Abstract
Common bean, Phaseolus vulgaris, is an important food and cash crop in Africa. Its production is seriously affected by the bean stem maggot (BSM), Ophiomyia spp., which attacks seedlings. We evaluated the ability of eleven fungal isolates to colonize bean plants and the effects of inoculation on BSM feeding and oviposition, pupation, and adult emergence. All fungal isolates were able to colonize different bean plant parts (root, stem, and leaves), except isolates of Metarhizium anisopliae and Beauveria bassiana isolate ICIPE 273. Colonization was generally higher on the roots than on the stem and leaves and varied significantly between the fungal isolates. BSM feeding and oviposition were significantly reduced in all the fungus-inoculated bean plants which in turn affected pupation and adult emergence as compared to the control. Metarhizium anisopliae ICIPE 20 outperformed the other isolates in interfering with BSM lifecycle. Although M. anisopliae ICIPE 78 recorded a high number of punctures similar to the control, a significant reduction in the number of pupae and adult emergence was observed, suggesting possible BSM growth inhibition. This study clearly demonstrates that fungal endophytes can be considered as promising tools for the management of BSM in East Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The bean stem maggot (BSM) is an important pest in East Africa; due to its cryptic feeding and its ability to pupate in stem, its management is extremely difficult.
-
It is hypothesized that fungal entomopathogens as endophytes can be effectively be used to control leaf mining pests such as BSM.
-
Our results showed the ability of some fungal endophytes to colonize bean plant parts and their effect in reducing BSM feeding and oviposition as well as pupation and adult emergence as compared to the control.
-
Metarhizium anisopliae isolates which did not colonize the bean plants were also able to reduce BSM feeding, oviposition, pupation, and emergence.
-
The outcomes of this study suggest the possibility to use fungal endophytes as tools for the management of BSM in East Africa.
Introduction
The common bean, Phaseolus vulgaris L. (Fabaceae), is an important food and cash crop in Africa, particularly in the Eastern, Southern, and Great Lakes regions of the continent where it is considered as an important source of human food in terms of calories (Pachico 1993), protein, oil, and micronutrients (Singh 1990; Pyndji and Trutmann 1992; Blair et al. 2007). It is also a source of income for resource-poor households where a significant proportion is exported to European markets (Abate and Ampofo 1996; Wortmann et al. 1999). Annual production of beans in Kenya is estimated to be over 90,000 MT covering around 150,000 hectares. However, serious decline due to insect pests have been observed over the years (USAID 2010). Among the various insect pests of bean, the bean stem maggot (BSM), Ophiomyia spp. (Diptera: Agromyzidae), and bruchids are the most important field and storage pests, respectively (Greathead 1969; Karel 1985; Abate 1991). BSM is typical of dry conditions and low fertility soils (Karel and Autrique 1989; Abate and Ampofo 1996; Songa and Ampofo 1998). Bean flies oviposit on young seedlings and larval feeding and tunneling interfere with nutrient transport and creates avenues for entry of disease organisms (Ampofo and Massomo 1998). The presence of BSM pupae in the plant results in the swelling and rotting and plant suffers from premature leaf fall which causes up to 100 % loss (Wickramasinghe and Fernando 1962; Ochilo and Nyamasyo 2011).
The management of BSM is difficult because of the cryptic behavior of the pest. Generally, the control is achieved through the use of a traditional IPM approach that consists of appropriate sowing dates, optimum plant density, resistant varieties, intercropping, and good crop husbandry (Abate and Ampofo 1996). Chemical control is also commonly used, however, it is discouraged due to environmental and health risks, and resistance development by the BSM. There is the need therefore to look for alternative strategies that are safe, environmentally friendly, and cost-effective.
