Abstract
A resistant strain of Nilaparvata lugens has been continuously selected in the presence of nitenpyram for 27 generations under laboratory conditions in order to study the fitness cost of nitenpyram resistance. The resistance to nitenpyram in N. lugens was at a high level (resistance ratio = 144.7-fold). Life-tables of the nitenpyram-resistant strain and the susceptible strain (SS) of N. lugens were studied by using the age-stage, two-sex life-table approach. Compared to the SS, the relative fitness of the resistant strain of N. lugens was 0.55, with a lower intrinsic rate of increase (r) and net reproductive rate (R0). Changes in some life-history traits of the resistant strain were also observed. The duration of the egg stage, the development times of the first-, third- and fourth-instar nymphs, the adult preoviposition period and the total preoviposition period of the resistant strain were significantly increased, whereas the longevity, egg survival rate and fecundity (eggs/female) (absolute fitness) were significantly decreased in the resistant strain of N. lugens. The lower intrinsic rate of increase (r) and net reproductive rate (R0) seemed to be largely due to number of eggs laid, developmental time and egg survival rate. These results showed that the development of nitenpyram resistance may lead to significant fitness costs in resistant populations of N. lugens. This study provided valuable information for facilitating the development of nitenpyram-resistance management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The brown planthopper (BPH) Nilaparvata lugens (Stål) is the most common pest of rice around the world. Nitenpyram is a popular insecticide for N. lugens control, and insecticide resistance was inevitable. Thus, understanding the fitness costs associated with insecticide resistance is critical to resistance management.
-
A laboratory-selected, nitenpyram-resistant strain and a susceptible strain were used to construct two-sex life-tables and investigated the fitness costs associated with nitenpyram resistance in N. lugens.
-
The changes in life-history traits were exactly those responsible for the fitness differences.
Introduction
The brown planthopper (BPH) Nilaparvata lugens (Stål) (Hemiptera: Delphacidae) is the major pest of rice in Asia, and this pest has caused large losses in yield through direct sucking, ovipositing and virus transmission. The average loss in rice yield in China due to N. lugens has been approximately 1.2 million tons during the past 10 years (2006–2015) (Liu et al. 2016). Insecticides have been regarded as an important tool for controlling N. lugens in order to reduce yield loss (Zhang et al. 2014, 2017; Liu et al. 2015; Tong and Feng 2016). Currently, N. lugens is resistant to more than 31 active ingredients of insecticides, including organochlorine, organophosphates, carbamates, pyrethroids, neonicotinoids, phenylpyrazoles, insect growth regulators and pyridine azomethine insecticides (Nagata and Moriya 1974; Tang et al. 1982; Wang et al. 2008, 2013; Zhao et al. 2011; Matsumura et al. 2014; Min et al. 2014; Zhang et al. 2014, 2016a, b, 2017; Malathi et al. 2017; Liao et al. 2017).
Nitenpyram, a second-generation neonicotinoid insecticide, was commercialized in China in 2007 (Liang et al. 2012). It was a popular insecticide for N. lugens control because it was highly efficient, showed systemic action and long-lasting effects and good environmental safety profile (Akayama and Minamida 1999). However, the most recent report on nitenpyram showed that field populations of N. lugens had developed resistance to nitenpyram (Zhang et al. 2017). In addition, field populations of other pests, such as Aphis gossypii Glover, Bemisia tabaci Gennadius, Leptinotarsa decemlineata Say, etc., have also developed resistance to nitenpyram (Mota-Sanchez et al. 2006; Yuan et al. 2012; Matsuura and Nakamura 2014; Abbas et al. 2015a, b; Naeem et al. 2016; Saddiq et al. 2015; Ullah et al. 2016; Mansoor et al. 2017; Wang et al. 2017). These findings suggest that nitenpyram resistance has developed to become widespread in populations of some insect pests.
The fitness cost of insecticide resistance is a high energetic cost or a significant disadvantage accompanying the development of resistance to an insecticide that could diminish the resistant insect’s survival and fecundity in the absence of insecticides compared with its susceptible counterpart in the population (Kliot and Ghanim 2012). Fitness costs associated with resistance to insecticides have been documented in N. lugens and other insects belonging to different orders (Liu and Hang 2006; Feng et al. 2009; Gassmann et al. 2009; Kliot and Ghanim 2012; Wang and Wu 2014; Abbas et al. 2015a, b; Afzal et al. 2015; Gordon et al. 2015; Shen et al. 2017; Tieu et al. 2017). These studies also indicated that these insects adapted to insecticides suffer a fitness cost derived from a pleiotrophic genetic effect (Kliot and Ghanim 2012). These pleiotropic effects associated with resistance include changes in survival rate, egg hatching, fecundity, and development time, among others (Abbas et al. 2012; Kliot and Ghanim 2012).
