et al. 2018).
Edible coatings reduce fruit losses by creating a semi-permeable barrier on the surface of the fruit, which regulates moisture and gas exchange between the inside of the fruit and the outside atmosphere (Ncama et al. 2018.) As a result, edible coatings may increase fruit postharvest life by delaying fruit ripening and physicochemical changes, as well as preventing the development of physiological abnormalities (Kumar et al. 2017). The ability of essential oils to reduce postharvest waste, as well as their naturalness and lower level of harm to human health, has led to increased interest in and attention on essential oils (Vergis et al. 2015). One of these edible coatings is fennel essential oil. The antimicrobial and antioxidant properties of fennel essential oil are due to flavonoids, terpenoids, carotenoids, and coumarins (Singh et al. 2006).
Several researchers recorded the chemical composition of fennel oils of different origin and the major components were reported to be methyl chavicol, trans-anethol, limonene, fenchone, α‑terpinene, and piperitonene oxide (Özcan and Akgül 2001; Özcan and Chalchat 2006). The oil from the fruit of Foeniculum vulgare subsp. piperitum from Turkey has been studied, with the main components reported as being methyl chavicol (40.49% and 21.69%), with limonene (17.66% and 22.44%) and fenchone (16.9% and 12.98%) being the other important components (Özcan and Akgül 2001).
In order to maintain the quality of the fruit and ensure its marketability, detailed protocols should be developed for the postharvest operations of ‘Valencia’ oranges. Since the demand for the ‘Valencia’ cultivars is high up to several months after harvest in different provinces of Iran, it is necessary to use appropriate methods to increase the shelf life for use in all seasons. On the other hand, due to wide variety of citrus cultivars, general recommendations for all cultivars are not acceptable under any circumstances. Therefore, the methods used to maintain and store these cultivars should be that the fruit is of the best quality when marketed. Thus, the objective of this study was to evaluate the effects of various concentrations of 24-epibrassinolide and the essential oil extracted from F. vulgare on ‘Valencia’ orange during postharvest cold storage and observation of phytochemical contents, enzymatic activities and increasing the shelf life of ‘Valencia’ orange.
Materials and Methods
Plant Material, Fruit Treatment, and Storage Conditions
The present study was performed on ‘Valencia’ orange cultivar. Orange fruits of the ‘Valencia’ variety were harvested at commercial maturity stage from a commercial garden located in Qaem shahr, Mazandaran province (north of Iran) in late January according to soluble solids content/titratable acidity (SSC/TA) ratio from mature trees and transferred into cold storage at the laboratory of the Horticulture Department, Urmia University. The diameter of the fruits at the time of harvesting was 70–80 mm.
The fruit was rinsed with distilled water and dried at room temperature. Then, 24-epibrassinolide treatments (Sigma-Aldrich Co., Madrid, Spain) in three levels including 0, 4, and 8 μlL−1 and fennel essential oil in three levels of 0, 300, and 600 μlL−1 as well as fruits with simultaneous treatment of fennel essential oil and 24-epibrassinolide was applied by immersion for 5 min. After drying the treated fruits at normal laboratory temperature, which lasted 10 min, they were then put in clear disposable polystyrene containers and left for 0, 45, and 90 days at 5 °C and kept in the relative cold at 85 to 95% relative humidity. Sampling was performed to measure the desired indicators on days 0, 45, and 90 days after storage in polystyrene containers. The traits were evaluated at three times: 0 or harvest time, 45 days after treatment, and 90 days after treatment.
Essential Oil Analysis
A total of 30 g dried fennel leaves (drying at room temperature and in the dark) were used for water distillation using a Clevenger apparatus (for 3 h). The prepared extract was then stored in the dark at 4 °C before analysis. Gas chromatography-mass spectrometry (GC-MS) analyses were performed on a Thermo Finnigan capillary gas chromatograph directly joined to the mass spectrometer system (model Trace GC/Trace MS Plus system). First, the prepared samples were injected into a chromatographic apparatus, and a suitable temperature programming column was obtained for complete separation of the essential oil compounds. Also, the percentage of constituents of each essential oil was calculated. The essential oils were then injected into a gas chromatographic apparatus connected to a mass spectrometer, and the mass spectra of the compounds were obtained. Essential oil compounds were identified using the Quats inhibition index, and the mass spectra were compared with the proposed mass spectra by the computer libraries of the gas chromatograph connected to the mass spectrometer.
Measuring the Firmness of Fruit Tissue
In order to determine the firmness of the fruit tissue of the decomposition device and texture measurement of the XTPluss-TA model (Stable, UK) microsystem was used. The probe used was a cylindrical type with a flat base and a diameter of 6 mm. The amount of pressure in kilograms due to the resistance of fruit tissue to the tip of the stiffness meter was read from the device (Vargas et al. 2006).
Measurement of Total Soluble Solids (TSS)
For this purpose, the ATAGO manual refractometer was used. At first, the refractometer was calibrated, then a few drops of fruit juice were poured on the device, the relevant number was read from the column, and the data were recorded in Brix (Ayala-Zavala et al. 2007).
Measurable Titratable Acidity (TA)
To measure TA, the titration method with 0.1 N NaOH solution was used to reach pH = 8.2, and the results were expressed in grams of citric acid in 100 ml of fruit juice. For this purpose, 10 ml of fruit juice was mixed with 20 ml of distilled water and then titrated. The amount of titratable acidity was calculated according to the percentage of citric acid according to the following formula:
In this formula: A = amount of organic acids in fruit extract, S = amount of NaOH consumed (ml), N = normality of NaOH, F = factor NaOH, C = amount of fruit extract (ml), and E = equivalence the target acid (malic acid) (Ayala-Zavala et al. 2007).