In recent years, there is a shift in emphasis towards utilization of entomopathogenic fungi (EPF) for the management of crop insect pests such as leaf miners (Migiro et al. 2010), thrips (Ekesi and Maniania 2002), and aphids (Jandricic et al. 2014). Some EPF have versatile attributes; they can be used as biopesticides applied in inundative approach or as endophytes. As endophytes, EPF can directly and indirectly promote plant growth and development through plant defense against herbivorous insects (Vega et al. 2009). For instance, fungal endophytes have been reported to deter feeding, oviposition, and performance of sap-sucking insects such as thrips (Muvea et al. 2014, 2015) and leaf mining insects (Akutse et al. 2013). On the other hand, the effect of entomopathogenic endophytes Beauveria bassiana (Bals.-Criv.) Vuill. and Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones, and Samson (Ascomycota: Hypocreales) in enhancing plant growth has been reported by Lopez and Sword (2015). However, no studies have been conducted on BSM. The objective of this study was therefore initiated to screen fungal isolates for endophytic colonization of bean plants and assess the effect of inoculation on BSM feeding and oviposition, pupation, and adult emergence.
Materials and methods
Ethical considerations
The study was carried out at the International Centre of Insect Physiology and Ecology (icipe) laboratories in Nairobi, Kenya (S 03.35517°, E 037.33861°, and 1616 m.a.s.l.). Bean plants, fungal isolates, and the pest (BSM) used in this study are not endangered species. The fungal isolates were obtained from the icipe’s Arthropod Germplasm Centre and no permission was required since icipe operates under a Headquarters’ agreement with the Kenyan Government. The study was not undertaken in protected areas of land therefore no special permits were required to undertake the study. Permission was only obtained from the farmers’ field prior to sampling.
Bean plant
Phaseolus vulgaris, variety Brown Rose Coco was used in this study. Bean seeds were sowed in 15-cm pots (5–8 plants per pot) and maintained at room temperature (25 ± 3 °C and 60 % R.H.) using a mixture of manure and soil in a ratio of 1:5. The substrate was sterilized in an autoclave for 2 h at 121 °C and allowed to cool for 72 h prior to planting. Pots were maintained immediately in a screen house (2.8 m length × 1.8 m width × 2.2 m height) at 25 ± 3 °C, for 2 weeks. Seedlings were thinned to three per pot after germination and were watered once per day in the afternoon.
BSM colony establishment
The initial stock of BSM originated from Kabaru (0.2833°S, 37.1667°E, 2309 m.a.s.l.) around Mt Kenya region. Bean plants showing symptoms of BSM attacks were uprooted and kept in cages for a period of 3–4 weeks for emergence of bean flies. The newly emerged adults were collected using an aspirator and transferred to the infestation cage which contained clean bean plants to allow females to oviposit. After emergence, specimens of adult bean flies were placed in 95 % alcohol for taxonomical identification. Based on Spencer’s key (Spencer 1973), samples were identified as Ophiomyia phaseoli (Tryon) (Diptera: Agromyzidae) on morphological features using taxonomic keys. This was later confirmed using molecular tools by amplification of COI (Cytochrome Oxidase 1) region. The purified PCR products were sent to Macrogen Inc, Europe Laboratories, Netherlands, for sequencing. DNA sequences were edited using bio-edit software and subjected to a Basic Local Alignment Search Tool (BLAST) algorithm search (NCBI). Gene accession numbers for the all the characterized specimens were EF104664.1 with 98 % percent identity and E = 0.
Insects were reared on 1-week-old fresh potted bean plants in a Plexiglas cage (50 × 50 × 45 cm) in screen house to allow female BSM to oviposit. Bean plants were changed every 48 h and infested plants were transferred into clean cages for larval development until pupation and adult emergence. The newly emerged flies were again placed in the infestation cage (30 × 30 × 25 cm) with fresh bean plants as previously described. The colony was maintained at 27 ± 2 °C with a photoperiod of 12L: 12D and relative humidity of approximately 40 %. Flies were fed on a 10 % natural honey solution provided in cotton balls placed at the bottom corner of the infestation cage.