In addition, fitness costs as a result of resistance to insecticides could be beneficial for pest management. If the insect pest has the potential to develop high levels of resistance to insecticides, but this lowers the fitness of this resistant strain, it is easy to recover susceptibility to insecticides when the population is not exposed to those insecticides (Liu and Hang 2006; Feng et al. 2009; Kliot and Ghanim 2012). In other words, fitness costs that occur in insecticide-resistant individuals can delay the development of resistance in the population under certain conditions (Carriere and Tabashnik 2001). Therefore, studies of fitness costs may be valuable for designing an integrated pest management (IPM) program for managing the spread of the resistant population (Abbas et al. 2012; Sun et al. 2012; Santos-Amaya et al. 2017). To the best of our knowledge, a fitness cost of nitenpyram resistance has not yet been reported in N. lugens. Thus, there is an urgent need to evaluate the fitness of nitenpyram-resistant strains of N. lugens.
Here, we used a susceptible strain selected for its high level of resistance to nitenpyram using rice seedling containing this insecticide solution to minimize confounding effects related to genetic differences between susceptible and resistant populations. Laboratory-selected nitenpyram-resistant and susceptible strains were used to construct life-tables and to investigate development, reproduction and survival. Consequently, we hypothesized potential fitness costs associated with nitenpyram resistance in N. lugens and expected negative effects on life-history traits in the resistant strain leading to such costs. Assessment of such effects will be relevant to document the impact of nitenpyram on N. lugens more thoroughly, and thus to optimize its use against this pest.
Materials and methods
Resistant and susceptible strains
The susceptible strain of N. lugens was collected from a rice paddy at the Hunan Academy of Agricultural Sciences and reared on rice seedlings in the laboratory without exposure to any insecticide for more than 10 years. The resistant strain derived from this susceptible strain was continuously selected through 27 generations of exposure to nitenpyram (Table 1). The concentrations of nitenpyram used for selection in the different generations were determined as LC30–LC50 of their parent’s generation. Rice seedlings of 14–20 days were sprayed with 200 ml nitenpyram solution. These rice seedlings were air-dried at room temperature for 2–4 h. Then, the solution on the plate was discarded and distilled water was added tothe plate. Then, the treated rice seedlings were moved into clear cages and reared to the third-instar nymph for 96 h. The number of nymphs selected per generation ranged between 1000 and 2000. The mortality during individual selection cycles was about 50–80%. These strains were reared on rice seedlings at 27 ± 1 °C under 70–80% relative humidity and a 16-h/8-h light/dark photoperiod.
Insecticide
The insecticide nitenpyram (96%, technical grade, CAS 150824-47-8) was purchased from Hubei KangBaoTai Fine Chemical, Wuhan, China.
Bioassays
Bioassays were performed with third-instar nymphs of N. lugens using a previously described rice-stem dipping method (Wang et al. 2008). Briefly, a nitenpyram stock solution was prepared by dissolving nitenpyram in double-distilled water containing 0.1% Triton X-100 (v/v). Rice plants in the tillering to early booting stage were pulled from the soil, washed thoroughly, cut to a length of approximately 10 cm including the roots, and air-dried. Three rice stems were grouped together and immersed in the appropriate insecticide solution for 30 s and then air-dried at room temperature for at least 30 min. The stems were then wrapped with water-impregnated cotton and placed into 500-mL plastic cups (one group of three stems per cup). Third-instar nymphs were collected with a homemade aspirating device, and 15 nymphs were transferred into each cup. There were three replicates for each dose (concentration) and 6–9 doses for each insecticide. The control rice stems were treated with only the 0.1% Triton X-100 water solution. All treatments were maintained at 27 ± 1 °C under 70–80% relative humidity and a 16-h/8-h light/dark photoperiod. Mortality was assessed after exposure to nitenpyram for 96 h. The nymphs were considered dead if they were unable to move after being gently prodded with a fine brush.
