Abstract
Forests provide important ecosystem services, and the need for these services is expected to increase. Although disturbances are often important components of management plans for forest ecosystems, management efforts often target the prevention and mitigation of disturbances that threaten these services. Exotic insects are an increasingly important disturbance agent in terms of ecological and economic impacts, particularly large woodborers in the family Cerambycidae. Semiochemical-baited traps are important components of management programs. This study looked at the effect of trap location along a horizontal edge—forest gradient with traps deployed in forest clearings adjacent to forests, at the forest—clearing edge and in the forest. Nine species were captured in high enough numbers for analysis, and significant effects of trap location relative to the edge were observed in all but one. Three patterns of edge effects were observed. In general, Acmaeops proteus proteus (Kirby) was more abundant at the edge than in the forest interior; male and female Monochamus mutator LeConte and Monochamus scutellatus (Say) were more abundant in the clearing adjacent to the forest than in the forest; and Anelaphus pumilus (Newman), Euderces pini (Fabricius), Neoclytus acuminatus (Fabricius), male and female Monochamus carolinensis (Olivier) and female Monochamus titillator (Fabricius) were more abundant in the forest than the adjacent clearing. These results demonstrate that non-targeted surveys for Cerambycidae should deploy traps in multiple locations along the forest edge-interior gradient and that taxa-specific surveys should be developed based on knowledge of how this gradient impacts trap performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Trap location effects differed among species with some captured in higher numbers in open fields adjacent to forest edges (Monochamus mutator and Monochamus scutellatus), some at the forest edge (Acmaeops proteus proteus) and others in the forest (Anelaphus pumilus, Euderces pini, Neoclytus acuminatus, Monochamus carolinensis and Monochamus titillator).
-
These results suggest that generic surveys of Cerambycidae communities should deploy traps along edge-interior gradients and that taxa-specific surveys need to consider this gradient when deploying survey traps.
Introduction
Although estimates of the global annual value of ecosystem services vary (Constanza et al. 1997; Pimentel et al. 1997; Ninan and Inoue 2013), their importance is universally recognized and they continue to receive increasing research and policy attention. Historically, the value of forest and woodland ecosystem services has been underestimated, but recognition of the importance of forest and woodland ecosystems is increasing (Wingfield et al. 2015). Among terrestrial ecosystems, wetlands and forests are estimated to be the largest contributors to the annual value of global ecosystem services (Ninan and Inoue 2013). It has been estimated that globally ca. 1 in 6 people rely on forests for food and many more rely on non-food forest ecosystem services [e.g. carbon storage, wood and wood products resources (Vira et al. 2015; Wingfield et al. 2015)]. The significance of forest and woodland ecosystem services is expected to increase as human population levels increase globally.
Coincident with the increase in demand for forest ecosystem services has been an increase in the frequency and severity of disturbances experienced by forest ecosystems. For example, the frequency and severity of insect and fungal pathogen outbreaks have increased dramatically (Aukema et al. 2010; Boyd et al. 2013; Roy et al. 2014; Wingfield et al. 2015). Altered distributions and patterns of interactions among forest insects and fungal pathogens as a consequence of range expansions and introductions of species outside of their native ranges have contributed to these dramatic increases. Woodboring beetles from the family Cerambycidae (e.g. longhorned beetles) are among the most significant groups of invasive forest insects, both in terms of frequency and impact (Aukema et al. 2010, 2011). For example, Nowak et al. (2001) estimated the potential costs associated with Anoplophora glabripennis attacks on urban trees in nine US cities to be between $72 million and $2.3 billion. Nationally, A. glabripennis impacts were estimated to be as high as ca. 35% loss of total canopy cover, ca. 30% tree mortality with an associated maximum total cost of $669 billion. The direct and indirect impacts of invasive large woodboring beetles in North America include altered nutrient and successional cycles, facilitation of spread of invasive plants due to gap creation associated with tree mortality and the extirpation of species (Gandhi and Herms 2010a, b).
Invasive species management plans in North America attempt to limit the arrival of exotic forest insects with management strategies that integrate regulatory and preventative measures and when these fail eradication and containment tactics are used to manage establishment and/or spread (Liebhold and Tobin 2008). The success of eradication and containment efforts is contingent on the availability of affordable, effective survey and detection tools because: (1) there is a negative correlation between the probability of successful containment and/or eradication and target population size; (2) ongoing and accurate delineation of target species distribution is essential for successful containment and/or eradication; and (3) it is not possible to evaluate the success of containment and/or eradication efforts without them (Myers et al. 2000; Liebhold and Bascompte 2003; Liebhold and Tobin 2008).
Although alternate methods exist for the surveillance of forest insects (e.g. ground-based and aerial surveys, trap trees), flight intercept traps are the most commonly used in operational surveys and monitoring and management programs targeting forest insects. The performance of semiochemical-baited intercept traps is largely determined by the probability of capture of the target taxon if present and the target taxon distribution relative to the scale of sampling. The first factor is a function of trap active space and the capture and retention efficiency of the trap. Studies of intercept trap active space primarily consider quantitative and qualitative features of the semiochemicals used to bait the trap (e.g. Allison et al. 2004, 2012). Trap type and design features affect the abundance (McIntosh et al. 2001; Morewood et al. 2002; Allison et al. 2014; Allison and Redak 2017) and community (Dodds et al. 2015) of Cerambycidae sampled, likely due to combined effects on trap active space and capture and retention efficiencies.