Fungal cultures
The list of fungal species used in this study and their origin are presented in Table 1. Fungal isolates were mainly from the genera Metarhizium (5), Beauveria (3), Hypocrea (1), and Trichoderma (2). The Metarhizium and Beauveria isolates were obtained from the International Centre of Insect Physiology and Ecology (icipe)’s Arthropod Germplasm Centre. All the isolates were cultured on potato dextrose agar (PDA), except Metarhizium isolates which were cultured on Sabouraud dextrose agar (SDA). They were maintained at 25 ± 2 °C in complete darkness. Conidia were harvested by scraping the surface of 2–3 week-old sporulating cultures with a sterile spatula. The harvested conidia were then mixed in 10 mL sterile distilled water containing 0.05 % Triton X-100 in universal bottles containing 3 mm glass beads. The conidial suspensions were vortexed for 5 min to produce a homogenous suspension. Conidial counts were done using an improved Neubauer Hemocytometer (Goettel and Inglis 1997). A final concentration of 1 × 108 conidia mL−1 was used for the inoculation of bean seeds.
The viability of conidia was assessed before any bioassay by spread plating 0.1 mL of 3 × 106 conidia mL−1 onto 90-mm Petri dishes containing SDA or PDA (Goettel and Inglis 1997). The plates were incubated at 25 ± 2 °C and were examined after 16–20 h under a light microscope at a magnification of 400X. Conidia were considered as germinated when the germ tube was twice the diameter of the conidium. Four replicate plates were used per isolate. In viability tests, >88 % of conidia of all the isolates germinated (Table 1).
Inoculation of bean plants
For each treatment, five bean seeds were surface sterilized in 70 % ethanol for 2 min followed by 1.5 % sodium hypochlorite for 3 min and rinsed with sterile distilled water three times. The last rinse water was plated out to assess the reliability of the surface sterilization procedure (Schultz et al. 1998). Inoculation was done by soaking bean seed in conidial suspensions (1 × 108 conidia ml−1) for 2 h (Akutse et al. 2013). For the control, sterilized seeds were soaked in sterile distilled water for 2 h. Seeds were then dried on sterile paper towel for 30 min and planted in plastic pots containing the planting substrate (mixture of manure and red soil 1:5). The substrate was sterilized in an autoclave for 2 h at 121 °C and then allowed to cool for 72 h prior to planting. Five seeds were sowed per pot and then maintained at 25 ± 3 °C, 60–80 % R.H. and with a 12-h photoperiod in a screen house for 2 weeks. Seedlings were thinned to three per pot and were watered twice per day (morning and afternoon).
Evaluation of the endophytic colonization
Two weeks after inoculation of the seeds, three bean plants per treatment and per replicate were carefully uprooted and washed with tap water. The seedlings were cut into different sections, i.e., leaves, stems, and roots. Five randomly selected leaf, stem, and root sections from each plant were surface sterilized as already described above. The different plant parts were aseptically cut under a laminar flow hood into 1 × 1 cm pieces before placing them, 4 cm apart, onto PDA plates supplemented with a 0.05 % solution of streptomycin sulfate (Istifadah and McGee 2006; Gurulingappa et al. 2010). The plates were incubated at 25 ± 1 °C for 10 days, after which the presence of endophytes was observed. The last rinse water was also plated out in order to assess the reliability of the surface sterilization procedure as described earlier. The colonization of the different plant parts was recorded by counting the number of pieces of the different plant parts that showed the presence of inoculated fungal growth/mycelia (Petrini and Fisher 1987). Only the presence of endophytes that were inoculated was scored. Microscope slides were prepared from the mother plates and used for morphological identification. Fungal structures such as conidia and conidiogenous cells obtained from fungal cultures were mounted in lactophenol cotton blue (0.01 % w/v) and observed under a Leica microscope (EZ4 HD, Germany) equipped with a digital camera microscope. The treatments were randomized in complete block design and the experiment replicated five times at three different occasions.