Measuring the pH of the Fruit
The filtered fruit extract was used to measure the pH of the juice. The Juice was measured with a digital pH meter (pH-Meter model CG824) (Jalili Marandi 2010).
Measuring the Amount of Weight Loss
Weight loss of fruits was measured with a digital scale model CANDGL300; for this purpose, the difference in fruit weight after 45 and 90 days with the first-day fruits was calculated. Weight loss is due to the decrease in fruit moisture, calculated from the following formula (Ali et al. 2011):
Measurement of Ascorbic Acid
To measure ascorbic acid, 1269 g of iodine was mixed with 16.6 g of potassium iodide in distilled water, and its volume was reduced to 1 l. In this mixture, the iodine normality is 0.01 normal, but its factor must be measured before the test. For this purpose, the prepared mixture was stored for 1–2 days, then 20 ml of the above mixture was poured into another container, to which 2 ml of 1% starch solution was added. This mixture was titrated with the pure ascorbic acid solution, such that at the endpoint of the solution, it turns pale gray. To prepare the ascorbic acid solution, 100 ml of pure powder was dissolved in 100 ml of distilled water. The following equation was used to calculate the iodine mixture factor (Omaye et al. 1979):
- F:
-
= iodine mixture factor and
- N:
-
= iodine mixture normality
- A:
-
= amount of pure ascorbic acid (mg)
- B:
-
= amount of iodine mixture consumed (mg)
Measurement of Total Antioxidant Activity of Fruit Extract (DPPH)
In the evaluation of total antioxidants by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, the first 50 μl of the prepared extract was mixed with 950 μl of DPPH, and after 30 min, it was read by a spectrophotometer with a wavelength of 517 nm and placed in the following formula:
Abs sample is the amount of DPPH absorption in the presence of the sample, and Abs control is the absorption of DPPH without extract (Navarro et al. 2006).
Measurement of Total Anthocyanin Content
The amount of total anthocyanin in fruit juice was determined by the pH difference method (Giusti and Wrolstad 2001). At first, the fruit juice was centrifuged at 11,000 × g for 20 min at 4 °C, and then 100 μl of a supernatant was used. The absorption was measured via UV–vis spectrophotometer at 520 and 700 nm wavelengths of buffers containing 1.0 and 4.5 pHs based on A = [(A530 − A700) pH1.0 − (A530 − A700) pH4.5] with molar extinction coefficients of cyanidin-3-O-glucoside for orange juice. The results were expressed based on cyanidin three glucoside in mg L−1 fresh weight.
Measurement of Total Phenol Content (TPC)
To determine the total phenol content, colorimetric determination by Folin-Ciocalteu phenol reagent was used (Slinkard and Singleton 1977). The juice was centrifuged at 11,000 × g for 15 min at 4 °C. Then, 30 μl concentrated extract of fruit juice was mixed with 90 μl of distilled water. Later, 600 μl of 10% folin was added to the solution and was kept for 10 min. Then, 480 μl of 7.5% sodium carbonate (Na2CO3) was added to this solution and placed in darkness for 1.5–2 h. Finally, using gallic acid as a standard, the color change of the extracts was calorimetrically determined at a wavelength of 765 nm by UV–vis spectrophotometer. The data are expressed as milligram gallic acid equivalent per ml fruit extract.
Measuring the Amount of Total Flavonoids
The amount of total flavonoids was determined using a colorimetric assay (Shin et al. 2007). At first, 500 μl of the prepared extract was mixed with 150 μl of 5% sodium nitrite and was kept at room temperature for 5 min. Next, it was mixed with 300 μl of 10% aluminium chloride (Alcl3) and was kept for 5 min. Then, 1 ml of 1 mol l−1 sodium hydroxide was added to the solution and was brought to 5‑ml volume with adding distilled water. The absorption mixture was read in 510 nm wavelength. The amount of total flavonoids in fruit juice is reported according to mg quercetin equivalent per ml fruit juice.
Measurement of Enzyme Activity
To measure enzyme activity, protein extract must first be prepared.
Protein Extraction
First, 1 g of fruit tissue was ground in a mortar containing 5 ml of 0.05 M Tris-HCL buffer at pH = 7.5 to obtain a homogeneous solution. The solution was transferred to a centrifuge tube. Then, after centrifugation, the resulting solution was allowed to stand for 10 min. It was then centrifuged for 25 min at 10,000 rpm at 4 °C using a refrigerated centrifuge. The tubes were then gently removed from the device and the supernatant solution was poured into the test tube to obtain a protein extract, which was used to measure the activity of antioxidant enzymes (Bradford 1976).
Measurement of Catalase Enzyme
Catalase activity was measured based on the reduction of hydrogen peroxide adsorption at a wavelength of 240 nm. According to this method, the reaction mixture (3 ml) consisted of 50 mM potassium phosphate buffer (PH = 7.0), 15 mM hydrogen peroxide, and 100 μl of enzyme extract. By adding hydrogen peroxide to the reaction mixture, the reaction was started, and the reduction in hydrogen peroxide uptake was measured for 60 s at 240 nm using a spectrophotometer (Dhindsa et al. 1981).
Measurement of Ascorbate Peroxidase
The activity of this enzyme was measured based on the oxidation of ascorbic acid and reduction of adsorption at 290 nm. Initially, samples were powdered in liquid nitrogen. The enzyme extract was extracted in sodium-potassium buffer at a concentration of 50 mM and pH = 7 and centrifuged at 10,000 g for 10 min. To measure enzyme activity, 300 μl of sodium phosphate buffer (pH = 7) containing 0.2 mM EDTA, 200 μl of ascorbic acid with 0.5 mM concentration, 200 μl of bovine serum albumin (BSA), and 50 μl of enzyme extract were mixed, and the reaction was started by adding 50 μl of hydrogen peroxide at a concentration of 250 mM. Absorption changes were measured by spectrophotometer for 3 min, and enzyme activity was calculated using ascorbic acid extinction coefficient (mM−1 cm−1) in units of µM ascorbic acid mg−1protein min−1 (Nakano and Asada 1981).