The impact of trap location relative to environmental gradients and landscape features (i.e. target taxon distribution relative to the scale of sampling) on Cerambycidae captures has received far less attention than semiochemical development and trap type and design effects. The two gradients that have received limited attention are horizontal edge-interior and vertical understory-canopy gradients (Harman and Harman 1987; Vance et al. 2003; Ulyshen et al. 2004; Wermelinger et al. 2007; Dodds 2011, 2014; Graham et al. 2012; Vodka and Cizek 2013; Maguire et al. 2014; Su and Woods 2014; Schmeelk et al. 2016; Li et al. 2017; Ulyshen and Sheehan 2017; Rassati et al. 2018). The objective of this study was to characterize the impact of trap location relative to forest edges (horizontal clear-cut—edge—interior gradient) on the capture of Cerambycidae by semiochemical-baited intercept traps. Many of the most damaging cerambycid pests and species on North American regulatory agency (e.g. USDA-APHIS, CFIA) watch lists belong to the subfamilies Cerambycinae and Lamiinae. To document patterns of effects of these gradients on species from both subfamilies, field-trapping experiments were replicated in central Louisiana and northern Ontario using blends of semiochemicals attractive to species from the two subfamilies.
Materials and methods
Three field-trapping experiments were conducted to examine the distribution of target species of Cerambycidae across 60 m, linear transects perpendicular to forest edges. Intercept traps baited with blends of semiochemicals targeted species from the two largest subfamilies of Cerambycidae, the Lamiinae in Northern Ontario and Central Louisiana (Experiments 1 and 3) and the Cerambycinae in Central Louisiana (Experiment 2). Experiments 1 and 3 targeted species of Monochamus (a genus from the subfamily Lamiinae) by baiting traps with ethanol, α-pinene and 2-(undecyloxy)-ethanol (hereafter monochamol) (Experiment 1) or α-pinene, monochamol, ipsdienol, ipsenol, cis-verbenol and lanierone (Experiment 3), semiochemicals known to be attractive to Monochamus spp. (see Pajares et al. 2010; Allison et al. 2001, 2003, 2004, 2012, 2013; Miller et al. 2016; Boone et al. 2018). Experiment 2 targeted Anelaphus pumilus (Newman), Euderces pini (Fabricius) and Neoclytus acuminatus (Fabricius) (all species of Cerambycinae) by baiting traps with racemic 3-hydroxy-2-hexanone, racemic 3-hydroxy-2-octanone and syn-2,3-hexanediols, compounds known to be attractive to several species in the Cerambycinae (Hanks and Millar 2016). These species were targeted because semiochemical attractants are known for them and they could be captured in high numbers, they represent the two largest subfamilies of the Cerambycidae and most invasive Cerambycidae of concern belong to these two subfamilies. Although experiments 1–3 targeted these species, all Cerambycidae were counted and any species for which we captured ≥ 1 individual per trap was subjected to statistical analyses.
Twelve-unit multiple-funnel traps were deployed in linear transects perpendicular to abrupt forest edges marking the transition between an open field [two clear-cuts in Experiment 1 and a gas (n = 4) or railroad/highway (n = 1) right-of-way or clear-cut (n = 2) in Experiments 2 and 3] and forest. Each transect had seven traps and a symmetrical design with a single trap at the edge and at ± 30, ± 10 and ± 3 m from the edge (i.e. seven total traps with three in both the open field and forest habitats adjacent to the edge and one at the edge). Although horizontal edge gradients are normally described from the forest edge to the interior, intercept traps in survey programs for forest insects are often deployed in clearings adjacent to forest edges for convenience. All traps were multiple-funnel traps treated with Fluon and equipped with a wet collection cup containing 150–200 ml of propylene glycol to increase trap performance (Graham et al. 2010; Allison et al. 2011, 2014, 2016; Graham and Poland 2012; Allison and Redak 2017). Metal conduit pipe was driven into the ground and traps suspended individually from a bend near the top so that the bottom of the collection cup of each trap was 0.5–1.0 m above the ground.
Lures
Ultra-high release (UHR) ethanol (release rate ca. 0.5 g/d at 23 °C), UHR α-pinene (chemical purity ≥ 95%, enantiomeric purity 95% (−); release rate ca. 2 g/d at 20 °C) and monochamol (99.3% pure; release rate ca. 0.4 mg/d at 20 °C) were purchased from Contech Enterprises, Inc. (Delta, B.C.). Ipsenol (chemical purity > 98%, racemic; release rate ca. 0.4 mg/d at 20 °C), ipsdienol (chemical purity > 98%, racemic; release rate ca. 0.4 mg/d at 20 °C), lanierone (chemical purity > 98%, release rate ca. 0.01–0.03 mg/d at 22–24 °C) and cis-verbenol (chemical purity > 98%; 13:87 mixture of plus and minus enantiomers; release rate ca. 0.3–0.6 mg/d at 22 °C) were purchased from Synergy Semiochemicals. Racemic 3-hydroxy-2-hexanone (99% pure, release rate ca. 20–25 mg/d at 20 °C) and racemic 3-hydroxy-2-octanone (99% pure, release rate ca. 20–25 mg/d at 20 °C), purchased from Bedoukian Research (Danbury CT), and syn-2,3-hexanediols (99% pure, release rate ca. 1–2 mg/d at 20 °C), synthesized by Peter Mayo (Atlantic Forestry Centre. Fredericton, NB), were loaded into pouch lures by Contech Enterprises, Inc. (Delta, BC). Purity and release rate data were supplied by the suppliers.