Effects of fungus-inoculated bean plants on BSM
The effects of fungus-inoculated bean plants on feeding, oviposition punctures on leaves, pupation, and adult emergence were evaluated. Thirty (30) two-day-old presumably mated adult female BSM flies were exposed for 72 h to 2-week-old fungus-inoculated host plant seedlings in Plexiglas cages (30 × 30 × 25 cm) and maintained at 26 ± 1 °C, 50–70 % RH and 12L: 12D photoperiod. After 72 h post-exposure, plants were replaced with fresh non-inoculated seedlings to prevent excessive oviposition and feeding damage by adult flies.
For feeding and oviposition, inoculated plants were maintained until the leaves were well developed (approximately 5–8 days after exposure). In the control, plants were not inoculated with fungal pathogens. Two leaves were cut from each pot and stained in boiling lactophenol-acid fuchsin solution for 5 min (Nuessly et al. 1995). After staining, the leaves were placed in 90-mm Petri dishes for 1 h before destaining. The stain was removed by immersing the leaves in warm water for 3 min. The number of feeding and oviposition punctures were counted under a stereomicroscope and recorded as described by (Muvea et al. 2014).
For pupation and fly emergence, bean plants were maintained until larvae reached the pupation stage. The number of pupae on the stem and the number of emerged adults were recorded under a dissecting microscope. All the treatments were randomized and experiments were replicated five times at three different occasions.
Data analysis
Colonization frequency (CF) was calculated as described by (Petrini and Fisher 1987) using the formula: Colonization (%) = (PF/TP) X100, where PF = Number of plant pieces colonized, TP = Total number of plant pieces. The proportion of fungal colonization per plant part was arcsine-transformed before subjecting it to ANOVA and means were separated using SNK (Student–Newman–Keuls) and multiple comparison tests. The number of oviposition and feeding punctures, number of pupae, and adult emergence data were log transformed before being subjected to same procedure (ANOVA/SNK). R statistical software, 2.15.4 version (R Development Core Team 2013), was used for all the analysis. The level of significance was fixed at 95 % confidence interval.
Results
Endophytic colonization of P. vulgaris by fungal isolates
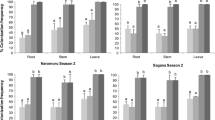
The endophytic colonization of bean plants varied significantly between the fungal isolates
(F11, 165 = 18.7, P < 0.0001) and plants parts (root, stem, and leaves) (F 2, 312 = 8.5, P = 0.0002). The interaction between fungal isolates and plant parts was significant (F 22, 165 = 4.1, P < 0.0001). For instance, T. atroviride (F5S21) showed the highest percentage colonization of the stem (86.6 %); whereas T. asperellum (M2RT4) exhibited high colonization of the roots (85.3 %) (Fig. 1). Beauveria bassiana ICIPE 279 and H. lixii F3ST1 had the lowest percentage colonization of the leaves (10.8 and 10.7 %, respectively) (Fig. 1). All the isolates of M. anisopliae and B. bassiana ICIPE 273 failed to colonize any of the bean plant parts.
Colonization of different bean plant parts (root, stem, and leaves) by fungal pathogens, Beauveria bassiana (Bb) G1LU3, 279 and 273, Trichoderma atroviride (Trich) F5S21, T. asperellum M2RT4, Hypocrea lixii (Hypo) F3ST1, and Metarhizium anisopliae (Met) 20, 30, 69, 78, and GZP after 12 days post-inoculation. Bars indicate mean ± one standard error at 95 % CI (P < 0.0001)
Effects of fungus-inoculated bean plants on BSM feeding and oviposition
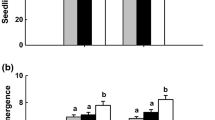
The feeding and oviposition punctures differed between fungal isolates (F 11, 165 = 19.82, P < 0.0001) (Fig. 2). The number of feeding and oviposition punctures by BSM was significantly lower in all the fungus-inoculated plants, except M. anisopliae ICIPE 78 which was similar to the control (Fig. 2).
