Abstract
Over the last few decades, important advances have been made in understanding of host–parasitoid relations and their applications to biological pest control. Not only has the number of agent species increased, but new manipulation techniques for natural enemies have also been empirically introduced, particularly in greenhouse crops. This makes biocontrol more complex, requiring a new mathematical modeling approach appropriate for the optimization of the release of agents. The present paper aimed at filling this gap by the development of a temperature- and stage-dependent dynamic mathematical model of the host–parasitoid system with an improved functional response. The model is appropriate not only for simulation analysis of the efficiency of biocontrol agents, but also for the application of optimal control methodology for the optimal timing of agent releases, and for the consideration of economic implications. Based on both laboratory and greenhouse trials, the model was validated and fitted to the data of Chelonus oculator (F.) (Hym.: Braconidae) as a biological control agent against the beet armyworm, Spodoptera exigua Hübner (Lep.: Noctuidae). We emphasize that this model can be easily adapted to other interacting species involved in biological or integrated pest control with either parasitoid or predator agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The paper aimed at filling the gap between existing host–parasitoid models and the empirically introduced new manipulation techniques of biocontrol.
-
The result is a temperature- and stage-dependent dynamic model of the host–parasitoid system, appropriate for simulation analysis and optimization of the release of biocontrol agents
-
For an illustration, the model is validated and fitted to the data of a concrete host–parasitoid system.
-
Our model building can be easily adapted to biocontrol by predatory agents.
Introduction
Recently, a large amount of high-quality data is collected in laboratory and greenhouse conditions. This facilitates the development of mathematical models based on fine-scale details of biological situations. These kinds of models have important advantages. First, we can determine whether the parameters (in this study, degree-day dependence, functional response, and population dynamics) measured in laboratory correspond with the appropriate theoretical population dynamics. If not, different degree-day dependences and functional responses can be considered, respectively, or the theoretical model improved. Second, like in physics, a theoretical model of population dynamics can be used to predict experimental results by simulation, and these predictions can be experimentally tested. Of course, based on the general principles, in each concrete biological situation, new models are required for each specific biological situation.
Over the last few decades, important advances have been made concerning host–parasitoid relations and their applications to biological pest control. Not only has the number of agent species increased, but new manipulation techniques for natural enemies have also been introduced, particularly for greenhouse crops. This makes biocontrol more complex, requiring a new mathematical modeling approach appropriate for the optimization of the release of agents. The present paper aimed at filling this gap by the development of a temperature- and stage-dependent dynamic mathematical model of a host–parasitoid system with an improved functional response. This model is appropriate not only for simulation analysis of the efficiency of biocontrol agents, but also for the application of control methodology for optimal timing of agent releases, and for the consideration of economic implications.
Based on both laboratory and greenhouse trials, the model was validated and fitted to the data of Chelonus oculator (F.) (Hym.: Braconidae), a biological control agent (koinobiont parasitoid) for the beet armyworm, Spodoptera exigua Hübner (Lep.: Noctuidae). We emphasize that this model can be easily adapted to other interacting species involved in biological or integrated pest control with either parasitoid or predator agents.
Theoretical preliminaries
Mathematical models are widely used in population biology, particularly in entomology. Fecundity, rates of development, and mortality are strongly affected by temperature in insects (Chown and Nicolson 2004). These effects are included in most deterministic dynamic stage-dependent models of insect populations (e.g., Birt et al. 2009; Bommarco 2001; Prasad et al. 2002; Schmidt et al. 2003; Son and Lewis 2005; Söndgerath and Müller-Pietralla 1996; Wagner et al. 1985). For stochastic models, see Castañera et al. (2003) and the references therein. To describe interacting insect populations, functional response has also been built into stage-dependent models. Murdoch et al. (1997) studied the effects of host size and parasitoid state-dependent attacks. Alto et al. (2009) investigated the coexistence of competing mosquitoes when the predation efficiency depends on the size of instars in a marine aquaculture model (Barbeau and Caswell 1999). In our study, we are interested in a concrete mechanism underlying host–parasitoid interaction that can be applied in biological control practice. In our dynamic population model, two factors play an important role: temperature dependence of the developmental rates and density dependence of the functional response.
