Abstract
The rape stem weevil, Ceutorhynchus napi Gyll., is a serious pest of winter oilseed rape (Brassica napus L.) crops in Europe that causes stem distortion, severe disruption of stem growth, and significant yield loss. No resistance has been identified in current B. napus breeding material. Resynthesized lines of oilseed rape can be used to introduce genetic material from the parent species Brassica oleracea L. and B. rapa L. The susceptibility to C. napi of five resynthesized lines and three cultivars of oilseed rape was compared in a multi-choice field plot experiment. Morphological stem traits and stem levels of glucosinolates, carbon, and nitrogen were assessed as potential mediators of resistance. Eggs and larvae of C. napi in stems were counted weekly from the beginning of oviposition to larval maturation and stem pith degeneration in response to oviposition was assessed. Egg counts per stem were negatively dependent on stem length and positively dependent on the levels of five glucosinolates, glucobrassicanapin, glucoalyssin, glucobrassicin, neoglucobrassicin, and gluconasturtiin. The resynthesized line S30 was significantly more resistant to C. napi than the commercial cultivar Sollux, having fewer eggs and larvae. This resistance appeared to be largely antixenotic, due to the combination of a long stem and a paucity of the five key glucosinolates. We propose that resynthesized lines such as S30 should be used to introduce genes determining traits such as long stem length and glucosinolate profile into breeding programs to enhance resistance against C. napi in oilseed rape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Rape stem weevil is a serious pest of winter oilseed rape in Europe against which there is little resistance in crop breeding lines.
-
Do resynthesized lines of oilseed rape carry resistance genes from parent species?
-
In a field experiment, resynthesized line S30 was more resistant than commercial cultivar Sollux.
-
This resistance depends on a long stem and a paucity of five glucosinolates.
-
Resynthesized lines have the potential for breeding rape stem weevil resistance, reducing pesticide use.
Introduction
In oilseed rape (OSR) breeding, resynthesized lines are developed by interspecific crossing of the two progenitor species of Brassica napus L., Brassica oleracea L. and Brassica rapa L., and are already being used to broaden the genetic variation available to breeders of oilseed rape (Olsson et al. 1980; Girke et al. 2012). Several studies have reported the potential of resynthesized lines as sources of traits to improve resistance to disease (Diederichsen and Sacristan 1991; Rygulla et al. 2007) and resistance to insect pests (Seyis et al. 2001; Eickermann et al. 2011). For example, resynthesized lines of OSR are genetic resources for modifying the profiles of glucosinolates (Cleemput and Becker 2012), important plant defense compounds in Brassicaceae (Giamoustaris and Mithen 1995; Ali and Agrawal 2012).
The rape stem weevil, Ceutorhynchus napi Gyll. (Coleoptera, Curculionidae), is one of the most destructive pests in European crops of winter OSR, B. napus (Alford et al. 2003). Egg deposition by females into developing stems may cause them to become twisted, splitted, and S-shaped, resulting in plant distortion and considerable loss of yield (Dosse 1951; Deubert 1952; Le Pape and Bronner 1987; Buechi 1988).
There is an urgent need for OSR cultivars resistant to C. napi and other insect pests as part of integrated pest management systems to minimize the number of insecticide applications in Europe, where over-reliance on insecticide use has led to increasing incidence of insecticide resistance in OSR pests (Zimmer et al. 2014). Plant resistance to pest insects may be due to reduced host finding and host acceptance (antixenosis) or reduced performance and increased mortality (antibiosis) (Kogan and Ortman 1978; Sarfraz et al. 2006).
So far, only few studies have been published on screening brassicaceous genotypes for insect resistance traits (Renwick 2002; Eickermann et al. 2011; Tansey and Dosdall 2011; Hervé et al. 2014). However, two such studies seeking cultivars resistant to C. napi found only minor variation in weevil infestation among the cultivars and lines tested (Mrowczynski 1998; Dechert and Ulber 2004). The use of resynthesized lines presents a potential opportunity to break this impasse.
The life cycle of C. napi presents opportunities to identify potential resistance traits that act either on adult or on larval stages of the pest (Guenthart 1949; Dosse 1951; Ballanger 1987; Lerin 1993; Williams 2010). This univoltine weevil overwinters as an adult in a pupal cocoon in the soil beneath the previous year’s OSR crop, in obligate pre-reproductive diapause. Adults must therefore locate new OSR crops to migrate to in early spring. Herbivores that specialize in Brassicaceae make use of host plant volatiles to locate their hosts (Cook et al. 2007). Many of these volatiles are isothiocyanates, the breakdown products of glucosinolates that are hydrolyzed by the endogenous enzyme myrosinase following cell disruption (Hopkins et al. 2009). Glucosinolates can also play an important role in the acceptability of the Brassica host for feeding and oviposition when the host has been located (Renwick et al. 1992; Huang and Renwick 1994; Chew and Renwick 1995; Giamoustaris and Mithen 1995; Cook et al. 2006; Ulmer and Dosdall 2006). In March/April, after approximately 2 weeks of feeding, females deposit single eggs into the tips of elongating stems, close to the terminal buds. Thereafter, the larvae feed within the pith of stems for three to 5 weeks. As obligate stem feeders, the larvae are therefore also vulnerable to the defense compounds in the host plant chosen by their mother. Chief among these are again the glucosinolates. Currently used OSR cultivars contain low glucosinolate contents in seeds, because glucosinolates reduce the quality of the seed meal (Mithen 2001). However, glucosinolate contents in seeds are not correlated with the glucosinolate contents in stems (Cleemput and Becker 2012).