Measurement of Guaiacol Peroxidase Activity
After the preparation of protein extract, the following reagents are needed to measure peroxidase activity:
2 ml of 100 mM Tris buffer (PH = 7.5), 300 μl of 5 mM hydrogen peroxide, and 200 μl of 10 mM guaiacol were all mixed in the ice bath. Then 50 μL of enzyme extract were added. The absorption change curve at 425 nm was read with a spectrophotometer (Kochba et al. 1977).
Measurement of Phenylalanine Ammonia-Lyase (PAL) Enzyme Activity
The method of Ke and Saltveit (1986) was used to measure the activity of the phenylalanine ammonia-lyase (PAL) enzyme with some modifications. Initially, 0.5 g of fresh fruit tissue was extracted using 1.5 ml of buffer (0.1 M borate buffer, 0.1% polyvinylpyrrolidone, and 1.4 mM mercaptoethanol with pH = 7). This was then centrifuged for 15 min at 12,000 rpm at 4 °C. After centrifugation, the supernatant extract was used to assay the enzyme. Sample contents for the enzyme assay contained 30 μl of enzyme extract and 1 ml of buffer (0.1 M borate buffer with pH 8.8, 0.1% polyvinyl pyrrolidone, 1.4 mM mercaptoethanol, and 1 mL 12 mM L-phenylalanine). Finally, the samples were placed in a hot water bath at 30 °C for 30 min and the absorbance was read at 290 nm using a spectrophotometer.
Experimental Design and Statistical Analysis
This factorial experiment was performed in a completely randomized design with three replications, and its mean was compared with Duncan’s multiple range test. The data obtained from the experiment were analyzed by SAS software, and the graphs were drawn using Excel software.
Results
Essential Oil Composition
The results of the gas chromatography (GC) revealed that fennel essential oil contains 16 different compositions; major compositions identified include: E‑anethole (60.01%), fenchone (22.35%), α‑pinen (4.75%), methyl chavicol (3.08%), sabinene (1.6%), and limonene (1.28%). All chemical compositions of fennel are shown in Table 1. In a previous investigation on the volatiles of F. vulgare subsp. piperitum fruits, methyl chavicol (47.09%), limonene (29.07%), fenchone (13.43%), and fenchyl acetate (1.95%) were found to be the major components (Özcan and Chalchat, 2006). The main identified components of the flower and unripe and ripe fruit oils were estragole (53.08%, 56.11%, and 61.08%), fenchone (13.53%, 19.18%, and 23.46%), and α-phellandrene (5.77%, 3.30%, and 0.72%), respectively (Özcan et al. 2006).
The Effect of 24-Epibrassinolide and Fennel Essential Oil on the Firmness of Fruit Tissue
The amount of firmness of fruit tissue decreased during the storage period compared to the first day (Fig. 1). The combined treatment of fennel essential oil and 24-epibrassinolide (especially at a concentration of 600 μlL−1 of fennel essential oil) in 45 days of storage showed the highest firmness of the fruit (Fig. 1).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the firmness of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on Soluble Solids (TSS)
With increasing storage time, the amount of fruit TSS increased so that the highest amount of TSS was obtained in treatments of 300 μlL−1 (45 days of storage) and 600 μlL−1 (90 days of storage) of fennel essential oil with 8 μlL−1 of 24-epibrassinolide (Fig. 2). The possible mechanism by which sugar levels increase in harvested citrus fruit is synthesis of sugars from organic acids due to the presence and increase of glycolytic enzymes during fruit storage (Rapisarda et al. 2008).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on soluble solids (TSS) of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of Epibrassinolide and Fennel Essential Oil on the Total Acid Content
A downward trend in the amount of organic acids was observed during storage (Fig. 3). The highest amount of organic acids was observed in fruits related to fennel essential oil of 600 μl L−1 and 24-epibrassinolide with a concentration of 4 μl L−1 in 45 days of storage. Compared to the fruits on the first day of storage, 18.9% showed an increase in the amount of organic acids.
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1) and storage time on total acid content of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of Epibrassinolide and Fennel Essential Oil on pH Content
Fig. 4 illustrates that with increasing storage time, the pH content of fruits has an upward trend (Fig. 4). Thus, the highest amount of this trait is in 90 days of storage in the treatment of 24-epibrassinolide with a concentration of 8 μl L−1 without the use of fennel essential oil. Compared to the fruits of the first day, it increased by 9.7%.
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on pH content of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
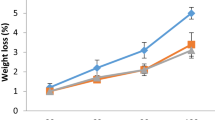
Effect of 24-Epibrassinolide and Fennel Essential Oil on Weight Loss
As shown in Fig. 5, the interaction of fennel essential oil and 24-epibrassinolide significantly reduced the amount of fruit weight loss in storage. The highest percentage of fruit weight loss during storage was observed in fennel essential oil treatment with a concentration of 600 μl L−1 and 24-epibrassinolide treatment with a concentration of 8 μl L−1 on day 90 of storage. In contrast, the interaction of fennel essential oil and 24-epibrassinolide in reducing fruit weight loss, especially on the 45th day of storage, was significant compared to the fruits on the first day.