Effects of exposure to Phaseolus vulgaris plants inoculated with different fungal pathogens, Beauveria bassiana (Bb) G1LU3, 279 and 273, Trichoderma atroviride (Trich) F5S21, T. asperellum M2RT4, Hypocrea lixii (Hypo) F3ST1, and Metarhizium anisopliae (Met) 20, 30, 69, 78, and GZP on oviposition and feeding of adult Ophiomyia phaseoli after 72 h. Bars denote means ± one standard error at 95 % CI (P < 0.0001)
Effects of fungus-inoculated bean plants on BSM pupation and adult emergence
Fewer BSM pupae were produced in fungus-inoculated plants compared to the control (F 11, 165 = 17.28, P < 0.0001) (Fig. 3). Metarhizium anisopliae ICIPE 20 recorded the lowest number of pupae; but was not significantly different from the other fungal isolates, except B. bassiana ICIPE 273, T. atroviride F5S21, and M. anisopliae GZP (Fig. 3). More adult BSM emerged from the control than from the fungus-treated plants (F 11, 165 = 10.46, P < 0.0001) (Fig. 4). Metarhizium anisopliae isolates ICIPE 20 and ICIPE 30 had significantly lower numbers of adult emergence than T. atroviride F5S21, M. anisopliae GZP, and B. bassiana ICIPE 273 as observed with the number of pupae (Fig. 4).
Effects of exposure to Phaseolus vulgaris plants inoculated with different fungal pathogens, Beauveria bassiana (Bb) G1LU3, 279 and 273, Trichoderma atroviride (Trich) F5S21, T. asperellum M2RT4, Hypocrea lixii (Hypo) F3ST1, and Metarhizium anisopliae (Met) 20, 30, 69, 78, and GZP on pupation of adult Ophiomyia phaseoli after 35 days post-inoculation. Bars denote means ± one standard error at 95 % CI (P < 0.0001)
Effects of inoculation of bean plants by fungal pathogens, Beauveria bassiana (Bb) G1LU3, 279 and 273, Trichoderma atroviride (Trich) F5S21, T. asperellum M2RT4, Hypocrea lixii (Hypo) F3ST1, and Metarhizium anisopliae (Met) 20, 30, 69, 78, and GZP on emergence of adult Ophiomyia phaseoli after 35 days post-inoculation. Bars denote means ± one standard error at 95 % CI (P < 0.0001)
Discussion
Endophytic colonization differed between the fungal species and isolates, and the bean plant parts. For instance, T. asperellum isolate M2RT4, T. atroviride isolate F5S21, H. lixii isolate F3ST1, and two B. bassiana isolates GILU3 and ICIPE 279 were able to colonize different parts of the bean plant, except B. bassiana isolate ICIPE 273. On the other hand, M. anisopliae isolates failed to colonize any of the parts of bean plant. The level of colonization of different parts of the bean plant varied according to fungal isolates. For example, inoculation of H. lixii F3ST1 resulted in higher colonization of roots and stem than leaves while the one of T. atroviride F5S21 resulted in higher colonization of the stem and leaves than roots (Fig. 1). Similar results have been reported on other host plants (Vega et al. 2008; Tefera and Vidal 2009; Gurulingappa et al. 2010; Akutse et al. 2013; Muvea et al. 2014). The variation in colonization rate could be due to the fact that fungal endophytes display preferential tissue colonization within their plant hosts as reported by Behie et al. (2014). Similar observations were made on other bean plants such as French bean, Faba bean, and onion (Akutse et al. 2013; Muvea et al. 2014). All the isolates of M. anisopliae failed to colonize any of the bean plant parts. Similar observation was reported earlier with M. anisopliae ICIPE 30 (the same used in the present study) on French bean and Faba bean (Akutse et al. 2013). On the other hand, endophyte colonization of Metarhizium sp. has been reported elsewhere. For instance, Akello (2012) reported that M. anisopliae could be endophyte inside maize and bean tissues. Sasan and Bidochka (2012) and Behie et al. (2014) reported endophyte colonization of Metarhizium in haricot bean plants while García et al. (2011) reported the same in tomato plants. The technique for M. anisopliae recovery used in our study might not have allowed the detection of this fungal species which is typically found in the plant rhizophere. Plant tissues were homogenized using a tissue homogenizer before plating (Sasan and Bidochka 2012; Behie et al. 2014), while whole pieces of plant parts were plated in our study. Furthermore, the inhibitor used in the selective media may also have an effect on Metarhizium competing with other microorganisms (García et al. 2011).