The presence of glucosinolates in brassicaceous plants serves as an effective defense against non-specific insect herbivores (Giamoustaris and Mithen 1995) but even Brassica specialists are not completely immune to them (Ali and Agrawal 2012) and may be negatively affected by specific glucosinolates, e.g., cabbage flea beetles (Phyllotreta spp.) (Siemens and Mitchelolds 1996), cabbage root fly (Delia radicum (L.)) (Tansey and Dosdall 2011) and cabbage stem weevil (C. pallidactylus (Marsh.)) (Eickermann et al. 2011). Within 24 h following oviposition by C. napi, the stem pith tissue surrounding the egg begins to degenerate (Dosse 1951; Deubert 1955). This pith tissue degeneration constitutes gall formation caused by the eggs of C. napi which induce translocation of water and nutrients from the plant tissue toward the egg (Deubert 1955; Le Pape and Bronner 1987). However, eggs deposited by herbivorous insects on or within a plant may trigger metabolic responses, resulting in changes in the plant tissue which may affect egg hatching and development of larvae (Hilker et al. 2002). There are no detailed studies about the response of different OSR genotypes to C. napi egg deposition.
The nutrient content of OSR can affect plant morphology and biochemical composition (Malhi et al. 2005; Veromann et al. 2013) and can also affect the oviposition preference and larval performance of herbivorous insects. For example, the field-scale distribution of plant nutrients has been shown to affect the distribution of cabbage seed weevil (C. obstrictus (Marsh.)) infestation (Blake et al. 2010) and the carbon to nitrogen (C:N) ratio in plant tissue influences the performance of insect herbivores (Awmack and Leather 2002).
Morphological plant traits can be cues for host plant acceptance in pests of Brassicaceae and they might also be manipulated for herbivore resistance in OSR (Sarfraz et al. 2006). It has been reported that C. napi females strongly prefer plants with stems up to 22 cm long for oviposition (Buechi 1996). Stems which are longer than 22 cm are more likely to be rejected, thus conferring some resistance to early varieties with longer stems at the time of C. napi immigration.
The objective of this study was to explore the potential of resynthesized lines of B. napus as sources of genetic resistance to C. napi in OSR breeding. The oviposition preference and larval performance of C. napi were compared among eight B. napus genotypes, comprising five resynthesized lines and three cultivars of OSR, in a multi-choice field plot experiment. The study primarily focused on antixenosis resistance (expressed as reduced oviposition) of the lines and cultivars. The length of plant stems, their biomass and glucosinolate profile were measured, together with their carbon and nitrogen contents as indicators of their nutritive status for herbivores. These data were analyzed in relation to levels of natural infestation by C. napi eggs, larvae, and larval development, to identify genotypes bearing traits with potential to confer resistance to this pest.
Materials and methods
Plant genotypes and experimental field plots
The study was conducted as a multi-choice field plot experiment at the experimental station of Georg-August University, Goettingen, Germany (N51°33′56.3 E9°56′49.9) in 2012–2013. Eight genotypes of B. napus, comprising five resynthesized lines and three cultivars of OSR, were tested because of their broad genetic background according to Girke (2002) (Table 1). Four replicates of each genotype were sown on 22 August 2012 into 1.5 m × 7.5 m plots arranged in a 4 × 8 array with an unsown space of 0.25 m between each plot, forming a randomized complete block design. To reduce border effects, the experiment was set within an area (30 m × 90 m) sown with a mixture of the OSR cultivars Trabant, Dimension and Elektra so that all plots were a minimum of 1.0 m from the crop edge. The sowing density throughout was 33 seeds/m2. All genotypes and plots were supplied with a total of 177 kg N/ha (54 kg N/ha on 4 March, 42 kg N/ha on 26 March and 81 kg N/ha on 23 April). The available mineral nitrogen (N min) in the soil was assessed to be 36.0 kg N/ha on 5 March 2013. All experimental sampling took place in April and May 2013.
C. napi colonization, plant responses, and larval performance
In order to time the start of experimental sampling from plants appropriately, the natural spring migration of C. napi adults was monitored using four yellow water traps (0.33 m × 0.25 m × 0.07 m) containing water with a small amount of detergent. One trap was placed on the ground in the margin beside each of the four sides of the experiment. Weekly from 15 April to 22 May, the stems of the five plants per plot sampled for stem length measurement were dissected under a stereo microscope (Zeiss, Stemi 2000-C) to count the number of eggs and larvae of C. napi and to assess the degeneration of the pith tissue in response to the oviposition/ presence of eggs. Pith degeneration was classified into three categories: no decomposition = eggs not surrounded by hollows, moderate decomposition = eggs surrounded by small hollows, and strong decomposition = no pith tissue surrounding the eggs. As an indicator of the relative rates of larval development in different plant genotypes, a larval instar index was calculated for the last samples on 22 May by subtracting the number of 2nd larval instars present from the number of 3rd larval instars present and adding a constant (K = 8), to avoid negative values (Kurtz et al. 2010). The value of the index is expected to increase as the rate of larval development increases. The three larval instars of C. napi were discriminated based on head capsule width (Guenthart 1949).
Physical traits and nutritive value of plants
On 15 April, before C. napi oviposition into stems had begun, plant density of the genotypes was assessed by counting all plants per plot; six randomly selected plants from each plot were collected to assess the BBCH growth stage (Lancashire et al. 1991) and the above-ground biomass of the main stems. Subsequently, main stem length above ground was measured on five randomly selected plants from each plot collected c. weekly on 15, 22, and 29 April and on 6, 13, and 22 May. Nitrogen and carbon contents, proxies for protein and carbohydrate and indicators of nutritive value, were measured in the stems of six randomly selected non-infested plants per plot sampled on 15 April. The stems were dried at 60 °C for 3 days, milled using a centrifugal mill and analyzed with a CN element analyzer (Vario EL, Fa. Elementar Analysensysteme GmbH, Hanau).