Fitness comparisons
Life-tables were constructed separately for the susceptible (SS) and highly resistant (RS) strains. Rice plants with eggs laid by each of these strains within a 24-h period were collected from at least 20 pairs of adults. After hatching, approximately 100 neonates were randomly selected and transferred individually to a new rice plant. As the nymphs became adults, pairs of males and females were placed in a glass tube with a single fresh rice seedling. The rice seedling was changed every day during the experiment. The newly hatched nymphs were counted and then discarded. The rice stems were then checked thoroughly using a microscope, and the numbers of neonates and unhatched eggs were noted. During this study, the following observations were made: the development rate of each instar stage, the emergence of adults, the life duration of the adult stage, fecundity and hatchability. The life-table experiment was conducted in an insectary at 27 ± 1 °C under 70–80% relative humidity and a 16-h/8-h light/dark photoperiod.
Statistical analysis
The LC50 values with 95% CI, resistance ratio with 95% CI, slopes with standard error (SE) and Chi square value (χ2) with degrees of freedom (df) were calculated using a regression model based on a probit transformation of mortalities and a logarithmic transformation of the concentrations tested, i.e., a log-probit model by Polo Plus software. The criteria of resistance level significant classification was that, if the 95% CI includes 1, then the LC50s are not significantly different (Robertson et al. 2007). The raw life-cycle data of each individual were analyzed using the age-stage, two-sex life-table approach (Chi and Liu 1985; Chi 1988). The basic life-table parameters, including intrinsic rate of increase (r), net reproductive rate (R0) and mean generation time (T), were calculated by using the computer program TWOSEX-MS Chart (Chi 2016). The variances and standard errors of the population parameters were estimated using the bootstrap procedure included in TWOSEX-MS Chart with 200,000 random resamplings. The development time, adult longevity, adult preoviposition period (APOP), total preoviposition period (TPOP), oviposition days, fecundity (absolute fitness) and population parameters (r, R0, and T) were compared by using the paired bootstrap test based on the confidence intervals of differences (Efron and Tibshirani 1993; Akköprü et al. 2015). The relative fitness of the resistant strain was calculated following Shen et al. (2017): Relative fitness (R f ) = R0 of the resistant strain/R0 of the susceptible strain.
Results
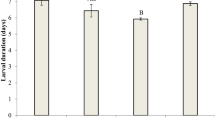
Means of life-history traits of RS and SS strains of N. lugens are given in Table 2. The survival rates from first to fifth instar, female and male of strains of RS and SS were not significantly different. There was also no significant difference observed in the developmental time of second-instar larvae, duration of female, longevity, nymph and adult survival rates (Table 2; Supplementary Figs. 1, 2, 3, 4). And the female ratio of RS was not significantly different compared with that of SS (Table 2). However, the development time of the eggs and the first-, third- and fourth-instar larvae as well as longevity, the APOP, and the mean of the TPOP, were significantly different between the RS and SS (Table 2; Supplementary Figs. 1, 2, 3, 4). Compared with the SS, the durations of the egg and the first-, third- and fourth-instar nymph stages as well as the APOP and TPOP were significantly prolonged in the RS by 0.49, 0.24, 0.26, 0.26, 0.31 and 1.71 days, respectively, while the longevity of the RS was significantly decreased by 4.46 days (Table 2). Moreover, the fecundity (eggs/female) (absolute fitness) of RS was also significantly different from that of SS. The RS females produced significantly fewer eggs per female compared to the SS females. Also, egg survival rate of RS was significantly different from that of SS (Table 2). The survival rate of eggs also showed a decline from 0.93 in the SS to 0.77 in the RS. The percent egg hatching of RR and SS were not significantly different. In this generation, RR and SS produced 4695 and 9713 larvae, respectively (Table 2).
In addition, similar effects of duration of nymphs, longevity (days) and fecundity (eggs per female) (absolute fitness) were seen at the intrinsic rate of increase (r), the net reproduction rate (R0) and the mean generation time (T). The intrinsic rate of increase (r) and the net reproduction rate (R0) of RS were also significant lower than that of SS (Table 3), a pattern that was consistent for fecundity (absolute fitness) measurements. However, the mean generation time (T) was significantly prolonged in RS (33.14 days) compared to the SS (31.35 days) (Table 3). The relative fitness of the resistant strain of N. lugens was 0.55 compared to the susceptible strain (SS) (Table 3).