With the change from chemical to biological control motivated principally by resistances to insecticides, pest control in greenhouse crops of northern Europe has experienced an important evolution in the last 30 years (van Lenteren 2007). A similar, more recent, shift has occurred in Spanish greenhouse crops (van der Blom 2010). Again, excessive use of chemical control (Cabello and Cañero 1994) led to increased resistance to insecticides (van der Blom 2010). This change was very rapid; out of approximately 24,000 Ha of crops, the use of biological control has increased from 1,400 Ha in the 2006–2007 crop season to 23,500 Ha in 2009–2010 (van der Blom 2010).
Factors modeled in the literature but not considered in the current study
Not only are the developmental rates of insects strongly affected by temperature, but the functional responses also depend on it (Logan et al. 2006; Logan and Wolesensky 2007). For simplicity, we did not account for this dependence. Furthermore, humidity (e.g., Choi and Ryoo 2003) and dispersal and/or spatial population structure (e.g., Ims and Andreassen 2005) also have an effect on insect population dynamics.
Our stage-dependent model is not a spatial model as it ignores the colonization process, since in a greenhouse, we can consider the pest and parasitoid populations uniformly distributed among plants (although we do not consider their distribution on a given plant). This is justified, firstly, by the relatively small crop areas in greenhouses compared with those in open-air crops. Secondly, after the initial infestation of the pest population, crop colonization is very quick because of the temperature and high plant density. Finally, the hypothesis of a uniform distribution of parasitoid populations is justified by their uniform release throughout the greenhouse, and because the released specimens, in our case, are adults with the capacity for flight.
The host–parasitoid system
The beet armyworm, S. exigua, is a lepidopteran species (Family Noctuidae); it is polyphagous and attacks herbaceous plants in greenhouses and open-air crops. It is a serious pest species in pepper and watermelon crops in greenhouses (Cabello 2009). In turn, Ch. oculator is a species in the genus Chelonus (Subfamily Cheloninae) that constitutes a wide group within the Braconidae family (Ichneumonoidea superfamily). All Cheloninae species are egg-larval lepidopteran solitary koinobiont endoparasitoids. Females oviposit inside the host egg, and subsequently, at the beginning of the third larval instar, the parasitoid leaves the egg to pupate (Gauld and Bolton 1996; Garcia-Martin et al. 2005). Several aspects of its biology, ecology, and functional response have been studied in laboratory conditions, but not on natural hosts (Garcia-Martin et al. 2005, 2008; Ozkan and Tunca 2005; Ozkan 2006; Cabello et al. 2011a). The species has already been used in the biological control of S. exigua in greenhouses of southeast Spain.
First, we summarize the methods of data collection and evaluation of the biological parameters of the host and parasitoid species, for laboratory and greenhouse trials. A dynamic mathematical model of the host–parasitoid system was developed for the analysis of the biological control mechanism, and its validation with the greenhouse data is presented. It describes the results of laboratory and greenhouse trials, as well as simulation results of the dynamic biological control model. Applications for predicting the effect of release of Chelonus during different stages of Spodoptera development are also considered. Finally, the results are summarized and discussed.
Materials and methods
Rearing conditions
The species used in the different trials come from populations maintained in laboratory conditions (25 ± 1 °C, 60–80 % R.H. and 16:8 h light:dark cycle) at the University of Almeria. S. exigua was reared on an artificial diet, according to the methodology described by Cabello et al. (1984a, b). The parasitoid species, Ch. oculator, was reared according to the methodology used by Cabello et al. (2011a), on alternative host, Ephestia kuehniella Zeller (Lep.: Pyralidae), which was reared according to Daumal et al. (1975).