Fungus-inoculated bean plants significantly reduced BSM feeding and oviposition, which is in agreement with previous reports (Quesada-Moraga et al. 2006; Akello et al. 2008; Muvea et al. 2014, 2015). In addition, the number of pupae produced by female BSM and their adult emergence were also significantly reduced. Similar results were reported on another agromyzid, L. huidobrensis (Akutse et al. 2013). Significant reduction in adult emergence, longevity, and oviposition period of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) following exposure of larvae to diet supplemented with ethyl acetate extract of Alternaria alternata (Fr.) Keissl. (Ascomycota: Pleosporales) has also been reported (Kaur et al. 2013).
The mechanism by which fungal endophytes interfere could be explained by the production of active metabolites by endophytes that could deter insect feeding (Bing and Lewis 1991; Vega et al. 2008). Studies by Cherry et al. (2004) on Sesamia calamistis (Lepidoptera: Noctuidae) seem to support the feeding deterrence/antibiosis hypothesis since larvae feeding on plants injected with B. bassiana were smaller than those in the control plants. Investigating the behavioral responses of T. tabaci to endophyte-inoculated onion plants, (Muvea et al. 2015) observed that thrips (adults and nymphs) orientate preferentially to endophyte-free host plants and it was hypothesized that volatiles produced as a result of endophyte–host plant interactions were responsible for thrips non-preference to endophyte-inoculated plant. Jasmonic acid (JA) is known to play essential role in plant defense against herbivore insects (Mcconn et al. 1997). Navarro-Meléndez and Heil (2014) found that endophytic colonization with Fusarium sp. (Ascomycota: Hypocreales) or Cochliobolus lunatus R.R. Nelson & Haasis, (Ascomycota: Pleosporales) enhanced the number of detectable volatile organic compounds (VOCs) (salicylic acid and JA) emitted from intact leaves of the wild Lima bean, Phaseolus lunatus L. (Fabales: Fabaceae).
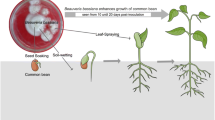
Fungal isolates, H. lixii isolate F3ST1 and B. bassiana isolate G1LU3, which were reported earlier to have effect on the number of pupae and emergence of L. huidobrensis (Akutse et al. 2013), produced similar effects against BSM. Although the effect of endophyte inoculation on the growth of bean plants was not carried out, it is well documented that fungal endophytes promote plant growth (García et al. 2011), as illustrated by Fig. 5 in our study.
The present study has demonstrated that fungal endophytes can be considered as promising tools for the management of BSM. Fungus-inoculated bean plants significantly reduced BSM feeding and oviposition which in turn affected pupation and adult emergence. Future research should focus on the traceability of the endophytes within plant tissue and their performance in field conditions.
Author contribution
All authors conceived and designed the research. BMM conducted the experiments. NKM and SE provided the reagents, fungal isolates, analytical tools, and the facilities. BMM, NKM, SE, and NS analyzed the data and wrote the manuscript. All authors read and approved the manuscript.