Experiment 1: Lamiinae in northern Ontario
Traps were baited with ethanol, α-pinene and monochamol on 16 July 2014 and targeted Monochamus mutator LeConte and Monochamus scutellatus (Say), two species from the subfamily Lamiinae. Insects were collected ca. weekly on 24 and 30 July; 6, 13, 20 and 27 August; and 3, 10, 17 and 26 September 2014. A total of six transects (42 traps total) were established perpendicular to forest edges marking the transition between clear-cuts and adjacent forest. The six transects were divided equally between two clear-cuts (cutting occurred in fall 2012) separated by ca. 5 km beginning ca. 20 km north of Highway 129 on Hinkler Road north of Aubrey Falls, ON and within sites transects were spaced 1–2 km apart. The clear-cut (prior to harvest) and adjacent stands were located in the Great Lakes—St. Lawrence forest region and predominately jack pine, Pinus banksiana Lamb. (ca. 70%). Cerambycidae were identified to species using morphology, and all Monochamus were sexed using antennal and abdominal morphology (Yanega 1996; Lingafelter 2007).
Experiment 2: Cerambycinae in central Louisiana
Traps were baited with racemic 3-hydroxy-2-hexanone, racemic 3-hydroxy-2-octanone and syn-2,3-hexanediols on 16–17 April 2014. Insects were collected on 21–22 and 24 April; 1, 8, 15, 22, and 29 May; and 5 June at which time lures were collected from traps. This experiment targeted the Cerambycinae species Anelaphus pumilus, Euderces pini and Neoclytus acuminatus. The seven sites in Central Louisiana took advantage of existing forest openings, being right-of-ways for a gas pipeline (n = 4), railroad and highway (n = 1) or clear-cuts (n = 2) in both Experiments 2 and 3 (the rights of way were at least 120 m wide). A total of seven transects (49 traps total) were deployed (the closest distance between transects was 0.6 km). The right-of-way (prior to cutting) and adjacent forest were in the Kisatchie National Forest, Catahoula Ranger District and was predominately loblolly pine, Pinus taeda L. and mixed hardwoods that experienced a prescribed burn in 2010. Voucher specimens from a previous study on the Cerambycidae of Central Louisiana (Allison et al. 2014), were used to identify A. pumilus, Elaphidion mucronatum (Say), E. pini and N. acuminatus. Monochamus carolinensis (Olivier) and M. titillator (Fabricius) were identified to species and sexed using morphology (Yanega 1996; Lingafelter 2007).
Experiment 3: Lamiinae in central Louisiana
Traps were baited with monochamol, α-pinene, ipsenol, ipsdienol, lanierone and cis-verbenol on 23 June 2014. Insects were collected every-other-week: 1, 15 and 29 July; 14 and 27 August and 10 September 2014. This experiment targeted the species M. carolinensis and M. titillator from the subfamily Lamiinae and used the same transects described above and used in Experiment 2. Individual Cerambycidae were identified as described in Experiment 2.
Statistics
The experimental designs were the same for all three field experiments, and therefore, the data were analysed similarly. Sums of each species for which ≥ 1 individual per trap was captured were divided by the number of trapping days to standardize to catch per day; this response variable (# of individuals captured/trap day), transformed by ln + 0.1 to normalize variances (as evaluated by residual plots), was subjected to generalized linear mixed model analysis of variance with block as the random variable using JMP®, version 13.1. SAS Institute Inc., Cary, NC. Means were compared using Tukey’s HSD and considered different when P < 0.05.
Results
Experiment 1
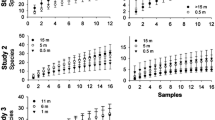
In Experiment 1, a total of 290 A. p. proteus (Kirby), 1790 male M. mutator, 3556 female M. mutator, 3870 male M. scutellatus and 4028 female M. scutellatus were captured. There was a significant treatment effect on the abundance of A. p. proteus (F6,30 = 2.9, P < 0.05), male and female M. mutator (male: F6,30 = 25.7, P < 0.0001; female: F6,30 = 31.9, P < 0.0001) and male and female M. scutellatus (male: F6,30 = 20.9, P < 0.0001; female: F6,30 = 21.1, P < 0.0001) (Fig. 1). In general, A. p. proteus were more abundant at or near the forest edge. The only significant difference in the abundance of A. p. proteus was observed between traps 30 m from the forest edge in the forest and those at the forest edge. In general, the abundance of male and female M. mutator and M. scutellatus in traps was greatest at the forest edge and in the open field (i.e. clear-cut), and declined significantly as few as 3 m from the edge inside the forest (Fig. 1). Mean numbers per trap of both male and female M. mutator and M. scutellatus were significantly greater in traps located in the open clear-cuts 30 m from the forest edge, than in traps located inside the forest, 3, 10 or 30 m from the edge, but did not differ significantly from traps located on the edge, or 3 and 10 m from the edge in the open (Fig. 1).