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on weight loss content of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of Epibrassinolide and Fennel Essential Oil on Ascorbic Acid
The highest amount of fruit ascorbic acid (5.5 mg acid ascorbic 100 g−1fw) was observed in the treatment of fennel essential oil with a concentration of 300 μlL−1 and 24-epibrassinolide with a concentration of 4 μlL−1 in 90 days of storage, meaning that the rate of increase of this trait compared to the fruits of the first day was 25% (Fig. 6).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the ascorbic acid content of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on Fruit Antioxidant Activity (DPPH)
Looking at Fig. 7, it is apparent that the amount of antioxidant activity increased with increasing storage time. The highest amount of total fruit antioxidants during storage (49%) was observed in the treatment of fennel essential oil with a concentration of 600 μlL−1 plus 24-epibrassinolide with a concentration of 8 μlL−1 in 90 days of storage, and the lowest (29.6%) was obtained in the fruits of the first day (Fig. 7).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the antioxidant activity content (DPPH) of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on Total Anthocyanin Content
The highest amount of whole fruit anthocyanin (127 mg cyanidin-3-o-glucoside 1 ml−1 extract) was observed in the treatment of fennel essential oil with a concentration of 300 μlL−1 and 24-epibrassinolide with a concentration of 8 μlL−1 in 90 days of storage, and its lowest amount (83 mg cyanidin-3-o-glucoside 1 ml−1 extract) was obtained in the fruits of the first day (Fig. 8). The increase in anthocyanins in this study is probably due to the ripening of the fruits, as well as the increased amounts of sugars and enzymatic activity of phenylalanine amonyalias during the storage (Vargas et al. 2006).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on total anthocyanin content of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on Total Phenol Content
Fig. 9 illustrates that the amount of total phenol was affected by the duration of storage and different concentrations of fennel essential oil and 24-epibrassinolide treatments. The highest amount of total fruit phenol (32.18 mg GAL g−1 FW) was observed during storage in the treatment of fennel essential oil with a concentration of 600 μlL−1 with 24-epibrassinolide with a concentration of 8 μlL−1 in 90 days of storage. (Fig. 9).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the total phenol content of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on Total Flavonoid Content
With increasing storage time, the total flavonoid content increased significantly compared to the control, which reached its maximum using fennel essential oil and 24-epibrassinolide on the 90th day of storage. The highest amount of whole fruit flavonoids (3.69 mg QUE g−1 FW) was observed during storage in the treatment of fennel essential oil with a concentration of 600 μlL−1 and 24-epibrassinolide with a concentration of 8 μlL−1 in 90 days of storage (Fig. 10).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the total flavonoid content of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on Catalase Activity
According to Fig. 11, catalase activity was different depending on the type of treatment and storage time. According to the results of the present study, the highest activity of catalase enzyme (1 U g−1 FW) was during the storage period of 45 days in essential oil treatments with a concentration of 300 μlL−1 and 24-epibrassinolide with a concentration of 8 μlL−1, and its lowest (0.37 U g−1 FW) was observed during the storage period of 90 days in essential oil treatments with a concentration of 600 μlL−1 and 24-epibrassinolide with a concentration of 0 μlL−1 (Fig. 11).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the catalase activity of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on Ascorbate Peroxidase Activity
Ascorbate peroxidase activity increased with increasing storage time. Thus, the highest enzyme activity was observed on the 90th day of storage (0.94 U g−1 FW), and the lowest level of the enzyme was observed in the fruits of the first day of storage (0.68 U g−1 FW) (Fig. 12).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the ascorbate peroxidase activity of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on the Activity of Guaiacol Peroxidase
According to the results, the highest level of guaiacol peroxidase was observed during the storage period of 90 days in the treatment of essential oil of 300 μlL−1 and 24-epibrassinolide 8 μlL−1 (Fig. 13).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the guaiacol peroxidase activity of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Effect of 24-Epibrassinolide and Fennel Essential Oil on the Activity of Phenylalanine Ammonia-Lyase (PAL)
The highest level of PAL was observed during the storage period of 90 days in the treatment of essential oil with a concentration of 300 μlL−1 and 24-epibrassinolide with a concentration of 4 μlL−1 (2.08 uMol g−1fw), meaning that the rate of increase of this enzyme compared to the fruits of the first day was 18.2% (Fig. 14).
Interactions between fennel essential oil (Eo) treatments (0, 300, and 600 μl L−1), 24-epibrassinolide (0, 4, and 8 μl L−1), and storage time on the activity of phenylalanine ammonia-lyase (PAL) of ‘Valencia’ orange fruits stored for 90 days at 5 °C with 85–95% relative humidity. Control refers to untreated ‘Valencia’ orange fruits during storage. Different letters indicate statistical significance (p < 0.05)
Discussion
Fruit texture firmness is one of the vital quality indicators in marketing. In the present study, the best treatment in preserving the firmness of ‘Valencia’ orange fruits on the 90th day of storage was reported at a concentration of 600 μlL−1 of fennel essential oil and 24-epibrassinolide with concentrations of 4 and 8 μlL−1. He et al. (2018) showed that the treatment of persimmon fruits with brasinazole (10 μmolL−1), from the expression of genes related to fruit softening enzymes such as polygalacturonase, pectate lyase, galactosidase, and endo 1‑4-beta-glucanase prevented and maintained the firmness of the fruits during storage. Essential oils reduce the activity of cell wall softening enzymes such as polygalactronase and galactoxidase, maintaining fruit firmness (Batisse et al. 1996). The use of cinnamon essential oil in tomatoes maintained the firmness of the fruit (Nikos and Tzortzakis 2007). In the present study, it is likely that 24-epibrassinolide and fennel essential oil reduced the activity of cell wall-destroying enzymes (pectin methyl esterase and polygalactronase), causing strength and delay in membrane breakdown, thus maintaining fruit firmness.
Soluble solids are another helpful indicator in evaluating the quality of fruits. In a study using thyme essential oil treatment on cherry fruits, the amount of soluble solids after a storage period was lower than the control treatment (Vilaplana et al. 2018). Based on the results of this study, the amount of soluble solids during the storage period in fruits treated with fennel essential oil and 24-epibrassinolide was less than the control in each storage period. These results may be related to the low respiration rate of the covered fruits. The best concentration of soluble solids was observed on the 45th day of storage in the treatment of fennel essential oil with a concentration of 300 μlL−1 along with 24-epibrassinolide with a concentration of 8 μlL−1. However, during fruit storage after harvest, organic sugars and acids are reduced due to the use of these compounds as a respiratory substrate as well as the synthesis of new molecules (Habibi et al. 2021).