Discussion
The use of chemical insecticides to control insect pests is extremely widespread, which often causes the development of insecticide resistance within insect populations (Yang et al. 2017). The development of resistance to insecticides is accompanied by a high energetic cost or significant disadvantage that diminishes the insect’s fitness in the absence of insecticides (Kliot and Ghanim 2012). The fitness cost of insecticide resistance is considered to be a factor affecting the evolution of resistance, and a better understanding of the fitness cost could be invaluable in devising effective resistance management strategies (Liu and Hang 2006; Kliot and Ghanim 2012; Sun et al. 2012; Shen et al. 2017). Therefore, to examine the fitness of a resistant strain is important for creating a resistance management strategy.
It is vitally important to keep a similar genetic background when testing for the fitness costs associated with resistance, due to the genetic background of populations confusing the determination of the fitness costs (Wang and Wu 2014). Having a similar genetic background, the resistant and susceptible strains differ only in small regions of the genome, which facilitates the assessment of fitness costs (Shen et al. 2017). Therefore, the resistant strain derived from a susceptible strain was selected in this study, and their genetic backgrounds were similar and homogenized. Although laboratory experiments do not completely reflect field conditions, the present analysis can serve as an early warning for pest managers. Previous studies have indicated that the detoxification enzyme cytochrome P450 monooxygenase is a more likely contributing factor to nitenpyram resistance in field populations of N. lugens (Zhang et al. 2017). The same was found for the laboratory-selected strain of N. lugens (RS). In general, cytochrome P450 monooxygenase was involved in nitenpyram resistance in RS (Zhang et al. 2017). These may illustrate the resistance profile of field populations, and the laboratory-selected strain of N. lugens was similar.
Fitness in N. lugens strains resistant to nitenpyram were examined in this study, which demonstrated that the development of increased nitenpyram resistance could decrease fitness in the resistant strain of N. lugens, involving a longer development time, lower survival rate, or reduced reproductive performance in the absence of insecticides. Similarly, fitness costs associated with resistance in the absence of insecticides are common in N. lugens (Liu and Hang 2006; Ling et al. 2009; Yang et al. 2017). Deleterious effects on some life-history traits (larval survival, egg hatching and fecundity) and reduced mating success of resistant males have been reported in resistant strains of N. lugens (Liu and Hang 2006; Ling et al. 2009; Kliot and Ghanim 2012; Yang et al. 2017). A fitness analysis using life-tables demonstrated that imidacloprid-resistant N. lugens had obvious disadvantages in their reproduction, including significantly lower larval survival rate, adult emergence rate, copulation rate, fecundity (absolute fitness) and hatchability, and the field strain of N. lugens with imidacloprid resistance also showed reduced fitness (0.169 and 0.104) (Liu and Hang 2006). In another laboratory strain of N. lugens with 253-fold resistance to chlorpyrifos, relative fitness was 0.206 with a low survival rate, low emergence rate, low female ratio, low fecundity (absolute fitness) and low hatchability compared with a susceptible strain (Yang et al. 2017).
In the current study, in the absence of nitenpyram, the development time of the egg incubation period, APOP and TPOP, as well as the durations of the first-, third- and fourth-instar larvae were prolonged in the resistant strain compared to the susceptible strain; in contrast, the longevity and ovi-days in the resistant strain were shorter than those in the susceptible strain. The fecundity (eggs/female) (absolute fitness) and egg survival rate of RS were decreased. Therefore, these indicated that nitenpyram resistance in N. lugens corresponds with a significant disadvantage in developmental duration, and also showed the presence of a trade-off in the distribution of resources between resistance and fitness of N. lugens. Also, these life-history traits suggest the dominance of fitness costs due to significant differences in the traits of RS and SS. These fitness effects have also been reported in other resistant strains (Kliot and Ghanim 2012). The mean generation time (T) is an important indicator of population dynamics, which if prolonged in the resistant strain would lead to apparent fitness costs (Shen et al. 2017). The mean generation time (T) of RS were significantly prolonged compared with SS, and the relative fitness of RS was 0.55. Moreover, the resistant strain also showed lower survival of eggs and lower fecundity (absolute fitness) compared with the susceptible strain, and this was also consistent for a significantly lower intrinsic rate of population increase (r) and a lower net reproductive rate (R0) compared with the susceptible strain. This lower intrinsic rate of population increase (r) seemed to be largely due to the number of eggs laid, the developmental time and the survival rates of the eggs. These demographic parameters (r, R0 and T) are important indices for evaluating the biological characteristics of insect populations. Based on these parameters, it was concluded that the impact of resistance on life-history traits seems to be very strong, which could help delay the evolution of resistance.