Analysis of glucosinolate profiles of plants
In order to determine the effect of glucosinolates on oviposition by C. napi, the glucosinolate content of non-infested stems was analyzed at the beginning of the oviposition period. Five randomly selected non-infested main stems were sampled per plot on 17 April for glucosinolate analyses. To test for any changes in glucosinolate levels that might signal a defensive response to stem herbivory, on 24 May five randomly selected stems infested by C. napi larvae were sampled per plot and stem glucosinolate levels were analyzed. The stems were frozen on dry ice and stored at −20 °C. After freeze-drying for 96 h, the stems from each plot were homogenized using a mill (Krups KM 75). Stem glucosinolates were separated and individual compounds were identified and quantified by HPLC (Thies 1977). They were analyzed using a Shimadzu Prominence LC20AT series HPLC (Shimadzu Deutschland GmbH) equipped with a Nucleodur 100-3 C18 column (Macherey–Nagel). Desulfoglucosinolates were extracted as described in Cleemput and Becker (2012) and were separated using a water-acetonitrile gradient (solvent A water, solvent B acetonitrile; 0–20 min 1–20 % B; 20–25 min 20 % B; 25–27 min 20 % B; 27–34 min 1 % B) at a flow rate of 0.6 ml/min. Retention times of known standards were used to identify desulfoglucosinolates. The content of glucosinolates is expressed in µmol/g dry weight (DW).
Data analysis
Univariate data analysis was done using Statistica 10 (StatSoft®, Tulsa, USA) and tested for normal distribution with the Shapiro–Wilk W Test. Corresponding graphs were made with SigmaPlot for Windows, Version 11.0. Multivariate data analysis and graphs were done with R 3.0.1.
Relationships between genotype and C. napi infestation
To analyze the influence of physical plant traits on infestation by C. napi, linear regression was used to test the effect of stem biomass on the number of eggs in samples during the period of peak oviposition (22 and 29 April, 6 May) and to test the effect of stem length on egg number on 29 April. The egg number was analyzed only on one of the six sampling dates because oviposition had peaked by 29 April. Pearson Product-Moment Correlation was used to test the relationship between the number of larvae on 22 May and the larval instar index on the same date.
The effect of plant genotype on the numbers of C. napi eggs present in stems on 22 and 29 April and 6 May and on the number of larvae in stems on 13 May was tested by ANOVA for each date. Counts of eggs on 6 May were log x + 1 transformed to normalize the residuals. Numbers of eggs on 13 May and numbers of larvae on 22 and 29 April, and 6 and 22 May were too small and non-normally distributed for analysis by ANOVA and so the non-parametric test KW-ANOVA was used to analyze the effect of plant genotype on these data.
To investigate the influence of stem glucosinolate profiles on the numbers of C. napi eggs, the numbers of larvae, and on the larval instar index, partial least squares regression (PLSR; Geladi and Kowalski 1986) was used. Data were scaled to unit variance, and were mean centered by default in the analyses. Numbers of larvae on 29 April and 6 May were not analyzed by PLSR because the plant genotype did not significantly affect the numbers of larvae on these sampling dates. The total glucosinolate content of non-infested stems and infested stems was compared using the Mann–Whitney U test. Effects of plant genotype on the larval instar index and of genotype on stem pith degeneration in response to the oviposition/egg presence were analyzed by KW-ANOVA.
Effect of genotype on plant characters
The effects of plant genotype on the plant density, stem biomass, and on stem length on 15 and 29 April and 6 May were analyzed by ANOVA; the differences among means were evaluated by the Bonferroni test. The stem length on 22 April was analyzed differently from 15 and 29 April and 6 May because of non-normal distribution of residuals on 22 April. Genotype effects on stem length of 22 April were analyzed by KW-ANOVA; the differences among ranks were evaluated by Kruskal–Wallis test. The stem length was analyzed only on four of the six measuring dates because oviposition had peaked by 29 April and so this early period was of most importance to the relationship with C. napi oviposition. The effects of genotype on stem nitrogen content, carbon content, and C:N ratio on 15 April were each assessed using KW-ANOVA; the differences among ranks were evaluated by Kruskal–Wallis test. To test both non-infested and infested stems for between-genotype differences in glucosinolate profiles, partial least squares-discriminant analysis (PLS-DA; Barker and Rayens 2003) was used, and the significance of discrimination was tested by multivariate analysis of variance (MANOVA).
Results
Responses of C. napi to plant genotypes
Oviposition by C. napi
The main immigration of C. napi into the OSR field was detected in water traps on 7 April and oviposition into stems of all genotypes had begun by 22 April (Table 2). The stem samples of 22 and 29 April and 6 May encompassed the main egg-laying period, maximum numbers of eggs being found on 29 April, and there were significant differences between the genotypes in the number of eggs on each of these dates (Table 2). During this period, the resynthesized line S30 consistently had the fewest eggs of any genotype, significantly fewer compared to cultivars Oase and Mendel on 22 April, compared to Sollux on 29 April and 6 May, and compared to line H30 on 6 May. By 13 May, numbers of eggs were too few to show differences between genotypes (Table 2).
Numbers and performance of C. napi larvae
Larvae of C. napi were first found in stem samples on 22 April. Their numbers reached a maximum on 13 May and had already begun to decline on 22 May (Table 3), by which time larval exit holes in the stems indicated that larvae had begun to emerge from stems to pupate in the soil. In the resynthesized line S30, the first larvae were counted on 6 May, a week later than they were found in each of the other genotypes, indicating delayed larval hatching in this line. In accordance with the small number of eggs laid into stems of S30, numbers of C. napi larvae per stem were consistently lowest in this line (Table 3). Significant differences between plant genotypes were found on both 13 and 22 May and on both dates only S30 differed significantly from other lines, having fewer larvae than H30 and Sollux on both occasions and fewer than Mendel on 22 May (Table 3).