Coating guava fruits with gum arabic delayed aging and starch decomposition, TSS slightly increased due to the delay in aging and reduced respiration, and ultimately reduced starch decomposition into sugars (Anjum et al. 2020). In the present study, it is possible that fennel essential oil and epibrassinolide treatments prevented increased respiration and thus, by preventing the decomposition of pectins and other polysaccharides, reduced the consumption of soluble solids. Decreased levels of organic acids are associated with the aging process of the fruit.
This study showed that the amount of organic acids in the fruits had a downward trend from the 45th to the 90th day of storage. With the onset of fruit ripening, the activity of the enzymes responsible for converting sucrose to organic acids decreases, while that of sucrose phosphate synthetase increases (Rathore et al. 2007). Any treatment that reduces fruit respiration prevents the reduction of organic acids due to reduced sugar consumption. The highest amount of total acid was reported in the treatment of fennel essential oil with a concentration of 600 μlL−1 plus the treatment of 24-epibrassinolide with a concentration of 4 μlL−1 on the 45th day of storage. In this study, it is possible that treatment of 24-epibrassinolide and fennel essential oil, by reducing respiration and reducing ethylene production, may reduce the rate of consumption of organic acids and their rate of conversion to sugars, thus preserving organic acids and preventing depletion.
Similar to the results obtained in the present study in guava fruit, application of gum Arabic and aloe vera coatings restricted access to oxygen and reduced oxidation of organic acids, which in turn showed higher titratable acidity and lower pH in treated fruits compared to controls (Anjum et al. 2020). Similar results were obtained in banana fruits coated with Arabic gum (Khaliq et al. 2019). The findings of the present study showed that with the application of 24-epibrassinolide and fennel essential oil on 45 and 90 days of storage, the upward trend of pH was slowed down. The increase in pH of orange fruit extract in this study is due to the conversion of organic acids to sugars during the respiration process. The combination of 24-epibrassinolide and fennel essential oil was able to slow down the breakdown of organic acids by reducing respiration, thus maintaining the pH of the fruit extract. The results of this study are consistent with the results of Vilaplana et al. (2018) on the use of thyme essential oil in pineapple in maintaining fruit pH.
The main reason for fruit weight loss during storage is postharvest physiological activities, such as respiration and transpiration (Deshi et al. 2021). On day 45 of storage, application of 8 μl L−1 of 24-epibrassinolide and 600 μl L−1 of fennel essential oil significantly prevented fruit weight loss, but on day 90 of storage, it had no significant effect compared to the control. Application of lemon essential oil as wax on mango fruit (Geng et al. 2011), Arabic gum in Guava fruits (Anjum et al. 2020), and chitosan in mango (Zahedi et al. 2019) led to a reduction in transpiration and prevention of fruit weight loss, which was consistent with the results of the present study.
The highest amount of ascorbic acid was obtained in the treatments of fennel essential oil with a concentration of 300 μlL−1 plus 24-epibrassinolide with a concentration of 4 μlL−1 on the 90th day of storage. It is possible that the combination of 24-epibrassinolide with fennel essential oil reduces the respiration of fruit and the consumption of organic acids, and thus prevents the reduction of ascorbic acid.
Zahedi et al. (2019) showed in strawberries that edible coatings increase the activity of the cytochrome oxidase enzyme by reducing the internal oxygen content of the fruit. This enzyme can significantly reduce the breakdown of ascorbic acid. Habibi et al. (2021) showed in ‘blood orange’ fruits that brassinosteroids, by reducing the rate of respiration and thus reducing the activity of polyphenol oxidase enzyme, maintained the ascorbic acid of the fruit. Reactive oxygen species (ROS) are produced as dangerous and destructive factors during normal metabolism. The activity of these molecules may lead to oxidative damage that accelerates the respiration and production of ethylene, resulting in aging and tissue loss increase (Huwei et al. 2021). The results of this study revealed that the use of essential oil of fennel essential oil as an edible coating and 24-epibrassinolide could reduce the damage caused by ROS with increasing the antioxidant activity. In this study, the combination of 24-epibrassinolide and fennel essential oil increased the level of antioxidant activity in the treated samples compared to the control. So that the best total antioxidant concentration was observed on the 90th day of storage in the combined treatment of fennel essential oil with a concentration of 600 μlL−1 and 24-epibrassinolide with a concentration of 8 μlL−1. In research, the antioxidant activity, as determined by DPPH radical scavenging activity, was 58.7 (oven)–67.84% (infrared) in lemon peel powder and 61.65 (microwave)–63.54% (infrared) in orange peel powder (Özcan et al. 2021).
In ‘blood oranges’, brassinosteroid treatment reduced the production of ROS by increasing their antioxidant activity and preventing their frost damage (Habibi et al. 2021). The use of lemon essential oil as wax on mango fruit increases the level of antioxidant activity, thus increasing the shelf life of the fruit (Longo and Vasapollo 2006). Similar to the findings of the present study, Wang et al. (2007) reported that strawberries treated with thymol and agenol had higher levels of antioxidant content, including anthocyanins and phenolic compounds, than untreated fruits.