In summary, we found fitness costs associated with nitenpyram resistance in the RR strain. The delayed development time and reduced fecundity (absolute fitness) and fertility of the resistant strain were the major factors substantially impacting the population growth potential, leading to fitness costs. These data may also provide a wealth of interesting and useful information that will be invaluable in IPM programs and in delaying N. lugens resistance. The frequencies of resistance alleles could decline when the selection pressure is removed (Ferre and Van Rie 2002). Previous studies have also indicated that low fitness could result in a quick recovery of population susceptibility when the application of the insecticide was suspended (Liu and Hang 2006; Feng et al. 2009). In the current study, the nitenpyram-resistant strain had a disadvantage compared to the susceptible strain, suggesting that the development of resistance to nitenpyram would be delayed. These data indicate a favorable condition for nitenpyram resistance management; for example, managing resistance to insecticides depends on associated fitness costs such that the frequency of resistance alleles will decline when selection is reduced (Abbas et al. 2012). In addition, field populations of N. lugens have developed resistance to nitenpyram. In those populations, an effective resistance management strategy (use of suitable rotation partners for nitenpyram) should be implemented to retard the further development of resistance to nitenpyram in N. lugens.
Author contributions
XLZ, HW and JHL designed the experiment; XLZ, KKM and XL collected data; XLZ analyzed the data and wrote the manuscript; and XLZ, KKM, XL, HW and JHL read, corrected and approved the manuscript.
References
Abbas N, Shad SA, Razaq M (2012) Fitness cost, cross-resistance and realized heritability of resistance to imidacloprid in Spodoptera litura (Lepidoptera: Noctuidae). Pestic Biochem Physiol 103:181–188
Abbas N, Ijaz M, Shad SA, Khan H (2015a) Stability of field-selected resistance to conventional and newer chemistry insecticides in the house fly, Musca domestica L. (Diptera: Muscidae). Neotrop Entomol 44:402–409
Abbas N, Khan H, Shad SA (2015b) Cross-resistance, stability, and fitness cost of resistance to imidacloprid in Musca domestica L., (Diptera: Muscidae). Parasitol Res 114:247–255
Afzal MBS, Shad SA, Abbas N, Ayyaz M, Walker WB (2015) Cross-resistance, the stability of acetamiprid resistance and its effect on the biological parameters of cotton mealybug, Phenacoccus solenopsis (Homoptera: Pseudococcidae), in Pakistan. Pest Manag Sci 71:151–158
Akayama A, Minamida I (1999) Discovery of a new systemic insecticide, nitenpyram and its insecticidal properties. In: Yamamoto I, Casida JE (eds) Nicotinoid insecticides and the nicotinic acetylcholine receptor. Springer, Tokyo, pp 127–148
Akköprü PE, Atlihan R, Okut H, Chi H (2015) Demographic assessment of plant cultivar resistance to insect pests: a case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J Econ Entomol 108:378–387
Carriere Y, Tabashnik BE (2001) Reversing insect adaptation to transgenic insecticidal plants. Proc R Soc Lond B 268:1475–1480
Chi H (1988) Life-table analysis incorporating both sexes and variable development rate among individuals. Environ Entomol 17:26–34
Chi H (2016) TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. http://140.120.197.173/Ecology/Download/TWOSEX-MSChart.rar. Accessed 6 Sept 2016
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool Acad Sin 24:225–240
Efron B, Tibshirani RJ (1993) An introduction to the Bootstrap. Chapman and Hall, London
Feng YT, Wu QJ, Xu BY, Wang SL, Chang XL, Xie W, Zhang YJ (2009) Fitness costs and morphological change of laboratory-selected thiamethoxam resistance in the B-type Bemisia tabaci (Hemiptera: Aleyrodidae). J Appl Entomol 133:466–472