Evaluation of biological parameters of the host species
Laboratory trial
The experimental design was randomized with one factor (temperature) at four levels (15, 20, 25, and 30 ± 1 °C). The number of replications was variable for each temperature tested, with a minimum of 515 eggs, 477 larvae, 240 pupae, and 99 couples of adults. Rearing conditions were 60–80 % R.H. and 16:8 h of light:dark cycle. Egg masses were obtained from the adult couples reared at one of the four temperatures for at least one generation. Small egg masses (presenting less than 50 eggs arranged in a single layer) were cleaned of scales and isolated in a container (25 ml) with a lid that was provided with a hole (0.75 cm in diameter) and closed with a metallic mesh. Then, neonate larvae were isolated from the hatched eggs in the same type of container, and an artificial diet was used and replaced every 24 h. The containers were placed in climatic cabinets at each of the four temperatures. After pupation, the sexes were separated by their morphological characters and maintained at each temperature until they reached the adult stage. Later, pairs of adults (1♀ + 1♂) were placed in cylindrical containers (Ø 85 mm × 70 mm) of filter paper used as the ovipositional container. As food, honey in water (10 %) was provided on cotton and was replaced every 24 h.
The times spent in the egg, pupa, and larval stages were recorded. Longevity and fecundity of adults were also recorded for each temperature. The data were statistically analyzed using a generalized linear model (GLM) and Tukey’s test with the SPSS software package (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, version 21.0, Armonk, NY, USA). To calculate the thermal units required for growth and development, we used the improved linear model of Ikemoto and Takai (2000):
where D = length of stage in days, T = temperature in °C, k = thermal units required for growth and development in accumulated degree-days (ADD), and t = minimum threshold temperature in °C. The linear regressions were realized using SPSS.
Greenhouse trial
The greenhouse trial was carried out between June and August in an “Almeria” type greenhouse with a soil mulch system and pepper crop (variety INIA) located at La Mojonera, Almeria, Spain, on a 750 m2 surface. The crop management was the same as traditional practice in the area except that no phytosanitary treatment was applied. Four cages were made with nonwoven fabric (2.25 m above and 0.15 m below the level of the soil; 2.0 m wide and 5.0 m long) located at random inside the crop surface. Each cage contained five pepper plants (1.2 m height). A mature adult couple (1 ♀ + 1 ♂) of S. exigua from the laboratory population was also released into every cage.
The plants inside every cage were numbered, and the phenological states were recorded (height, number of side branches, and number of leaves, flowers, buds, and fruits in the different branches). In the first 5 days, samples were collected every day to count the eggs oviposited by the females. Later, even in the pupal stage, sampling was conducted twice per week to count the larvae and record their instars. Then, the entire soil in every cage of the greenhouse up to 15 cm depth was sifted to obtain the pupae. Immediately after, the pupae of each cage were introduced in a wooden box with walls of a metallic mesh (# 0.5 mm) that was buried in the same soil of every cage in the greenhouse; these were observed, every 2 days, until the emergence of the adults. During the trial period, a thermohygrometer was placed in each cage.
To analyze the stage-frequency data of the host species, we have considered a simplified version of the Kiritani, Nakasuji, and Manly’s method (Manly 1990; Southwood and Henderson 2000) as it was described for zooplankton instar development (Rigler and Cooley 1974; Hairston 1985). However, we consider single cohort data (i.e., for populations where all individuals enter stage one at about the same time; Manly 1990).
We consider \(\bar{b}_{j + 1,j}\), the number of individuals entering stage j + 1:
where A j is the area under a stage-frequency curve for stage j, and T j is the developmental time for stage j + 1, expressed in ADD.
We also consider \(\bar{b}_{j,j}\), the number of individuals that remain in state j:
For each stage, values were obtained from the experiments. They were interpolated in order to have equidistant times for function fitting to calculate the transition coefficients between the stages.