Also, this study demonstrated that the combination of 24-epibrassinolide and fennel essential oil enhanced the amount of phenolic compounds such as phenolics, flavonoids, anthocyanins, and antioxidant capacity in the fruits. The amount of phenol and the total flavonoid had an upward trend. The highest amount of phenol and total flavonoid were observed in the treatment of fennel essential oil with a concentration of 600 μlL−1 and 24-epibrassinolide with a concentration of 8 μlL−1 on 90 days of storage. Total phenolic and total flavonoid amounts of Citrus seeds were determined to range from 411.43 mg GAE100 g−1 (lemon) and 814.84 mg GAE100 g−1 (bitter orange) to 97.84 mg100 g−1 (grapefruit) and 126.48 mg100 g−1 (lemon) (Özcan et al. 2022). In research on two fruits, lemon and orange, it was shown that the total phenol contents varied between 114.58 (fresh) and 179.69 mg GAE 100−1 g (oven) in lemon peel powder (LPP) and 158.54 (fresh) and 177.92 mg GAE 100−1 g (infrared) in orange peel powder (OPP). The total flavonoid contents were 380.44 (fresh)–1043.04 mg 100−1 g (oven) in LPP and 296.38 (fresh)–850.54 mg100−1 g (oven) in OPP (Özcan et al. 2021).
In plants, shikimate-phenylpropanoid-flavonoids pathways are the biosynthesis pathways of phenolic compounds. PAL enzyme is considered as a critical enzyme in the phenylpropanoid pathway, and this enzyme converts phenylalanine to trans-cinnamic acids (Pérez-Balibrea et al. 2011). According to our findings, the amount of total anthocyanin increased in the fruits during the storage process, by increasing the concentration of 24-epibrassinolide and fennel essential oil, especially on the 90th day. Increased levels of anthocyanins during storage can be associated with increased phenolic compounds and activation of the enzyme PAL. Also, increasing the amount of anthocyanin has a high correlate with increasing the amount of soluble solids in the fruit during storage.
Phenolic compounds are one of the most important secondary metabolites that are produced directly through the phenylpropanoid pathway and the key enzyme PAL. Accumulation of phenolic compounds such as hesperidin in oranges is considered as an adaptation mechanism to prevent oxidative damage, which is capable of detoxifying ROS (Cebadera-Miranda et al. 2019). The phenolic constituents of citrus are dependent on the cultivar and the fruit part (peel, seed, and pulp) and may vary in fruit products obtained using different techniques (Özcan et al. 2021). Concentrations of phenolic compounds and flavonoids in postharvest treatment of epibrassinolide increased in ‘Satsuma tangerines’ by increasing PAL activity and transcription factors related to the biosynthesis of phenols and anthocyanins, such as transcription factors MYB and MYC (Zhu et al. 2015). Therefore, epibrassinolide can increase the accumulation of anthocyanins and phenols by increasing PAL activity. In the present study, the highest amount of total phenol was obtained on the 90th day of storage at the highest concentrations of 24-epibrassinolide and fennel essential oil (32.18 mg GALg−1 FW). Therefore, in this study, 24-epibrassinolide may have increased phenolic compounds by activating phenylpropanoid pathways and reducing cellular respiration. Our results are supported by those of Jin et al. (2012). According to their observations, raspberries treated with plant essential oils had higher phenolic substances, anthocyanin content, and higher antioxidant activity than untreated raspberries. In previous studies, it was also shown in strawberries that the application of tea essential oil stimulates PAL enzyme activity and increases phenolic compounds, which is consistent with the results obtained in this study (Shao et al. 2013). According to our data, the activity of defense enzymes including PAL, CAT, GPX, and APX was enhanced by EBL (Epibrassinolide), and, in a concentration-dependent manner, with an increase in EBL levels, the activity of these enzymes was increased. In this study, PAL enzyme in the combined treatment of fennel essential oil with a concentration of 300 μlL−1 and 24-epibrassinolide with a concentration of 4 μlL−1 was reported at its maximum level on the 90th day of storage. PAL enzyme is activated by various plant hormones, including jasmonates, brassinosteroids, and salicylates in various plants (Asghari and Aghdam 2010). These findings indicate that brassinosteroids play substantial roles in mediating fruit defense responses. Previous observations have concluded that epibrassinolide treatments enhance antioxidant capacity in fruits. For example, studies in peach fruit showed that the activity of the guaiacol peroxidase enzyme in fruits treated with brassinosteroids was higher than the control (Tareen et al. 2012). Furthermore, the application of brassinosteroids increased the activity of catalase, ascorbate peroxidase, and guaiacol peroxidase enzymes in grapefruit (Anuradha and Rao 2007).