Ferre J, Van Rie J (2002) Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu Rev Entomol 47:501–533
Gassmann AJ, Carriere Y, Tabashnik BE (2009) Fitness costs of insect resistance to Bacillus thuringiensis. Annu Rev Entomol 54:147–163
Gordon JR, Potter MF, Haynes KF (2015) Insecticide resistance in the bed bug comes with a cost. Sci Rep-UK 5:10807
Kliot A, Ghanim M (2012) Fitness costs associated with insecticide resistance. Pest Manag Sci 68:1431–1437
Liang P, Tian YA, Biondi A, Desneux N, Gao XW (2012) Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 21:1889–1989
Liao X, Mao KK, Ali E, Zhang XL, Wan H, Li JH (2017) Temporal variability and resistance correlation of sulfoxaflor susceptibility among Chinese populations of the brown planthopper Nilaparvata lugens (Stål). Crop Prot 102:141–146
Ling SF, Zhang J, Hu LX, Zhang RJ (2009) Effect of fipronil on the reproduction, feeding, and relative fitness of brown planthopper, Nilaparvata lugens. Appl Entomol Zool 44:543–548
Liu ZW, Hang ZJ (2006) Fitness costs of laboratory-selected imidacloprid resistance in the brown planthopper, Nilaparvata lugens Stål. Pest Manag Sci 62:279–282
Liu ZW, Wu JC, Zhang YX, Liu F, Xu JX, Bao HB (2015) Mechanism of rice planthopper resistance to insecticides. In: Heong KL, Cheng JA, Escalada MM (eds) Rice planthopper: ecology, management, socio economics and policy. Zhejiang Universty Press, Hangzhou, pp 117–141
Liu WC, Liu ZD, Huang C, Lu MH, Liu J, Yang QP (2016) Statistics and analysis of crop yield losses caused by main diseases and insect pests in the recent 10 years. Plant Prot 42:1–9
Malathi VM, Jalali SK, Gowda DKS, Mohan M, Venkatesan T (2017) Establishing the role of detoxifying enzymes in field-evolved resistance to various insecticides in the brown planthopper (Nilaparvata lugens) in South India. Insect Sci 24:35–46
Mansoor MM, Raza ABM, Abbas N, Aqueel MA, Afzal M (2017) Resistance of green lacewing, Chrysoperla carnea Stephens to nitenpyram: cross-resistance patterns, mechanism, stability, and realized heritability. Pestic Biochem Phys 135:59–63
Matsumura M, Morimura SS, Otuka A, Ohtsu R, Sakumoto S, Takeuchia H, Satoha M (2014) Insecticide susceptibilities in populations of two rice planthoppers, Nilaparvata lugens and Sogatella furcifera, immigrating into Japan in the period 2005–2012. Pest Manag Sci 70:615–622
Matsuura A, Nakamura M (2014) Development of neonicotinoid resistance in the cotton aphid Aphis gossypii (Hemiptera: Aphididae) in Japan. Appl Entomol Zool 49:535–540
Min S, Lee SW, Choi BR, Lee SH, Kwon DH (2014) Insecticide resistance monitoring and correlation analysis to select appropriate insecticides against Nilaparvata lugens (Stål), a migratory pest in Korea. J Asia Pac Entomol 17:711–716
Mota-Sanchez D, Hollingworth RM, Grafius EJ, Moyer DD (2006) Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag Sci 62:30–37
Naeem A, Freed S, Jin FL, Akmal M, Mehmood M (2016) Monitoring of insecticide resistance in Diaphorina citri Kuwayama (Hemiptera: Psyllidae) from citrus groves of Punjab, Pakistan. Crop Prot 86:62–68
Nagata T, Moriya S (1974) Resistance in the brown planthopper, Nilaparvata lugens Stål, to lindane. Jap J Appl Ent Zool 18:73–80
Robertson JL, Russell RM, Preisler HK, Savin NE (2007) Bioassays with Arthropods. CRC, Boca Raton
Saddiq B, Shad S, Aslam M, Ijaz M, Abbas N (2015) Monitoring resistance of Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) to new chemical insecticides in Punjab, Pakistan. Crop Prot 74:24–29
Santos-Amaya OF, Tavares CS, Rodrigues JVC, Campos SO, Guedes RNC, Alves AP, Pereira EJG (2017) Fitness costs and stability of Cry1Fa resistance in Brazilian populations of Spodoptera Frugiperda. Pest Manag Sci 73:35–43
Shen J, Li DY, Zhang SZ, Zhu X, Wan H, Li JH (2017) Fitness and inheritance of metaflumizone resistance in Plutella xylostella. Pestic Biochem Phys 139:53–59