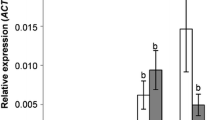
The rate of C. napi larval development, as reflected by the larval instar index on 22 May, differed significantly between the genotypes (Fig. 1). Larval development was most retarded in resynthesized line S30, with a larval instar index significantly smaller than line H30 in which larval development was the fastest. These two lines, respectively, had the smallest and the largest number of larvae per stem over the sampling period (Table 3). Over all genotypes, the number of larvae on 22 May was significantly positively correlated with the larval instar index (R = 0.886, N = 32, P = 0.003).
Comparison of the larval instar index for Ceutorhynchus napi larvae in the main stems of eight Brassica napus genotypes on 22 May. Mean values of four replicates, error bars are ± SE. Bigger values of the index reflect faster larval development rate. Analysis of variance (KW-ANOVA), H (7, N = 31) = 19.746, P = 0.006; genotypes not followed by the same letter differ significantly, P ≤ 0.05 (Kruskal–Wallis test). Larval instar index = (L3 − L2) + K; K = 8
Effect of physical plant traits on C. napi
The mean number of eggs per genotype on 29 April (peak oviposition) was negatively dependent on the stem length a week earlier on 22 April (Fig. 2). The mean number of eggs laid by C. napi in samples of 22 and 29 April or 6 May was not dependent on stem biomass on 15 April (R = 0.420, F = 1.283, P = 0.301; R = 0.330, F = 0.735, P = 0.424; R = 0.191, F = 0.228, P = 0.650, respectively).
Effect of glucosinolate profile on oviposition by C. napi
PLSR analyses of relationships between the glucosinolate profiles of non-infested stems on 17 April and egg counts in stems during the main oviposition period (samples of 22 and 29 April and 6 May) indicated that there was a significant relationship between glucosinolate profile and egg count only on 29 April, the date of peak egg numbers (Table 2); the factorial map 1–2 of the PLSR analysis explained 24.07, 51.17, and 29.31 % of the variance of egg counts on 22 and 29 April and 6 May, respectively. On 29 April, the number of eggs was most closely positively correlated with the glucosinolates glucoalyssin (ALY), gluconasturtiin (NAS), and glucobrassicanapin (GBN) and rather more weakly with glucobrassicin (GBC) and neoglucobrassicin (NEO) (Fig. 3).
PLSR analysis loading plot showing relationships between the glucosinolate profile of non-infested stems of eight Brassica napus genotypes on 17 April and the number of Ceutorhynchus napi eggs counted per stem on 29 April. The factorial map 1–2 explained 51.17 % of egg count variance. The smaller the angle between the egg arrow and the glucosinolate arrow, the tighter were the two variables correlated and vice versa. Glucosinolates with short arrows (≤radius) were found to be unrepresentative in the PLSR analysis. See legend of Table 6 for key to abbreviations for glucosinolates
Phenotypic differences between plant genotypes
Physical traits and nutritive value
The plant density (266.13 ± 9.90, mean number plants/plot ± SE) did not differ significantly between plant genotypes at the start of experimental sampling on 15 April (F 7, 24 = 2.136, P = 0.078). There was little variation between the BBCH growth stages of plant genotypes on 15 April; all plants were at early green bud stage with the resynthesized line S30 and the cultivar Oase slightly more advanced at BBCH growth stage 51 compared to the other genotypes at growth stage 50 (Table 4). The four first sampling dates, 15, 22, and 29 April and 6 May, were chosen for analysis of differences in plant physical traits and nutritive value as this encompassed the period of oviposition, and therefore host plant selection, by females (Table 2). On each of these dates, there was considerable variability in stem length and there were significant differences between the genotypes, the cultivar Sollux, and the resynthesized line H30 being notable for shortness and Oase, at a slightly more advanced growth on 15 April, having the longest stem on 15 and 22 April (Table 4). Stem biomass on 15 April showed a high degree of variability within genotype. Nevertheless, the two genotypes at the lower and upper extremes of stem biomass significantly differed from each other, the resynthesized line H113 accumulating significantly less biomass than the cultivar Oase (Table 5). The nutritive value of stem tissue was similar for each genotype, the nitrogen content, carbon content and the C:N ratios not differing significantly (Table 5).
Glucosinolate profiles
Glucosinolate levels in non-infested stems varied greatly between plant genotypes, the genotype with the highest total glucosinolate content, Sollux, having more than twice as much as that with the lowest, S30 (Table 6). Glucosinolate profiles also varied greatly (Table 6) and PLS-DA analysis showed that the profiles differed significantly between genotypes, the score plot explaining 69.79 % of intergenotypic variance (Fig. 4). The genotypes H30, S30, Mendel, and Oase fell into a cluster of similar profiles, more alike to each other than to those of Sollux, H113, G53, and S3 (Fig. 4).
Plant responses to C. napi infestation
Glucosinolate profiles in C. napi infested stems
There was great variation between genotypes in levels of total glucosinolates in infested stems on 24 May, as there had been in non-infested stems on 17 April. Almost all glucosinolates were less abundant as a proportion of dry weight than on 17 April, often markedly so (c. 5–20 fold difference; Table 6), and mean total glucosinolate levels were significantly lower in infested stems than in non-infested stems (Mann–Whitney U test, N = 31, df = 60, P = 0.000) (Table 6). However, the same three genotypes, S3, H113, and Sollux, had the highest levels of total glucosinolates both before and after infestation. PLS-DA analysis revealed that the glucosinolate profile of infested stems again differed between the genotypes (Fig. 5), the score plot explaining 72.37 % of the intergenotypic variance. The cluster pattern in the PLS-DA score plot was different in infested stems compared to non-infested stems on 17 April, indicating that the glucosinolate profile of S3 was clearly distinct, falling outside a broad cluster containing all seven other genotypes (Figs. 4, 5). PLSR analysis revealed no relationship between the glucosinolate profile of infested stems on 24 May and the number of larvae in stem samples of 13 or 22 May (20.07 and 29.17 % variance accounted for, respectively) nor with the larval instar index on 22 May (12.01 % variance accounted for) suggesting that glucosinolate profile had little influence on larval fitness.