References
Ali A, Abrar M, Sultan MT, Din A, Niaz B (2011) Postharvest physicochemical changes in full ripe strawberries during cold storage. J Anim Plant Sci 21(1):38–41
Anjum MA, Akram H, Zaidi M, Ali S (2020) Effect of gum rabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’guava fruits. Sci Hortic 271:109506. https://doi.org/10.1016/j.scienta.2020.109506
Anuradha S, Rao SSR (2007) The effect of brassinosteroids on radish (Vitis Venifera L.) seedlings growing under cadmium stress. Plant Soil Environ 53(11):465–472. https://doi.org/10.17221/2307-PSE
Asghari M, Aghdam MS (2010) Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci Technol 21(10):502–509. https://doi.org/10.1016/J.TIFS.2010.07.009
Asghari M, Rezaei-Rad R (2018) 24-Epibrassinolide enhanced the quality parameters and phytochemical contents of table grape. J Appl Bot Food Qual 91:226–231. https://doi.org/10.5073/JABFQ.2018.091.030
Ayala-Zavala JF, Wang SHY, Wang CY, Gonzalez-Aguilar GA (2007) High oxygen treatment increases antioxidant capacity and postharvest life of strawberry fruit. Food Technol Biotechnol 425:166–173
Batisse C, Buret M, Coulomb PJ (1996) Biochemical differences in cell wall of cherry fruit between soft and crisp fruit. J Agric Food Chem 44:453–457. https://doi.org/10.1021/jf950227r
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cebadera-Miranda L, Domínguez L, Dias MI, Barros L, Ferreira IC, Igual M, Martínez-Navarrete N, Fernández-Ruiz V, Morales P, Cámara M (2019) Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): Bioactive compounds and colour appearance of blood oranges. Food Chem 270:395–402. https://doi.org/10.1016/j.foodchem.2018.07.094
Deshi V, Homa F, Tokala VY, Mir H, Aftab MA, Siddiqui MW (2021) Regulation of pericarp browning in cold-stored litchi fruit using methyl jasmonate. J King Saud Univ 33:101445. https://doi.org/10.1016/j.jksus.2021.101445
Dhindsa RS, Dhindsa P, Thorpe AT (1981) Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decrease levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Duman E, Soltanbeigi A, Özcan MM (2016) Chemical compositions of essential oil of some Citrus spp. (sour, lemon, kumquat, Mandarin and orange) peels. J Med Spice Plants 21(4):153–159
FAO Statistics (2020) F.A.O. report. https://www.fao.org/3/cb6492en/cb6492en.pdf
Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54:137–164. https://doi.org/10.1146/annurev.arplant.54.031902.134921
Geng S, Cui Z, Huang X, Chen Y, Xu D, Xiong P (2011) Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind Crops Prod 33(1):248–252. https://doi.org/10.1016/j.indcrop.2010.10.018
Giusti MM, Wrolstad RE (2001) Unit F1.2.1-13: anthocyanins characterization and measurement with UV-visible spectroscopy. In: Wrolstad RE (ed) Currents protocols in food analytical chemistry. John Wiley & Sons, New York
Habibi F, Serrano M, Zacarías L, Valero D, Guillén F (2021) Postharvest application of 24-epibrassinolide reduces chilling injury symptoms and enhances bioactive compounds content and antioxidant activity of blood orange fruit. Front Plant Sci 12:145–150. https://doi.org/10.3389/fpls.2021.629733
He Y, Li J, Ban Q, Han S, Rao J (2018) Role of brassinosteroids in persimmon (Diospyros kaki L.) fruit ripening. J Agric Food Chem 66:2637–2644. https://doi.org/10.1021/acs.jafc.7b06117
Huwei S, Asghari M, Zahedipour-Sheshglani P, Alizadeh M (2021) Modeling and optimizing the changes in physical and biochemical properties of table grapes in response to natural zeolite treatment. LWT 141:110854. https://doi.org/10.1016/j.lwt.2021.110854
Jalili Marandi R (2010) Growing of temperate zone fruits. Jahad Daneshghahi, p 362
Jin P, Wang SY, Gao H, Chen H, Zheng Y, Wang CY (2012) Effect of cultural system and essential oil treatment on antioxidant capacity inraspberries. Food Chem 132:399–405. https://doi.org/10.1016/j.foodchem.2011.11.011
Kahramanoglu I, Rengasamy KR, Usanmaz S, Alas T, Helvacı M, Okatan V, Aşkın MA, Wan C (2021) Improving the safety and security of fruits and vegetables during COVID-19 pandemic with postharvest handling. Crit Rev Food Sci Nutr 10:1–11. https://doi.org/10.1080/10408398.2021.1935703
Ke D, Saltveit ME (1986) Effects of calcium and auxin on russet spotting and phenylalanine ammoni- alyase activity in iceberg lettuce. Hort Sci 21:1169–1171
Khaliq G, Abbas HT, Ali I, Waseem M (2019) Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Hortic Environ Biotechnol 60(5):659–669. https://doi.org/10.1007/s13580-019-00159-z
Kochba J, Lavee S, Spiegel-Roy P (1977) Differevces in peroxidase activity and isoenzymes in embryogenic and non-embryogenic ‘shamouti’ orange ovular callus lines. Plant Cell Physiol 18:463–497. https://doi.org/10.1093/oxfordjournals.pcp.a075455
Kumar P, Sethi S, Sharma RR, Srivastav M, Varghese E (2017) Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci Hortic 226:104–109. https://doi.org/10.1016/j.scienta.2017.08.037
Li T, Yun Z, Wu Q, Zhang Z, Liu S, Shi X, Duan X, Jiang Y (2018) Proteomic profiling of 24-epibrassinolide-induced chilling tolerance in harvested banana fruit. J Proteomics 187:1–12. https://doi.org/10.1016/j.jprot.2018.05.011
Longo L, Vasapollo G (2006) Extraction and identification of anthocyanins from Smilax aspera L. berries. Food Chem 94:226–231. https://doi.org/10.1016/j.foodchem.2004.11.008
Matthaus B, Özcan MM (2012) Chemical evaluation of citrus seeds, an agro-industrial waste, as a new potential source of vegetable oils. Grasas Y Aceites 63(3):313–320. https://doi.org/10.3989/gya.118411
Munhuweyi K, Mpai S, Sivakumar D (2020) Extension of avocado fruit postharvest quality using non-chemical treatments. Agronomy 10:212–218. https://doi.org/10.3390/agronomy10020212