Mean number of individuals captured in Experiment #1 run in Northern Ontario. Traps were deployed along a horizontal edge—forest gradient. Traps were either placed in the forest interior 30, 10 and 3 m from the forest edge (the − 30, − 10 and − 3 m trap positions), at the forest edge (the 0 trap position) or in a clear-cut adjacent to the forest (the 3, 10 and 30 m trap positions). A total of six transects were established with seven traps per transect (42 traps total). Letters indicate significant differences at P < 0.05 as determined by mixed model ANOVA and Tukey’s HSD for Acmaeops proteus proteus, Monochamus mutator (female = black bars; male = grey bars) and Monochamus scutellatus (female = black bars; male = grey bars)
Experiment 2
In Experiment 2, a total of 9267 A. pumilus, 74 E. mucronatum, 1432 E. pini and 1240 N. acuminatus were captured in the 49 traps. There was a significant effect of trap location on the abundance of A. pumilus (F6, 36 = 4.9, P < 0.001), E. pini (F6, 36 = 27.4, P < 0.0001) and N. acuminatus (F6, 36 = 17.0, P < 0.0001) (Fig. 2). Elaphidion mucronatum was by far the least abundant in our traps of the four species we analysed, and there was no effect of trap location observed with this species (F6, 36 = 1.3, P = 0.29). In general, the highest numbers of A. pumilus, E. pini and N. acuminatus were caught in traps located the furthest into the forest. With A. pumilus statistical differences were observed between traps 30 m into the forest and traps 3 m from the edge in the forest and traps 10 and 30 m from the edge in the open field. The abundance of both E. pini and N. acuminatus was greatest in traps located deep inside the forest and declined significantly in traps located nearer the forest edge or in the open field (Fig. 2).
Mean number of individuals captured in Experiment #2 run in central Louisiana. Traps were deployed along a horizontal edge—forest gradient. Traps were either placed in the forest interior 30, 10 and 3 m from the forest edge (the − 30, − 10 and − 3 m trap positions), at the forest edge (the 0 trap position) or in an opening [clear-cut (n = 2), gas pipeline right-of-way (n = 4), highway and railroad right-of-way (n = 1)] adjacent to the forest (the 3, 10 and 30 m trap positions). A total of seven transects were established with seven traps per transect (49 traps total). Letters indicate significant differences at P < 0.05 as determined by mixed model ANOVA and Tukey’s HSD for Anelaphus pumilus, Euderces pini and Neoclytus acuminatus
Experiment 3
In Experiment 3, a total of 3008 female M. carolinensis, 1987 male M. carolinensis, 3802 female M. titillator and 2371 male M. titillator were captured. There was a significant effect of trap location on both male and female M. carolinensis (male: F6, 36 = 4.5, P < 0.01; female: F6, 36 = 4.0, P < 0.01) and female M. titillator (F6, 36 = 3.4, P < 0.01) (Fig. 3) but not male M. titillator (F6, 36 = 1.2, P = 0.3409). In general, the abundance of male and female M. carolinensis and female M. titillator were higher in the forest and at the forest edge than in the open field. Female M. carolinensis were more abundant 30 m from the edge in the forest and at the forest edge, than 10 m from the edge in the open field. Male M. carolinensis were more abundant in traps 30 and 3 m from the edge in the forest, and at the forest edge, than in traps 10 m from the edge in the open field. Female M. titillator were more abundant at the forest edge than in traps 10 m from the edge in the open field.
Mean number of individuals captured in Experiment #3 run in central Louisiana. Traps were deployed along a horizontal edge—forest gradient. Traps were either placed in the forest interior 30, 10 and 3 m from the forest edge (the − 30, − 10 and − 3 m trap positions), at the forest edge (the 0 trap position) or in an opening [clear-cut (n = 2), gas pipeline right-of-way (n = 4), highway and railroad right-of-way (n = 1)] adjacent to the forest (the 3, 10 and 30 m trap positions). A total of seven transects were established with seven traps per transect (49 traps total). Letters indicate significant differences at P < 0.05 as determined by mixed model ANOVA and Tukey’s HSD for Monochamus carolinensis (female = black bars; male = grey bars) and Monochamus titillator (female = black bars; male = grey bars)
Discussion
Trap placement along a horizontal open field/clear-cut—edge—forest interior gradient had a significant effect on the abundance of several species from the two largest subfamilies of the Cerambycidae, the Cerambycinae and Lamiinae. Nine species were captured in high enough numbers for statistical analyses, and significant effects of the edge gradient on the number captured were observed for all but one (Elaphidion mucronatum, captured in very low numbers). The most common pattern observed was for abundance to be highest in the forest interior and to decrease from the forest edge out into the open field/clear-cut habitat adjacent to the forest edge. Alternate patterns observed include the mirror image of the most common pattern (i.e. higher catches in the open field/clear-cut habitat with decreasing captures moving along the gradient from the edge into the forest interior) and decreasing captures moving along the gradient from the forest edge in both directions.
Several studies have described patterns of effects of horizontal edge-forest interior gradients for forest insects. There is evidence that high taxonomic levels can provide meaningful estimates of species richness (Williams and Gaston 1994; Balmford et al. 1996a, b). Given the challenge of processing trap captures to the species level, it is not surprising that the majority of studies that have examined edge effects in forest insects have done so at high taxonomic levels (e.g. Deans et al. 2005). Responses to edges vary among communities, species and populations. While some taxa are reported to prefer forest edges [e.g. Carabidae, Staphylinidae and Cantharidae (Buse and Good 1993; Bedford and Usher 1994; Jokimäki et al. 1998; Magura et al. 2001; Phillips et al. 2006)], others have been reported to be more abundant in the forest interior [e.g. bark beetles (Peltonen and Heliövaara 1998)] or clearings adjacent to forests [e.g. Buprestidae (Wermelinger et al. 2007)]. Vodka and Cizek (2013) looked at the diversity of saproxylic species along a horizontal edge-forest gradient and observed that diversity was > 60% higher in the interior than at the edge. Guimarães et al. (2014) conducted a meta-analysis of the effects of forest edges on herbivore abundance and richness and reported that insect herbivores were 14% more abundant at forest edges than interiors and that richness was almost 65% higher at forest edges than interiors.