Pith tissue degeneration in the presence of C. napi eggs
The degeneration of pith tissue surrounding C. napi eggs in infested stems on 29 April significantly differed between plant genotypes (Table 7), being more severe in the most affected (H30) than in the least affected (S30) genotypes, and broadly reflecting the number of eggs present (Table 2). However, when the pith degeneration score was expressed relative to the number of eggs, no significant differences between the genotypes were found (Table 7).
Discussion
This study has demonstrated the potential of resynthesized lines of B. napus as potential sources of resistance against the stem-mining pest C. napi. We found considerable variability in the number of eggs and larvae in the material tested and identified the line S30 to be the least infested of the genotypes tested, including three commercial cultivars.
Responses of C. napi to plant genotypes
There was considerable variability both within and between genotypes in the number of C. napi eggs found in stems, but eggs were consistently fewest in resynthesized line S30 (Table 2). This suggests that S30 is likely to be a useful source of resistance traits for OSR and that its primary mechanism of resistance to C. napi is antixenosis. Additionally, the number of eggs per plant might also be considered as a measure to confirm antixenosis in this line.
Consistent with lower egg numbers, S30 was also the genotype with the lowest number of C. napi larvae, providing further evidence of antixenosis resistance to C. napi. Given the apparent parity in nutritional value of the different plant genotypes, comparison of larval development rates suggested that line S30 might also exhibit antibiosis resistance against C. napi, having the lowest value of the instar index, with only a small proportion of larvae having reached L3 by 22 May (Fig. 1). Antibiosis affects herbivore performance and may result in prolonged development, reduced weight, or increased mortality (Sarfraz et al. 2006). However, the larval count data showed a delayed presence of larvae in S30 (Table 3) which might have resulted from either a delayed or prolonged embryonic development. Delayed hatching and prolonged larval development might have contributed to the low larval instar index in S30 on 22 May (Table 3), indicating antibiosis resistance against C. napi. To determine potential host effects on the larval instar index in the advanced period of larval development, plant samples of 22 May were preferred to samples of 13 May, at peak larval abundance (Table 3).
Plant growth stages and the length of plant stems are known to have an influence on the oviposition preference of C. napi (Lerin 1993; Buechi 1996). In this study, there was no evidence that growth stage was in itself associated with the resistance of S30 to C. napi. Cultivar Oase and line S30 were both one point on the BBCH growth stage scale more advanced than the other genotypes on 15 April, yet on 22 April Oase had the most eggs of any genotype and S30 the fewest (Tables 2, 4). However, it has been reported that plants with stem length exceeding 22 cm are less preferred for oviposition (Buechi 1996) and stem length is of course linked with growth stage, the main stem rapidly extending during bud stage (BBCH 50–59) as can be seen in Table 4. Our data confirm the effect of stem length on infestation: on 29 April, egg numbers were negatively related to stem length on 22 April (Fig. 2). Line S30 had the second longest stem on 15 and 22 April and this may have contributed to its resistance to C. napi. OSR plants with a stem length longer than 20 cm have also been found to be less susceptible to stem splitting (Lerin 1993) and so the long stemmed trait may be valuable for tolerance to C. napi infestation as well as resistance to it. However, the main factor inducing stem splitting is the growth rate of the stem (Lerin 1993). Stem growth can also vary with climatic conditions (Ballanger 1987) and so it may be unwise to rely solely on this trait for resistance. Stem length is unlikely to be the only mechanism of resistance in S30 as cultivar Oase had equally long stems (>24 cm) on 22 April but more eggs than any other genotype and had seven times as many eggs as S30 on 29 April. Further, although the experiment was arranged in a randomized complete block design, the lower oviposition rates on long stemmed lines might have been due to fewer females finding these plots. The abundance of females could not be determined. Differences in stem biomass did not appear to have any influence on relative resistance to C. napi infestation.
At peak egg abundance, the distribution of C. napi eggs between plant genotypes was associated with the content of the glucosinolates ALY, NAS, and GBN and more weakly with GBC and NEO, measured in non-infested stems on 17 April (Fig. 3). The cultivar Sollux, the genotype with the largest number of C. napi eggs on 29 April and over the sampling period, had the highest total glucosinolate level in non-infested stems and ranked first or second out of the eight plant genotypes for the abundance of each of these five key glucosinolates (Tables 2, 6). By contrast, S30 had the lowest total glucosinolate abundance in non-infested stems and ranked no higher than fourth for the abundance of any of these key glucosinolates.
It is likely that low concentrations of glucosinolates, particularly of the five compounds identified as important in our analysis, play a large part in the antixenosis resistance of line S30 against C. napi oviposition, causing this genotype not to be recognized as a favored host. Low levels of glucosinolates have previously been identified as deterrents to feeding by two Brassicaceae-specialist flea beetles, Psylliodes chrysocephala (L.) and Phyllotreta cruciferae Goeze (Giamoustaris and Mithen 1995; Siemens and Mitchelolds 1996). Three of the five key glucosinolates identified here have also been found to be important for host plant acceptance responses in other Brassicaceae-specialist pests: GBC and NAS stimulated feeding on artificial medium by P. chrysocephala (Bartlet et al. 1994); oviposition by D. radicum correlated positively with GBC (Roessingh et al. 1992) and with NAS (Tansey and Dosdall 2011); GBN stimulated oviposition by D. radicum (Roessingh et al. 1992). In contrast, Hervé et al. (2014) found that the glucosinolate profile in buds of OSR is not important in feeding or oviposition behavior in pollen beetle (Meligethes aeneus F.). Five glucosinolates were found to be associated with antixenosis resistance of S30. However, other plant characteristics could also have an impact on host location and acceptance. Specific volatile isothiocyanates are known to attract crucifer-feeding insects (Renwick et al. 1992; Chew and Renwick 1995; Bartlet et al. 1997) and might be potential drivers for host acceptance (Moyes and Raybould 2001). However, even if the volatile profile emitted by an OSR crop is not obtrusive to specialist insects, plants can be located by other cues, such as color (Smart et al. 1997; Cook et al. 2013). Generally, low levels of glucosinolates in plants may increase their susceptibility to generalist herbivore pests. Thus, plants which are not well recognized by ovipositing C. napi due to low glucosinolate contents might be more apparent to generalist herbivores previously repelled by glucosinolates (Giamoustaris and Mithen 1995). Additionally, the flavonoid kaempferol has been identified to reduce oviposition by C. obstrictus (Lee et al. 2014) and crucifer-specific phytoalexins have been found to be important for host acceptance of D. radicum (Baur et al. 1998).