Evaluation of biological parameters of the parasitoid species
Laboratory trial
The experimental design was randomized with one factor (temperature) and two levels (20 and 30 ± 1 °C). The number of replications for each temperature treatment was variable, with a minimum of 350 larvae inside the host, 172 larvae in the third-instar outside the host, 125 pupae, and 21 parasitoid adult couples. Rearing conditions were 60–80 % R.H. and 16:8 h of light:dark cycle. Egg masses of the host, S. exigua, were obtained from laboratory rearing.
The eggs were parasitized by isolated Ch. oculator females that were mated previously with two males. Fifty eggs were offered for 4–5 h to every parasitoid female for both temperature treatments. Once the eggs hatched, the neonate host larvae were placed individually in a plastic container (20 ml). Host larvae were reared in similar conditions, up to the emergence of the parasitoid larvae, the third-instar. At this time, they were isolated in a clean container and preserved, at each test temperature until the emergence of the adult.
Later, the parasitoid adult couples (1♀ + 1♂) were placed in isolation in Petri dishes (Ø 85 mm). Food, honey diluted in water (50 %), was provided on cardboard and replaced every 24 h. In addition, every 24 h, a cardboard with 300 E. kuehniella eggs was added to each container. Later, the cardboard pieces with the parasitized eggs were moved to a plastic container (100 ml) with flour, beer yeast, and wheat germ, and they developed at 30 ± 1 °C until the emergence of the adult.
The times spent inside or outside the host larvae and in the pupa stage were recorded. Longevity and fecundity of adults were also recorded. These data were collected for both temperature treatments.
The data for each stage of host development as well the longevity and fecundity of adults were analyzed by GLM and the Tukey’s test by using the SPSS software package. From the values obtained, the transition coefficients of the species for each developmental stage were determined as described above.
Greenhouse trial
Trials were carried out with three different parasitoid release rates (0.5, 1.0, and 1.5 ♀/m2). For each trial, the experimental design was randomized with one factor (host density) at five levels (50, 100, 150, 200, or 250 host eggs). The number of replications was 12 for each host density. A multi-tunnel greenhouse (969 m2) located in La Cañada, Almeria, Spain, was used. There were two lines for each of the following crops: tomato, pepper, bean, cucumber, melon, and watermelon to minimize the effect of the plant in the behavior of parasitoid females. The three trials were realized between May and July and were separated by 15 days each. During the trial period, a thermohygrometer was placed in each cage.
The parasitism rate in each trial was evaluated by the sentinel method (Mills 1997). E. kuehniella eggs (factitious host) were used: They were glued on a white cardboard (70 × 30 mm) with water at the different host densities. The cardboard pieces with the host eggs were distributed uniformly at random in the greenhouse and placed over the leaves using a paper clip. The eggs were left for 72–90 h. Then, the cardboard was collected and transferred into plastic containers with flour, beer yeast, and wheat germ. They developed at 30 ± 1 °C until adult emergence. During the test period, temperature and relative humidity were measured using a thermohygrometer.
For each trial, the numbers of adult Ch. oculator and E. kuehniella that emerged from the cardboard were recorded.
Based on the collected data, we determined the type of functional response to adapt in our dynamic model. The functional response was fitted to the average number of parasitized host eggs according to the following equations:Type II (Hassell 1978):
Type III (Cabello et al. 2007):
where: x a = number of parasitized hosts, x = host density, a′ = instantaneous search rate (1/days), \(\hat{\alpha }\) = potential of mortality, T = total time available for search (days), and T h = handling time (days), y = number of parasitoids.
The least-squares method in TableCurve 2D and 3D software packages (TableCurve 2D version 5.01 and TableCurve 3D version 4.0, Systat Software, San Jose, CA, USA) was used for parameter estimation.
Dynamic mathematical model
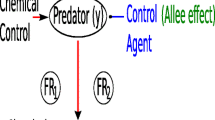
Our model is based on the mechanism displayed in the flow diagram in Fig. 1.
For the host species, we consider five model stages (simply called stages) of the life cycle: egg, small larvae (first- to third-instar), large larvae (fourth- and fifth-instar), pupa, and adults. Entomophagous parasites (parasitoid and predator species) are generally identified as small or large larvae (Cabello 1988).