Few studies have focused on edge effects on the Cerambycidae. Wermelinger et al. (2007) observed higher species richness for Cerambycidae and Scolytinae at soft edges than hard edges. Dodds (2011) examined the abundance and species richness of Cerambycidae along a horizontal clearing-edge-forest gradient and observed that the abundance of Cerambycidae was higher at the edge than the forest interior. Species richness did not differ between the edge and forest interior, but the number of species of Cerambycidae was higher at the edge than in the clearing. The development of predictive models of the effect of trap placement along horizontal edge-forest gradients will require the description of species level patterns of effects. Harman and Harman (1987) observed higher densities of Megacyllene robiniae at forest edges than in the clearings adjacent to the forests, and McCann and Harman (1990) reported that M. robiniae attack rate of black locust increased moving from interior mixed forest stands to the forest edge and into adjacent areas of clonal growth and open growth individual trees. Dunn et al. (2016) looked at Cerambycidae abundance along a horizontal forest edge—open field gradient and observed that total number of Cerambycidae, Xylotrechus colonus (Fabricius) and Prionus laticollis (Drury) decreased as you moved along the gradient out from the forest edge. Dunn et al. (2016) did not sample the forest habitat and consequently it is not clear if the higher trap captures at the edge indicate true edge species or forest species captured in higher numbers in edge traps because of their proximity to the forest interior. Cumulatively, the literature on horizontal edge—forest interior suggests that distribution patterns along environmental gradients of forest insects are variable and context dependent. Additional studies describing patterns at the species level are needed before patterns in effects among the Cerambycidae (e.g. by taxonomy, ecology) will begin to emerge.
This study observed that patterns of edge effects differed not only among species from different subfamilies, but also among congeners from the genus Monochamus. Surprisingly, species from this genus in Ontario (M. scutellatus and M. mutator) and Louisiana (M. carolinensis and M. titillator) had opposing patterns of edge effects. Two potential explanations for effects of horizontal edge gradients on abundance are differential survivorship of the previous generation along the gradient and adult movement (McGeoch and Gaston 2000). Resources associated with habitats adjacent to forest edges can also result in changes in species abundance and distribution. Movement of adults in response to resources located in the clear-cuts adjacent to forest edges at the Ontario sites likely contributes to the different edge effect patterns observed in Ontario and Louisiana. The clear-cuts used to establish transects in Ontario were created in the fall of 2012, and abundant logging debris (i.e. oviposition substrate) was left on site. At the sites used to establish transects in Louisiana, habitat adjacent to the forest did not have host resources available for oviposition. Although saproxylic beetle abundance and diversity increase in response to disturbance (most likely due to the increased availability of coarse woody debris), these responses are usually short-lived. The amount of coarse woody debris present in an area is negatively correlated with the time since disturbance (Spies et al. 1988; Siitonen 2001; Grove 2002) and likely explains the observed returns to pre-disturbance abundance and diversity levels. For example, Ulyshen et al. (2004) sampled forest Coleoptera in canopy gaps of different ages (and sizes) along a horizontal edge-forest gradient and observed that both the abundance and diversity of saproxylic beetles was higher in the centre of young than old gaps. Similarly, Wallace (1869) reported that young forest clearings were the most productive habitats for collecting Cerambycidae and other woodborers. Werner (2002) found that several groups of woodborers (including Cerambycidae) increased in abundance following a disturbance event but quickly declined to levels below pre-disturbance levels.
All of the species of Cerambycidae in this study feed as larvae on weakened and dying or recently dead trees (M. carolinensis, M. titillator, A. p. proteus on conifers and N. acuminatus, A. pumilus and E. pini on hardwoods) and sympatric species overlap in phenology (Yanega 1996; Lingafelter 2007). The mechanism(s) driving the edge effects observed in the remaining species in this study (M. carolinensis, M. titillator, A. p. proteus, N. acuminatus, A. pumilus and E. pini) are less clear. Despite numerous studies in several forest types, consensus on the mechanisms driving edge effects in forest habitats does not exist. It is clear that edges mark the transition from forest interior with a microclimate dominated by the crown canopy, to adjacent habitat matrices with climate usually controlled by the soil. This transition is usually associated with changes in microclimate. For example, clear-cut—forest edges delineate a significant change in the amount of solar radiation reaching and irradiating from the soil and as a result daily temperatures are higher and fluctuate more widely in the clear-cut than forest (Fetcher et al. 1985). Additionally, the orientation of and structure/density of vegetation [termed physiognomy by Murcia (1995)] at the edge can influence edge effects. Vegetative structure/density at forest edges and cardinal orientation can both reduce the penetration of solar radiation and influence edge zones associated with edges (i.e. increased structure/density and north-facing edges in the northern hemisphere can both reduce the size of the edge zone) (Palik and Murphy 1990; Matlack 1993, 1994). Additional factors that can influence edge effects include habitat matrix age and type, and the management history of the forest and adjacent habitat matrix (Murcia 1995). Given the diversity of factors that can influence forest edge effects and the challenges associated with locating adequate replicates, it is not surprising that consensus has not yet emerged.