Sun JY, Liang P, Gao XW (2012) Cross-resistance patterns and fitness in fufenozide-resistant diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag Sci 68:285–289
Tang ZH, Sun MG, Xu Q (1982) A preliminary study of the resistance to insecticides in brown planthopper (Nilaparvata lugens Stål). Acta Phytophy Sin 9:205–2010
Tieu S, Chen YZ, Woolley LK, Collins D, Barchia I, Lo N, Herron GA (2017) A significant fitness cost associated with ACE1 target site pirimicarb resistance in a field isolate of Aphis gossypii Glover from Australian cotton. J Pest Sci 90:773–779
Tong SM, Feng MG (2016) A mixture of putative sodium salts of camptothecin and bamboo tar is a novel botanical insecticide against rice planthoppers and stem borers. J Pest Sci 89:1003–1111
Ullah S, Shad SA, Abbas N (2016) Resistance of dusky cotton bug, Oxycarenus hyalinipennis Costa (Lygaidae: Hemiptera), to conventional and novel chemistry insecticides. J Econ Entomol 109:345–351
Wang R, Wu YD (2014) Dominant fitness costs of abamectin resistance in Plutella xylostella. Pest Manag Sci 70:1872–1876
Wang YH, Chen J, Zhu YC, Ma CY, Huang Y, Shen JL (2008) Susceptibility to neonicotinoids and risk of resistance development in the brown planthopper, Nilaparvata lugens (stål) (homoptera: delphacidae). Pest Manag Sci 64:1278–1284
Wang P, Ning ZP, Zhang S, Jiang TT, Tan LR, Dong S, Gao CF (2013) Resistance monitoring to conventional insecticides in brown planthopper, Nilaparvata lugens (hemiptera: delphacidae) in main rice growing regions in China. Chin J Rice Sci 27:191–197
Wang SL, Zhang YJ, Yang X, Xie W, Wu QJ (2017) Resistance monitoring for eight insecticides on the Sweetpotato whitefly (Hemiptera: Aleyrodidae) in China. J Econ Entomol 110:660–666
Yang BJ, Liu ML, Zhang YX, Liu ZW (2017) Effects of temperature on fitness costs in chlorpyrifos-resistant brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Sci. https://doi.org/10.1111/1744-7917.12432
Yuan LZ, Wang SL, Zhou JC, Du YZ, Zhang YJ, Wang JJ (2012) Status of insecticide resistance and associated mutations in Q-biotype of whitefly, Bemisia tabaci, from eastern China. Crop Prot 31:67–71
Zhang XL, Liu XY, Zhu FX, Li JH, You H, Lu P (2014) Field evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) in China. Crop Prot 58:61–66
Zhang XL, Liao X, Mao KK, Wan H, Lu P, Li JH (2016a) Resistance monitoring of the field populations of the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) to common insecticides in rice production areas of Hubei Province, central China. Acta Entomol Sin 59:1222–1231
Zhang XL, Liao X, Mao KK, Zhang KX, Wan H, Li JH (2016b) Insecticide resistance monitoring and correlation analysis of insecticides in field populations of the brown planthopper Nilaparvata lugens (stål) in China 2012–2014. Pestic Biochem Phys 132:13–20
Zhang XL, Liao X, Mao KK, Yang P, Li DY, Alia E, Wan H, Li JH (2017) The role of detoxifying enzymes in field-evolved resistance to nitenpyram in the brown planthopper Nilaparvata lugens in China. Crop Prot 94:106–114
Zhao XH, Ning ZP, He YP, Shen JL, Su JY, Gao CF, Zhu YC (2011) Differential resistance and cross-resistance to three phenylpyrazole insecticides in the planthopper Nilaparvata lugens (hemiptera: delphacidae). J Econ Entomol 104:1364–1368
Acknowledgements
This research was supported by a Grant from the National Natural Science Foundation of China (31471795), the Special Fund for Agro-Scientific Research in the Public Interest (201503107), and the National Key Research and Development Program of China (2016YFD0200500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Communicated by E. Roditakis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Mao, K., Liao, X. et al. Fitness cost of nitenpyram resistance in the brown planthopper Nilaparvata lugens. J Pest Sci 91, 1145–1151 (2018). https://doi.org/10.1007/s10340-018-0972-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-018-0972-2