For the life cycle of the parasitoid species, three model stages, egg, larva-pupa, and adult, were considered.
Since some coefficients in our model are functions of biological time (in ADD), we recall their general definitions in the discrete time form. Let T(t) (t = 0,1,2,3,…) be the temperature scenario, then biological time, \(\tau_{t}\), corresponding to physical time t is defined as
assuming that the temperature only changes within the physiological range of T min and T max valid for both species.
Let b j+1, j (τ) be the proportion of individuals passing from stage j to stage j + 1 during the biological time τ (j = 1,…,4), and b j,j (τ) be the proportion of individuals in stage j that remain in this stage; during the same biological time (j = 1,…,4), \(\alpha_{5} (\tau )\)is the number of eggs laid per host female during this period, and σ is the proportion of females among the host insects. Certainly, b j,j (τ) + b j+1, j (τ) ≤ 1 must hold (j = 1, 2, 3, 4, 5), and 1 − (b j,j (τ) + b j+1, j (τ)) is interpreted as the death rate for stage j during the period considered. For the dependence of the transition rates on biological time, we apply the Weibull cumulative distribution function that is widely used for the description of the development of ectothermic organisms (Wagner et al. 1984; Manly 1990; Söndgerath and Müller-Pietralla 1996; Choi and Ryoo 2003; Schmidt et al. 2003). Suppose that, after a long period of biological time, each individual passes to the next stage with probability \(\lambda \in ]0,1[.\)Then, the long-term death rate is 1 − λ. With constants α, β > 0, we define
Evidently, these constants may depend on the stage j. In the above expressions, for the ith cohort in a given stage, it is necessary to substitute
which is the accumulated degree-day during the last i time units and can be easily calculated using online software (Zalom et al. 1983). The number of eggs can also be expressed as
where μ is the maximal number of eggs per host female (after a long biological time), and ε and φ are constants. For the Weibull cumulative distribution function, the TableCurve 2D software package was used for parameter estimation by the Marquardt algorithm (Conway et al. 1970) and the least-squares method.
In our model, \(x_{1}\),…, \(x_{5}\) will denote the numbers (densities) of individuals in the above-specified stages of the host population. Similarly, for the parasitoid, \(y_{1}\), \(y_{2}\), \(y_{3}\) indicate the densities for each of the three considered stages.
Furthermore, we distinguish N and M cohorts in each stage of the host and the parasitoid, respectively, with respective densities
\(x_{ji}\) (j = 1, 2, 3, 4, 5; i = 1,…, N), and \(y_{ji}\) (j = 1, 2, 3; i = 1,…, M), and set \(x_{1} : = \sum\nolimits_{i = 1}^{N} {x_{1i} }\), \(y_{3} : = \sum\nolimits_{i = 1}^{M} {y_{3i} }\).
For the model, it is also supposed that the parasitoid female only parasitizes the host eggs (Garcia-Martin et al. 2008). Then, we applied the following functional response suggested by Cabello et al. (2007):
where T = 1 (day), T h , the estimated handling time of the host (days) and \(\hat{\alpha }\) are fitting parameters. A further model parameter is \(\alpha_{{^{5} }} (\tau ),\) the number of eggs laid per host female during this period.
Dynamics of the host population
To describe the development of the host population, we propose the following multistage dynamic model:
Suppose that, for a unit of time, individuals in each stage j = 1, 2, 3, 4, in each cohort i = 1, 2,…, N − 1, either pass into the first cohort of the next stage or pass into the next cohort of the same stage. Individuals of the Nth cohort in the stage j = 1, 2, 3, 4 either die or pass into the first cohort of the next stage. Individuals in the fifth stage (adults) either die or pass into the next cohort. Egg:
Small larva:
Large larva, pupa, and adult: the densities of the cohorts in these three stages are expressed similarly:
Dynamics of the parasitoid population
The corresponding multistage dynamic model for the parasitoid is as follows. Assuming that every host egg is parasitized at most once, consider rates \(d_{jj} (\tau )\) (j = 1, 2, 3) and \(d_{j + 1j} (\tau )\) (j = 1, 2) defined analogously as \(b_{j,j} (\tau )\) and \(b_{j + 1,j} (\tau )\), respectively. Then, for the parasitoid dynamics, we have the following: Egg:
Larva-pupa:
Adult:
The coefficients b i,j and d i,j correspond to transitions between the different stages of the life cycle of the host and parasitoid, respectively, and are estimated according to the Weibull cumulative distribution functions.