References
Abate T (1991) The bean fly Ophiomyia phaseoli (Tryon) (Diptera: Agromyzidae) and its parasitoids in Ethiopia. J Appl Entomol 111:278–285
Abate T, Ampofo JKO (1996) Insect pests of beans in Africa: their ecology and management. Ann Rev Entomol 41:45–73
Akello J (2012) Biodiversity of fungal endophytes associated with maize, sorghum and Napier grass and their influence of biopriming on resistance to leaf mining, stem boring and sap sucking insect pests. Ph.D., University of Bonn, Bonn
Akello J, Dubois T, Coyne D, Kyamanywa S (2008) Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomol Exp Appl 129:157–165
Akutse KS, Maniania NK, Fiaboe KKM, Van den Berg J, Ekesi S (2013) Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life-history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae). Fungal Ecol 6:293–301
Ampofo JKO, Massomo SMS (1998) Some cultural strategies for management of bean stem maggots (Diptera: Agromyzidae) on beans in Tanzania. Afr Crop Sci J 6:351–356
Behie S, Jones S, Bidochka M (2014) Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol 13:112–119
Bing L, Lewis L (1991) Suppression of Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ Entomol 20:1207–1211
Blair M, Fregene S, Beebe S, Ceballos H (2007) Marker-assisted selection in common beans and cassava. In: Guimarães E, Ruane J, Scherf B, Sonnino A, Dargie J (eds) Marker-assisted selection: current status and future perspectives in crops, livestock, forestry and fish. FAO, Rome, pp 81–115
Cherry AJ, Banito A, Djegui D, Lomer C (2004) Suppression of the stem-borer Sesamia calamistis (Lepidoptera: Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int J Pest Manag 50:67–73
Ekesi S, Maniania NK (2002) Metarhizium anisopliae: an effective biological control agent for the management of thrips in Horti- and Floricultural Crops in Africa. In: Upadhyay RK (ed) Advances in microbial control of insect pests. Kluwer Academic/Plenum Publishers, The Netherlands, pp 165–180
García JE, Posadas JB, Perticari A, Lecuona RE (2011) Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Adv Biol Res 5:22–27
Goettel MS, Inglis GD (1997) Fungi: Hyphomycetes. In: Lacey L (ed) Manual techniques in insect pathology. Academic press, San Diego, pp 213–214
Greathead DJ (1969) A study in East Africa of beanflies (Diptera: Agromyzidae) affecting Phaseolus vulgaris and of their natural enemies with the description of a new species of Melanagromyza Hend. Bull Entomol Res 59:541–556
Gurulingappa P, Sword GA, Murdoch G, McGee PA (2010) Colonization of crop plants by fungal entomopathogen and their effects on two insect pests when in planta. Biol Control 55:34–41
Istifadah N, McGee PA (2006) Endophytic Chaetomium globosum reduces development of tanspot in wheat caused by Pyrenophora triticirepentis. Aust Plant Pathol 35:411–418
Jandricic SE, Filotas M, Sanderson JP, Wraight SP (2014) Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J Invertebr Pathol 118:34–46
Karel AK (1985) A Bibliography of bean flies, Ophiomyia phaseoli (Tyron), O. centrosematis (de Meij) and Melanogromyza spencerella (Greathead) (Diptera: Agromyzidae). Michigan State University East Lansing
Karel AK, Autrique A (1989) Insects and other pests in Africa. In: Schwarts HF, Pasto-corales MA (eds) Bean production problems in the tropics. CIAT, Cali, pp 455–504
Kaur HP, Singh B, Kaur A, Kaur S (2013) Antifeedent and toxic activity of endophytic Alternaria alternata against tobacco caterpillar Spodoptera litura. J Pest Sci 86:543–550
Lopez DC, Sword GA (2015) The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol Control 89:53–60
Mcconn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Plant Biology Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci 94:5473–5477
Migiro L, Maniania N, Chabi-Olaye A, Van den Berg J (2010) Pathogenicity of entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana (Hypocreales: Clavicipitaceae) isolates to the adult pea leafminer (Diptera: Agromyzidae) and prospects of an autoinoculation device for infection in the field. Environ Entomol 39:468–475