The need for survey and detection tools to characterize the structure and change of geographical species ranges has increased in recent years as a consequence of concerns about: (1) how species will respond to climate change (e.g. Davis et al. 1998); (2) what the optimal strategy is for maintaining biodiversity (Gaston 1994); and (3) how to understand and manage the spread of invasive alien species (Williamson 1996). Coincident with the need for survey and detection tools is the development of protocol for effective deployment of these tools. To achieve this objective, additional studies describing patterns of gradient effects (e.g. horizontal edge-forest gradients and vertical understory-canopy gradients) at the species level are needed. Further, although the diversity of variables that must be considered makes obtaining adequate replicates challenging, studies that identify the mechanisms driving the patterns of edge effects observed at the species level are needed. The results of this study have obvious implications for the management of forest insect pests, particularly large woodborers. Early detection and rapid response surveys attempt to detect non-native species in the early stages of an invasion. This study suggests that these programs should deploy traps at multiple locations along the forest edge-interior gradient to increase the likelihood of early detection. Similarly, survey programs that target specific taxa (e.g. vectors of the pinewood nematode, Monochamus spp.) need to consider the impact of trap location along forest edge-interior gradients.
Author contributions
Research conceived by JA, BS and JS. BS conducted data analyses. PM synthesized pheromone. JA wrote the manuscript. All authors approved the final version of the manuscript.
References
Allison JD, Redak RA (2017) The impact of trap type and design features on survey and detection of bark and woodboring beetles and their associates: a review and meta-analysis. Ann Rev Entomol 62:127–146
Allison JD, Borden JH, McIntosh RL, de Groot P, Gries R (2001) Kairomonal responses by four Monochamus species (Coleoptera: Cerambycidae) to bark beetle pheromones. J Chem Ecol 27:633–646
Allison JD, Morewood WD, Borden JH, Hein KE, Wilson IM (2003) Differential bioactivity of Ips and Dendroctonus pheromone components for Monochamus clamator and M. scutellatus (Coleoptera: Cerambycidae). Environ Entomol 32:23–30
Allison JD, Borden JH, Seybold SJ (2004) A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14:123–150
Allison JD, Johnson CW, Meeker JR, Strom BL, Butler SM (2011) Effect of aerosol surface lubricants on the abundance and richness of selected forest insects captured in multiple-funnel and panel traps. J Econ Entomol 104:1258–1264
Allison JD, McKenney JL, Millar JG, McElfresh JS, Mitchell RF, Hanks LM (2012) Response of the woodborers Monochamus carolinensis and Monochamus titillator (Coleoptera: Cerambycidae) to known cerambycid pheromones in the presence and absence of the host plant volatile α-pinene. Environ Entomol 41:1587–1596
Allison JD, McKenney JL, Miller DR, Gimmel ML (2013) Kairomonal responses of natural enemies and associates of the southern Ips (Coleoptera: Curculionidae: Scolytinae) to ipsdienol, ipsenol and cis-verbenol. J Insect Behav 26:321–335
Allison JD, Bhandari BD, McKenney JL, Millar JG (2014) Design factors that influence the performance of flight intercept traps for the capture of longhorned beetles (Coleoptera: Cerambycidae) from the subfamilies Lamiinae and Cerambycinae. PLoS ONE 9:e93203
Allison JD, Graham EE, Poland TM, Strom BL (2016) Dilution of fluon before trap surface treatment has no effect on longhorned beetle (Coleoptera: Cerambycidae) captures. J Econ Entomol 109:1215–1219
Aukema JE, McCullough DG, von Holle B, Liebhold AM, Britton K, Frankel SJ (2010) Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60:886–897
Aukema JE, Leung B, Kovacs K, Chivers C, Britton KO, Englin J, Frankel SJ, Haight RG, Holmes TP, Liebhold AM, McCullough DG, von Holle B (2011) Economic impacts of non-native forest insects in the continental United States. PLoS ONE 6:e24587
Balmford A, Green MJB, Murray G (1996a) Using higher-taxon richness as a surrogate for species richness. I. Regional tests. Proc R Soc Lond B 263:1571–1575
Balmford A, Jayasuriya HM, Green MJB (1996b) Using higher-taxon richness as a surrogate for species richness. II. Local applications. Proc R Soc Lond B 263:1571–1575
Bedford SE, Usher MB (1994) Distribution of arthropod species across the margins of farm woodlands. Agric Ecosyst Environ 48:295–305
Boone CK, Sweeney J, Silk P, Hughes C, Webster RP, Stephen F, Maclauchlan L, Bentz B, Drumont A, Zhao B, Berkvens N, Casteels H, Gregoire JC (2018) Monochamus species from different continents can be effectively detected with the same trapping protocol. J Pest Sci. https://doi.org/10.1007/s10340-018-0954-4
Boyd IL, Freer-Smith PH, Gilligan CA, Godfray HCJ (2013) The consequence of tree pests and diseases for ecosystem services. Science 342:1235773
Buse A, Good JEG (1993) The effects of conifer forest design and management on abundance and diversity of rove beetles (Coleoptera: Staphylinidae): implications for conservation. Biol Conserv 64:67–76
Constanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hanna B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystems services and natural capital. Nature 387:253–260
Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S (1998) Making mistakes when predicting shifts in species range in response to global warming. Nature 391:783–786
Deans AM, Malcolm JR, Smith SM, Bellocq MI (2005) Edge effects and the responses of aerial insect assemblages to structural-retention harvesting in Canadian boreal peatland forests. For Ecol Manag 204:249–266
Dodds KJ (2011) Effects of habitat type and trap placement on captures of bark (Coleoptera: Scolytidae) and longhorned (Coleoptera: Cerambycidae) beetles in semiochemical-baited traps. J Econ Entomol 104:879–888
Dodds KJ (2014) Effect of trap height on captures of arboreal insects in pine stands of northeastern United States of America. Can Entomol 146:80–89
Dodds KJ, Allison JD, Miller DR, Hanavan RP, Sweeney J (2015) Considering species richness and rarity when selecting optimal survey traps: Comparisons of semiochemical baited flight intercept traps for Cerambycidae in eastern North America. Agric For Entomol 17:36–47
Dunn E, Hough-Goldstein J, Hanks LM, Millar JG, D’Amico V (2016) Range of attraction of pheromone lures and dispersal behavior of cerambycid beetles. Ann Entomol Soc Am 109:872–880
Fetcher N, Oberbauer SF, Strain BR (1985) Vegetation effects on microclimate in lowland tropical forest in Costa Rica. Int J Biometeorol 29:145–155
Gandhi JKJ, Herms DA (2010a) Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions 12:389–405
Gandhi JKJ, Herms DA (2010b) North American arthropods at risk due to widespread Fraxinus mortality caused by the alien emerald ash borer. Biol Invasions 12:1839–1846
Gaston KJ (1994) Rarity. Chapman Hall, London
Graham EE, Poland TM (2012) Efficacy of fluon conditioning for capturing cerambycid beetles in different trap designs and persistence on panel traps over time. J Econ Entomol 105:395–401
Graham EE, Mitchell PF, Reagel PF, Barbour JD, Millar JG, Hanks LM (2010) Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J Econ Entomol 103:641–647
Graham EE, Poland TM, McCullough DG, Millar JG (2012) A comparison of trap type and height for capturing cerambycid beetles (Coleoptera). J Econ Entomol 105:837–846
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annu Rev Ecol Syst 33:1–23
Guimarães C, Viana J, Cornelissen T (2014) A meta-analysis of the effects of fragmentation on herbivorous insects. Environ Entomol 43:537–545
Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Harman DM, Harman AL (1987) Distribution pattern of adult locust borers, (Coleoptera: Cerambycidae) on nearby goldenrod, Solidago spp. (Asteraceae), at a forest-field edge. Proc Entomol Soc Wash 89:706–710
Jokimäki J, Huhta E, Itämies J, Rahko P (1998) Distribution of arthropods in relation to forest patch size, edge, and stand characteristics. Can J For Res 28:1068–1072
Li Y, Meng Q, Silk P, Gao W, Mayo P, Sweeney J (2017) Effect of semiochemicals and trap height on catch of Neocerambyx raddei in Jilin province, China. Entomol Exp Appl 164:94–101
Liebhold AM, Bascompte J (2003) The Allee effect, stochastic dynamics and the eradication of alien species. Ecol Lett 6:133–140
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Ann Rev Entomol 53:387–408
Lingafelter SW (2007) Illustrated key to the longhorned woodboring beetles of the eastern United States. Coleopterists Society, North Potomac
Maguire DY, Robert K, Brochu K, Larrivee M, Buddle CM, Wheeler TA (2014) Vertical stratification of beetles (Coleoptera) and flies (Diptera) in temperate forest canopies. Environ Entomol 43:9–17
Magura T, Tothmeresz B, Molnar T (2001) Forest edge and diversity: carabids along forest-grassland transects. Biodivers Conserv 10:287–300
Matlack GR (1993) Microenvironment variation within and among forest edge sites in the eastern United States. Biol Conserv 66:185–194
Matlack GR (1994) Vegetation dynamics of the forest edge—trends in space and successional time. J Ecol 82:113–123
McCann JM, Harman DM (1990) Influence of the intrastand position of black locust trees on attack rate of the locust borer (Coleoptera: Cerambycidae). Ann Entomol Soc Am 83:705–711
McGeoch MA, Gaston KJ (2000) Edge effects on the prevalence and mortality factors of Phytomyza ilicis (Diptera, Agromyzidae) in a suburban woodland. Ecol Lett 3:23–29
McIntosh RL, Katinic PJ, Allison JD, Borden JH, Downey DL (2001) Comparative efficacy of five types of trap for woodborers in the Cerambycidae, Buprestidae and Siricidae. Agric For Entomol 3:113–120
Miller DR, Allison JD, Crowe CM, Dickinson DM, Eglitis A, Hofstetter RW, Munson AS, Poland TM, Reid LS, Steed BE, Sweeney JD (2016) Pine sawyers (Coleoptera: Cerambycidae) attracted to α-pinene, monochamol, and ipsenol in North America. J Econ Entomol 109:1205–1214
Morewood WD, Hein KE, Katinic PJ, Borden JH (2002) An improved trap for large wood-boring insects with special reference to Monochamus scutellatus (Coleoptera: Cerambycidae). Can J For Res 32:519–525
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Myers JH, Simberloff D, Kuris AM, Carey JR (2000) Eradication revisited: dealing with exotic species. Trends Ecol Evol 15:316–320
Ninan KN, Inoue M (2013) Valuing forest ecosystem services: what we know and what we don’t. Ecol Econ 93:137–149