Phenotypic differences between plant genotypes
Phenotypic differences between the genotypes tested were mainly found in stem length, stem biomass, total glucosinolate levels, and glucosinolate profiles. The variability found in stem length was probably related to variability in plant developmental rate, the two longest stemmed genotypes, Oase and resynthesized line S30, being at a slightly more advanced growth stage at the start of observations (Table 4). The considerable extent of between-genotype variability found in glucosinolate levels and profiles of non-infested stems (Fig. 4) is likely to be of particular significance in this study as these compounds play such a major role in host location and acceptance among Brassicaceae-specialists, as well as in antibiosis (Giamoustaris and Mithen 1995; Schoonhoven et al. 2005; Cook et al. 2006; Ulmer and Dosdall 2006; Cook et al. 2007; Ali and Agrawal 2012). The nitrogen and carbon content and the C:N ratio of stems did not significantly differ between genotypes and so differences in tissue concentrations of protein and carbohydrates are unlikely to have had a significant influence on infestation by C. napi in this study.
Plant responses to C. napi infestation
Glucosinolate profiles in C. napi larva infested stems on 24 May had changed considerably from their pre-infestation levels of 17 April, including marked falls in concentration, and there were again significant differences between genotypes. The glucosinolate content of brassicaceous plants has been found to be altered by herbivory (Bodnaryk 1992; Bartlet et al. 1999) and in this study, changes due to both plant development and herbivory might be expected when comparing pre- and post-infestation glucosinolate levels. However, there was no evidence that the changes observed were associated with any effect on C. napi larval abundance or larval performance as measured by the larval instar index. This might be due to analyses of glucosinolates profiles instead of specific glucosinolates. Ulmer and Dosdall (2006) suggested that high levels of specific glucosinolates were associated with larval performance in the cabbage seed weevil. However, some specialist herbivores of brassicaceous plants are able to detoxify glucosinolates (Després et al. 2007). This might be a further explanation why glucosinolate profiles of infested stems were not associated with abundance and performance of C. napi larvae. Additionally, assessment of non-infested control plants to proof that glucosinolate profiles were altered due to herbivory was not possible. Therefore, we have to speculate that changes in glucosinolate profiles were confounded with time and the glucosinolate profile might not be a key factor in terms of abundance and performance of C. napi larvae.
The severity of pith degeneration in response to the presence of C. napi eggs did not vary with plant genotype, the degree of stem degeneration simply being related to the number of eggs. There was no evidence for the type of response mounted by OSR in defense against infestation by C. pallidactylus, another stem-mining weevil, where neoplasm formation in petioles following oviposition may lead to increased mortality of egg batches (Nuss 2004), nor of a hypersensitive reaction, another defensive response to egg deposition (Hilker et al. 2002).
We have shown that OSR genotype can have a significant influence on oviposition by females of C. napi. The resynthesized line S30, was subject to a very low infestation rate and it is a strong candidate as a potential genetic source for breeding OSR cultivars with resistance to C. napi. The resistance of S30 against C. napi appears to be largely antixenotic. The low number of C. napi eggs in S30 was associated with specific glucosinolates, while the glucosinolates were not closely associated with larval abundance and performance. Future work should focus on the identification of this factor and on determining the potential effect of further chemical traits, including volatile isothiocyanates, on host location and acceptance by C. napi.
Author contribution statement
HLSK, HB, BU, and HCB conceived and designed research. HLSK and HB conducted experiments. HLSK analyzed data and wrote the manuscript. SV, BU, and HCB proofread the manuscript. All authors read and approved the manuscript.