Muvea AM, Meyhofer R, Subramanian S, Poehling HM, Ekesi S, Maniania NK (2014) Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS ONE 9(9):e108242
Muvea AM, Meyhofer R, Subramanian S, Poehling HM, Ekesi S, Maniania NK (2015) Behavioral responses of Thrips tabaci Lindeman to endophyte-inoculated onion plants. J Pest Sci 88:555–562
Navarro-Meléndez A, Heil M (2014) Symptomless endophytic fungi suppress endogenous levels of salicylic acid and interact with the jasmonate-dependent indirect defense traits of their host, Lima bean (Phaseolus lunatus). J Chem Ecol 40:816–825
Nuessly GS, Nagata RT, Skiles ES, Christenson JR, Elliott C (1995) Techniques for differentially staining Liriomyza trifolii (Diptera: Agromyzidae) eggs and stipples within Cos lettuce leaves. Fla Entomol 78:258–264
Ochilo W, Nyamasyo G (2011) Pest status of bean stem maggot (Ophiomyia spp.) and black bean aphid (Aphis fabae) in Taita district, Kenya. J Trop Subtrop Bot Agroecosyst 13:91–97
Pachico DH (1993) The demand for bean technology. Cali, Colombia
Petrini O, Fisher PJ (1987) Fungal endophytes in Salicornia perennis. Trans Soc Br Mycol 87:647–651
Pyndji MM, Trutmann P (1992) Managing angular leaf spot on common beans in Africa by supplementing farmer mixtures with resistant varieties. Plant Dis 76:1144–1473
Quesada-Moraga E, Landa BB, Muñoz-Ledesma J, Jiménez-Díaz RM, Santiago Alvarez C (2006) Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia 161:323–329
Sasan RK, Bidochka MJ (2012) The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am J Bot 99:101–107
Schultz B, Guske S, Dammann U, Boyle C (1998) Endophyte-host interactions II. Defining symbiosis of the endophyte-host interaction Symbiosis 25:213–227
Singh SR (1990) Fighting Insect Damage to Legumes http://www.anancy.net/tpl/fulltext.tpl.php?file_id=1088&language=english&acs 15. Accessed 25 Jul 2009
Songa JM, Ampofo JKO (1998) Ecology of bean stem maggot attacking dry bean (Phaseolus vulgaris L.) in semi-arid areas of eastern Kenya. Int J Pest Manag 45:35–40
Spencer KA (1973) Agromyzidae (Diptera) of economic importance. Junk, The Hague
R Development Core Team (2013) A Language and environment for statistical computing. http://www.r-project.org/. Accessed 20 Apr 2014
Tefera T, Vidal S (2009) Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. Biol Control 54:663–669
USAID (2010) Staple foods value chain analysis. In Country report Kenya. USAID, Kenya
Vega FE, Posada F, Aime MC, Pava-Ripoll M, Infante F, Rehner SA (2008) Entomopathogenic fungal endophytes. Biol Control 46:72–82
Vega FE, Goettel MS, Blackwell M, Chandler D, Jackson MA, Keller S, Koike M, Maniania NK, Monzo´n A, Ownley BH, Pell JK, Rangell DEN, Roy HE (2009) Fungal entomopathogens: news insights of their ecology. Fungal Ecol 2:149–159
Wickramasinghe N, Fernando HE (1962) Investigations on insecticidal seed dressings, soil treatments and foliar sprays for the control of Melanagromyza phaseoli (Tryon) in Ceylon. Bull Entomol Res 53:223–240
Wortmann CS, Kirkby RA, Eledu CA, Allen DJ (1999) Atlas of common bean (Phaseolus vulgaris L.) production in Africa. CIAT, Cali
Acknowledgments
This study was funded icipe’s Innovative Seed Research Grant “Bean Stem Maggot”. The authors are very grateful to Mrs Gloria Muthoni for technical assistance. We acknowledge the statistical analysis assistance from Dr. Daisy Salifu and taxonomical assistance from Dr. Robert Copeland of icipe and Dr. Fathiya Khamis for molecular analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by S. T. Jaronski.
Rights and permissions
About this article
Cite this article
Mutune, B., Ekesi, S., Niassy, S. et al. Fungal endophytes as promising tools for the management of bean stem maggot Ophiomyia phaseoli on beans Phaseolus vulgaris . J Pest Sci 89, 993–1001 (2016). https://doi.org/10.1007/s10340-015-0725-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-015-0725-4