Nowak DJ, Pasek JE, Sequeira RA, Crane DE, Mastro VC (2001) Potential effect of Anoplophora glabripennis (Coleoptera: Cerambycidae) on urban trees in the United States. J Econ Entomol 94:116–122
Pajares JA, Álvarez G, Ibeas F, Gallego D, Hall DR, Farman DI (2010) Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J Chem Ecol 36:570–583
Palik BJ, Murphy PG (1990) Disturbance versus edge effects in sugar-maple/beech forest fragments. For Ecol Manag 32:187–202
Peltonen M, Heliövaara K (1998) Incidence of Xylechinus pilosus and Cryphalus saltuarius (Scolytidae) in forest-clearcut edges. For Ecol Manag 103:141–147
Phillips ID, Cobb TP, Spence JR, Brigham RM (2006) Salvage logging, edge effects, and carabid beetles: connections to conservation and sustainable forest management. Environ Entomol 35:950–957
Pimentel D, Wilson C, McCullum C, Huang R, Dwen P, Flack J, Tran Q, Saltman T, Cliff B (1997) Economic and environmental benefits of biodiversity. Bioscience 47:747–757
Rassati D, Marini L, Marchioro M, Rapuzzi P, Magnani G, Poloni R, Di Giovanni F, Mayo P, Sweeney J (2018) Developing trapping protocols for wood-boring beetles associated with broadleaf trees. J Pest Sci. https://doi.org/10.1007/s10340-018-0984-y
Roy BA, Alexander HM, Davidson J, Campbell FT, Burdon JJ, Sniezko R, Brasier C (2014) Increasing forest loss worldwide from invasive pests requires new trade regulations. Front Ecol Environ 12:457–465
Schmeelk TC, Millar JG, Hanks LM (2016) Influence of trap height and bait type on abundance and species diversity of cerambycid beetles captured in forest of east-central Illinois. J Econ Entomol 109:1750–1757
Siitonen J (2001) Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol Bull 49:11–42
Spies TA, Franklin JF, Thomas TB (1988) Coarse woody debris in Douglas-fir forests of western Oregon and Washington. Ecology 69:1689–1702
Su JC, Woods SA (2014) Importance of sampling along a vertical gradient to compare the insect fauna in managed forests. Environ Entomol 30:400–408
Ulyshen MD, Sheehan TN (2017) Trap height considerations for detecting two economically important forest beetle guilds in southeastern US forests. J Pest Sci. https://doi.org/10.1007/s10340-017-0883-7
Ulyshen MD, Hanula JL, Horn S, Kilgo JC, Moorman CE (2004) Spatial and temporal patterns of beetles associated with coarse woody debris in managed bottomland hardwood forests. For Ecol Manage 199:259–272
Vance CC, Kirby KR, Malcolm JR, Smith SM (2003) Community composition of longhorned beetles (Coleoptera: Cerambycidae) in the canopy and understory of sugar maple and white pine stands in south-central Ontario. Environ Entomol 32:1066–1074
Vira B, Wildburger C, Mansourian S (2015) Forests, trees and landscapes for food security and nutrition. IUFRO World Ser 33:1–172
Vodka Š, Cizek L (2013) The effects of edge-interior and understory-canopy gradients on the distribution of saproxylic beetles in a temperate lowland forest. For Ecol Manage 304:33–41
Wallace AR (1869) The Malay Archipelago: the land of the orang-utan, and the bird of paradise. MacMillan and Co., London
Wermelinger B, Flückiger PF, Obrist MK, Duelli P (2007) Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges. J Appl Entomol 131:104–114
Werner RA (2002) Effect of ecosystem disturbance on diversity of bark and wood-boring beetles (Coleoptera: Scolytidae, Buprestidae, Cerambycidae) in white spruce (Picea glauca (Moench) Voss) ecosystems of Alaska. US Department of Agriculture, Forest Service, Pacific Northwest Research Station, Research Paper PNW-RP-546
Williams PH, Gaston KJ (1994) Measuring more of biodiversity: can higher-taxon richness predict wholesale species richness? Biol Conserv 67:211–217
Williamson M (1996) Biological invasions. Chapman Hall, London
Wingfield MJ, Brockerhoff EG, Wingfield BD, Slippers B (2015) Planted forest health: the need for a global strategy. Science 349:832–836
Yanega D (1996) Field guide to northeastern longhorned beetles (Coleoptera: Cerambycidae). Illinois Natural History Survey, Manual 6, Champaign
Acknowledgements
This work was funded by Natural Resources Canada, the US Department of Agriculture—Forest Service, US Department of Agriculture—Animal and Plant Health Inspection Service—Plant Protection Quarantine, and the Canadian Food Inspection Agency. The authors thank Nick Boyonoski, Isabelle Ochoa, Matt Cloud, Karen Reed and Steven Walters for field assistance and processing and identification of insects in the laboratory, and Deepa Abeysekara for work on the synthesis of the syn-2,3-hexanediols.
Funding
This work was supported in part by funding from Natural Resources Canada, the US Department of Agriculture—Forest Service, US Department of Agriculture—Animal and Plant Health Inspection Service—Plant Protection Quarantine, and the Canadian Food Inspection Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by B. Hurley.
Special Issue on Invasive Pests of Forests and Urban Trees.
Rights and permissions
About this article
Cite this article
Allison, J., Strom, B., Sweeney, J. et al. Trap deployment along linear transects perpendicular to forest edges: impact on capture of longhorned beetles (Coleoptera: Cerambycidae). J Pest Sci 92, 299–308 (2019). https://doi.org/10.1007/s10340-018-1008-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-018-1008-7