References
Alford DV, Nilsson C, Ulber B (2003) Insect pests of oilseed rape crops. In: Alford DV (ed) Biocontrol of oilseed rape pests, 1st edn. Blackwell Science Ltd., Oxford, pp 9–42
Ali JG, Agrawal AA (2012) Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci 17:293–302
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Ballanger Y (1987) Nuisibilité du charançon de la tige du colza. Phytoma 384:35–37
Barker E, Rayens W (2003) Partial least squares for discrimination. J Chemom 17:166–173
Bartlet E, Parsons D, Williams IH, Clark SJ (1994) The influence of glucosinolates and sugars on feeding by the cabbage stem flea beetle, Psylliodes chrysocephala. Entomol Exp Appl 73:77–83
Bartlet E, Blight MM, Lane P, Williams IH (1997) The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer. Entomol Exp Appl 85:257–262
Bartlet E, Kiddle G, Williams I, Wallsgrove R (1999) Wound-induced increases in the glucosinolate content of oilseed rape and their effect on subsequent herbivory by a crucifer specialist. Entomol Exp Appl 91:163–167
Baur R, Staedler E, Monde K, Takasugi M (1998) Phytoalexins from Brassica (Cruciferae) as oviposition stimulants for the cabbage root fly, Delia radicum. Chemoecology 8:163–168
Blake A, Dosdall LM, Keddie B (2010) Plant nutrients and the spatiotemporal distribution dynamics of Ceutorhynchus obstrictus (Coleoptera: Curculionidae) and its parasitoids. Environ Entomol 39:1195–1205
Bodnaryk RP (1992) Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry 31:2671–2677
Buechi R (1988) Neue Bekaempfungsschwelle fuer den Rapsstengelruessler Ceutorhynchus napi Gyll. Mitt Schweizerischen Landwirtsch 36:110–117
Buechi R (1996) Eiablage des Rapsstengelruesslers Ceutorhynchus napi Gyll., in Abhaengigkeit der Stengellaenge bei verschiedenen Rapssorten. Anz Schaedlingskunde Pflanzenschutz Umweltschutz 69:136–139
Chew F, Renwick J (1995) Host plant choice in Pieris butterflies. In: Carde RT, Bell WJ (eds) Chemical ecology of insects, 2nd edn. Chapman and Hall, New York, pp 214–238
Cleemput S, Becker HC (2012) Genetic variation in leaf and stem glucosinolates in resynthesized lines of winter rapeseed (Brassica napus L.). Genet Resour Crop Evol 59:539–546
Cook SM, Smart LE, Martin JL, Murray DA, Watts NP, Williams IH (2006) Exploitation of host plant preferences in pest management strategies for oilseed rape (Brassica napus). Entomol Exp Appl 119:221–229
Cook SM, Khan ZR, Pickett JA (2007) The use of push-pull strategies in integrated pest management. Annu Rev Entomol 52:375–400
Cook SM, Skellern MP, Doering TF, Pickett JA (2013) Red oilseed rape? The potential for manipulation of petal colour in control strategies for the pollen beetle (Meligethes aeneus). Arthropod Plant Interact 7:249–258
Dechert G, Ulber B (2004) Interactions between the stem-mining weevils Ceutorhynchus napi Gyll. and Ceutorhynchus pallidactylus (Marsh.) (Coleoptera: Curculionidae) in oilseed rape. Agric For Entomol 6:193–198
Després L, David J-P, Gallet C (2007) The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol Evol 22:298–307
Deubert K-H (1952) Ueber das durch die Eiablage von Ceutorrhynchus napi Gyll. (Col. Curc.) verursachte histologische Schadbild an Winterraps. Wissenschaftliche Z Martin-Luther-Universitaet Halle-Wittenberg 2:203–205
Deubert K-H (1955) Beitraege zu den Beziehungen zwischen Ceutorrhynchus napi Gyll. (Col. Curc.) und Winterraps hinsichtlich der Gallenbildung mit Ovarienuntersuchungen an verschiedenen Ceutorrhynchus-Arten. Wissenschaftliche Z Martin-Luther-Universitaet Halle-Wittenberg 5:909–932
Diederichsen E, Sacristan M (1991) Resynthesis of amphidiploid Brassica species and their clubroot disease reaction. In: Proceedings of the Rapeseed Congress, Saskatoon, Canada, 9–11 July 1991, pp 274–279
Dosse G (1951) Der grosse Kohltriebruessler Ceuthorrhynchus napi Gyll. Biologie, Schadenauftreten und Bekaempfung unter besonderer Beruecksichtigung der Gallbildung an Kohlpflanzen: Mit 41 Abb. Z Angew Entomol 32:32–566
Eickermann M, Ulber B, Vidal S (2011) Resynthesized lines and cultivars of Brassica napus L. provide sources of resistance to the cabbage stem weevil (Ceutorhynchus pallidactylus (Mrsh.)). Bull Entomol Res 101:287–294
Geladi P, Kowalski B (1986) Partial least squares regression: a tutorial. Anal Chim Acta 185:1–17
Giamoustaris A, Mithen R (1995) The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Ann Appl Biol 126:347–363
Girke A (2002) Neue Genpools aus resynthetisiertem Raps (Brassica napus L.) fuer die Hybridzuechtung. Dissertation, Georg-August University, Goettingen
Girke A, Schierholt A, Becker HC (2012) Extending the rapeseed genepool with resynthesized Brassica napus L. I: genetic diversity. Genet Resour Crop Evol 59:1441–1447
Guenthart E (1949) Beitraege zur Lebensweise und Bekaempfung von Ceutorrhynchus quadridens Panz. und Ceutorrhynchus napi Gyll. mit Beobachtungen an weiteren Kohl- und Rapsschaedlingen. Mitt Schweizerischen Entomologischen Ges 22:441–591
Hervé MR, Delourme R, Leclair M, Marnet N, Cortesero AM (2014) How oilseed rape (Brassica napus) genotype influences pollen beetle (Meligethes aeneus) oviposition. Arthropod Plant Interact 8:383–392
Hilker M, Rohfritsch O, Meiners T (2002) The plant’s response towards insect egg deposition. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition, 1st edn. Blackwell, Berlin, pp 205–233
Hopkins RJ, van Dam NM, van Loon JJA (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83
Huang X, Renwick J (1994) Relative activities of glucosinolates as oviposition stimulants for Pieris rapae and P. napi oleracea. J Chem Ecol 20:1025–1037
Kogan M, Ortman EF (1978) Antixenosis—a new term proposed to define Painters nonpreference modality of resistance. Bull Entomol Soc Am 24:175–176
Kurtz B, Karlovsky P, Vidal S (2010) Interaction between western corn rootworm (Coleoptera: Chrysomelidae) larvae and root-infecting Fusarium verticillioides. Environ Entomol 39:1532–1538