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Navarro JM, Flores P, Garrido C, Martinez V (2006) Changes in the contents of antioxidants compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem 96:66–73. https://doi.org/10.1016/j.foodchem.2005.01.057
Ncama K, Magwaza LS, Mditshwa A, Tesfay SZ (2018) Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag Shelf Life 16:157–167. https://doi.org/10.1016/j.fpsl.2018.03.011
Nikos G, Tzortzakis A (2007) Maintaining postharvest fungal rots on citrus fruit and sweet cherries using a pomegeranate peel extract. Postharvest Biol Technol 114:54–61. https://doi.org/10.1016/j.postharvbio.2015.11.012
Omaye ST, Turnbull JD, Sauberilich HE (1979) Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. In: Methods in enzymology. Academic Press, New York, pp 3–11 https://doi.org/10.1016/0076-6879(79)62181-x
Özcan M, Akgül A (2001) Chemical composition of the essential oil of bitter fennel (Foeniculum vulgare subsp. piperitum). J Spices Aromat Crop 10(1):49–50
Özcan MM, Chalchat JC (2006) Effect of collection time on chemical composition of the essential oil of Foeniculum vulgare subsp.piperitum growing wild in Turkey. Eur Food Res Technol 224:279–281. https://doi.org/10.1007/s00217-006-0309-x
Özcan MM, Chalchat JC, Arslan D, Ates A, Unver A (2006) Comparative essential oil composition and antifungal effect of bitter fennel (Foeniculum vulgare ssp. piperitum) fruit oils obtained during different vegetation. Natl Libr Med 9(4):552–561. https://doi.org/10.1089/jmf.2006.9.552
Özcan MM, Ghafoor K, Al Juhaimi F, Uslu N, Babiker EE, Mohamed Ahmed IA, Almusallam IA (2021) Influence of drying techniques on bioactive properties, phenolic compounds and fatty acid compositions of dried lemon and orange peel powder. J Food Sci Technol 58:147–158. https://doi.org/10.1007/s13197-020-04524-0
Özcan MM, Öztürk Ö, Lemiasheuski V (2022) Quality properties, fatty acid composition, and mineral contents of some citrus seeds and oils extracted by solvent extraction. Erwerbs-Obstbau. https://doi.org/10.1007/s10341-022-00675-w
Perez-Balibrea S, Moreno DA, Garcia-Viguera C (2011) Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem 129:35. https://doi.org/10.1016/j.foodchem.2011.03.049
Rapisarda P, Bianco ML, Pannuzzo P, Timpanaro N (2008) Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck. Postharvest Biol Technol 49:348–354. https://doi.org/10.1016/j.postharvbio.2008.02.002
Rathore HA, Masud T, Shehla XS, Soomro AH (2007) Effect of stronge on physico-chemical composition and sensory properties of Mango (Mangnifera indica L.,) variety Doseehari. Pak J Nutr 6:143–148
Shao X, Wang H, Xu F, Cheng S (2013) Effects and possible mechanisms of tea tree oil vapor treatmeant on the main disease in postharvest strawberry fruit. Postharvest Biol Technol 77:94–101. https://doi.org/10.1016/j.postharvbio.2012.11.010
Shin Y, Liu RH, Nock JF, Holliday D, Watkins CB (2007) Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol Technol 45:349–357. https://doi.org/10.1016/j.postharvbio.2007.03.007
Singh G, Maurya S, Lampasona MP, Catalan C (2006) Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Control 17:745–752. https://doi.org/10.1016/j.foodcont.2005.03.010
Slinkard K, Singleton VL (1977) Total phenol analysis automatin and comparison with manual methods. Am J Enol Vitic 28:49–55
Tadeo FR, Cercos M, Colmenero-Flores JM, Iglesias DJ, Naranjo MA, Rios G, Carrera E, Ruiz-Rivero O, Lliso I, Morillon R, Ollitrault P (2008) Molecular physiology of development and quality of citrus. Adv Bot Res 47:147–223. https://doi.org/10.1016/S0065-2296(08)00004-9
Tareen MJ, Abbasi NA, Hafiz IA (2012) Postharvest application of salicylic acid enhanced antioxidant enzyme activity maintained quality peach cv. Flor-daking: fruit during storage. Sci Hortic 142:221–228. https://doi.org/10.1016/j.scienta.2012.04.027
Vargas M, Albors A, Chiralt A, Gonzalez-Martinez C (2006) Quality of cold-stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol Technol 41:164–171. https://doi.org/10.1016/j.postharvbio.2006.03.016
Vergis J, Gokulakrishnan P, Agarwal RK, Kumar A (2015) Essential oils as natural food antimicrobial. Food Sci Nutr 55:1320–1323. https://doi.org/10.1080/10408398.2012.692127
Vilaplana R, Perez-Revelo K, Valencia-Chamorro S (2018) Essential oil as an alterntive Postharves treatment to control fusariosis, causd by Fusarium verticillioides, in fresh Pinneapples (Ananas comosum). Sci Hortic 238:255–563. https://doi.org/10.1016/j.scienta.2018.04.052
Wang CY, Wang SY, Yin JJ, Parry J, Yu LL (2007) Enhancing antioxidant, antiproliferation, and free radical scavenging activities in strawberries with essential oils. J Agric Food Chem 55(16):6527–6532. https://doi.org/10.1021/jf070429a
Zahedi SM, Hosseini MS, Karimi M, Ebrahimzadeh A (2019) Effects of postharvest polyamine application and edible coating on maintaining quality of mango (Mangifera indica L.) cv. Langra during cold storage. Food Sci Nutr 7(2):433–441. https://doi.org/10.1002/fsn3.802
Zhu F, Yun Z, Ma Q, Gong Q, Zeng Y, Xu J, Cheng Y, Deng X (2015) Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biology and Technology 100:8–15. https://doi.org/10.1016/j.postharvbio.2014.09.014
Ziv C, Fallik E (2021) Postharvest storage techniques and quality evaluation of fruits and vegetables for reducing food loss. Agronomy 11:1133–1137. https://doi.org/10.3390/agronomy11061133
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Rashidi, J. Amiri, and H. Shirzad declare that they have no competing interests.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Rashidi, H., Amiri, J. & Shirzad, H. Effect of Postharvest Treatment with 24-Epibrassinolide and Fennel (Foeniculum vulgare) Essential Oil on Quality Attributes and Storage Life of Orange (Citrus sinensis cv. ‘Valencia’). Erwerbs-Obstbau 65, 927–939 (2023). https://doi.org/10.1007/s10341-022-00791-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-022-00791-7