Lancashire PD, Bleiholder H, Boom T, Langelueddeke P, Stauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119:561–601
Le Pape H, Bronner R (1987) The effects of Ceutorhynchus napi (Curculionidae, Coleoptera) on stem tissue of Brassica napus var. oleifera. In: Labeyrie V, Fabres G, Lachaise D (eds) Insect-plants, 1st edn. Dr W Junk, Dordrecht, pp 207–212
Lee RWH, Malchev IT, Rajcan I, Kott LS (2014) Identification of putative quantitative trait loci associated with a flavonoid related to resistance to cabbage seedpod weevil (Ceutorhynchus obstrictus) in canola derived from an intergeneric cross, Sinapis alba × Brassica napus. Theor Appl Genet 127:419–428
Lerin J (1993) Influence of the growth rate of oilseed rape on the splitting of the stem after an attack of Ceutorhynchus napi Gyll. IOBC WPRS Bull 16(9):160–163
Malhi SS, Schoenau JJ, Grant CA (2005) A review of sulphur fertilizer management for optimum yield and quality of canola in the Canadian Great Plains. Can J Plant Sci 85:297–307
Mithen R (2001) Glucosinolates—biochemistry, genetics and biological activity. Plant Growth Regul 34:91–103
Moyes CL, Raybould AF (2001) The role of spatial scale and intraspecific variation in secondary chemistry in host-plant location by Ceutorhynchus assimilis (Coleoptera: Curculionidae). Proc R Soc Lond B Biol Sci 268:1567–1573
Mrowczynski M (1998) Studies on pest damage of cultivars and lines of winter oilseed rape. IOBC WPRS Bull 21(5):153–154
Nuss H (2004) Einfluss der Pflanzedichte und-architektur auf Abundanz und innnerpflanzliche Verteilung staengelminierender Schadinsekten in Winterraps. Dissertation, Georg-August University, Goettingen
Olsson G, Ellerstrom S, Tsunoda S, Hinata K, Gomez-Campo C (1980) Polyploidy breeding in Europe. In: Tsunoda S, Hinata K, Gomez-Campo C (eds) Brassica crops and wild allies, 2nd edn. Scientific Society Press, Tokyo, pp 167–190
Renwick JAA (2002) The chemical world of crucivores: lures, treats and traps. Entomol Exp Appl 104:35–42
Renwick JAA, Radke CD, Sachdev-Gupta K, Staedler E (1992) Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology 3:33–38
Roessingh P, Staedler E, Fenwick GR, Lewis JA, Nielsen JK, Hurter J, Ramp T (1992) Oviposition and tarsal chemoreceptors of the cabbage root fly are stimulated by glucosinolates and host plant extracts. Entomol Exp Appl 65:267–282
Rygulla W, Snowdon R, Eynck C, Koopmann B, von Tiedemann A, Luehs W, Friedt W (2007) Broadening the genetic basis of Verticillium longisporum resistance in Brassica napus by interspecific hybridization. Phytopathology 97:1391–1396
Sarfraz M, Dosdall L, Keddie B (2006) Diamond-back moth–host plant interactions: implications for pest management. Crop Prot 25:625–639
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology. Oxford University Press, Oxford
Seyis F, Friedt W, Luehs W (2001) Resynthese-Raps (Brassica napus L.) als genetische Ressource fuer die Qualitaets-und Ertragszuechtung. Schriften zu Genetischen Ressourcen 16:91–112
Siemens DH, Mitchelolds T (1996) Glucosinolates and herbivory by specialists (Coleoptera: Chrysomelidae, Lepidoptera: Plutellidae): consequences of concentration and induced resistance. Environ Entomol 25:1344–1353
Smart LE, Blight MM, Hick AJ (1997) Effect of visual cues and a mixture of isothiocyanates on trap capture of cabbage seed weevil, Ceutorhynchus assimilis. J Chem Ecol 23:889–902
Tansey JA, Dosdall LM (2011) Differential responses by some insect pests to novel insect-resistant Brassica napus L. In: Proceedings of the International Rapeseed Congress, Prague, Czech Republic, 5–9 July 2011, CD-ROM
Thies W (1977) Analysis of glucosinolates in seeds of rapeseed (Brassica napus L.): Concentration of glucosinolates by ion exchanges. Z Pflanzenzuechtung 79:331–335
Ulmer BJ, Dosdall LM (2006) Glucosinolate profile and oviposition behavior in relation to the susceptibilities of Brassicaceae to the cabbage seedpod weevil. Entomol Exp Appl 121:203–213
Veromann E, Toome M, Kaennaste A, Kaasik R, Copolovici L, Flink J, Ue Niinemets (2013) Effects of nitrogen fertilization on insect pests, their parasitoids, plant diseases and volatile organic compounds in Brassica napus. Crop Prot 43:79–88
Williams IH (2010) The major insect pests of oilseed rape in Europe and their management: an overview. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer Science and Business Media B.V., Dordrecht, pp 1–43
Zimmer CT, Koehler H, Nauen R (2014) Baseline susceptibility and insecticide resistance monitoring in European populations of Meligethes aeneus and Ceutorhynchus assimilis collected in winter oilseed rape. Entomol Exp Appl 150:279–288
Acknowledgments
We would like to thank B. Olberg and U. Ammermann (Georg-August University) for technical assistance in glucosinolate analyses and M. Hervé (INRA, France) for supplying the multivariate statistical macro and further assistance. We would also like to thank the technical staff in laboratory, greenhouse, and field: D. Mennerich, B. Tappe, E. Hodyl, M. Knobel, H. Reintke, G. Miotke and all student research assistants. We thank A. Ferguson (afscience.co.uk) for language editing of the manuscript and critical comments. The study was funded by German Federal Ministry of Food, Agriculture and Consumer Protection (BMELV) and coordinated by the Board of support for Private Plant Breeding in Germany (GFP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.J. Duan.
Rights and permissions
About this article
Cite this article
Schaefer-Koesterke, H.L., Brandes, H., Ulber, B. et al. The potential of resynthesized lines to provide resistance traits against rape stem weevil in oilseed rape. J Pest Sci 90, 87–101 (2017). https://doi.org/10.1007/s10340-016-0742-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-016-0742-y