The thermal parameter values used in the model are indicated in Table 2. For the minimum temperature of activity of adult females of S. exigua, we used a minimum threshold of 10 °C according to estimates of Aarvik (1981) and Belda (1994).
Data and method for validation of the model in the greenhouse
Trials were carried out, between August and September, in an “Almeria” type greenhouse with soil with gravel–sand mulch and pepper crop (Lamuyo variety) located at El Ejido, Almeria, Spain, with a surface of 5,000 m2. The traditional crop management practiced in the area was adopted for the trial except that no phytosanitary treatment was applied. Four cages were made with nonwoven fabric (3.0 m high, 18.0 m wide, and 18.0 m long) located at random on the crop surface; every cage contained 1,300 pepper plants (1.5 m height). During the trial period, a thermohygrometer was placed in each cage.
The pest infestation was artificial and was done by placing eggs of S. exigua that were less than 24 hours old obtained from laboratory cultures, and they were uniformly distributed at a density of 12.5 eggs/m2. At 24 h, adult parasitoids were released uniformly and at a dose of six females/m2. Later, seven samplings were performed, twice a week, for 20 plants per plot, in which all larvae found on the sampled plants were collected. The larvae were isolated and reared in the laboratory with an artificial diet, as described above, until the emergence of adults. Due to the death of some larvae, they were dissected under a binocular microscope to check for the presence of the parasitoid larva.
Data for the parasitized and non-parasitized larvae were compared with the data obtained in the mathematical model using the R 2 statistic, which was close to one indicating the validity of our model (Montgomery 2010).
Results
Biological parameters of the host species
In laboratory
The average values of the development as well as the longevity and fecundity of the adults are shown in Table 1. In the statistical analyses, significant effects of temperature on the duration were found for all stages of the host species (F 3,511 = 349.74, P < 0.0001; F 3,473 = 145.76, P < 0.0001; F 3,454 = 179.10, P < 0.0001; F 3,450 = 304.10, P < 0.0001; F 3,444 = 205.07, P < 0.0001; F 3,327 = 171.30, P < 0.0001; and F 2,237 = 200.34, P < 0.0001 for the duration of egg, first-, second-, third-, fourth-, and fifth-instar, larva, and pupa, respectively). Similarly, there were significant effects of temperature on longevity (F 3,95 = 16.83, P < 0.0001 and F 3,95 = 9.98, P < 0.0001) for female and male, respectively. However, there was no significant effect of temperature on the fecundity of females. Moreover, at 15 °C, the pupal stage is not completed, in any case, and adult emergence did not occur; therefore, longevity and fecundity of adults were calculated from the individuals that developed at 20 °C (Table 1). From the above data, the thermal relationships were calculated and are shown in Table 2.
In greenhouse
The transition coefficients of the host species, S. exigua, calculated for each developmental stage in the greenhouse pepper crop are shown in Table 3. The cumulative numbers of individuals entering each developmental stage are shown in Fig. 2.
For the parameters of \(\alpha_{5} (\tau )\), the following values were obtained: ε = 49.87, φ = 1.21, μ = 1292.8 (see the model description in 2.4).
Biological parameters of the parasitoid species
Laboratory
The average values for the rate of development as well as the longevity and fecundity of adults are shown in Table 4. In the statistical analyses, significant effects of temperature were found on the duration of the stages of the parasitoid species (F 1,348 = 5,345.25, P < 0.0001; F 1,170 = 64.82, P < 0.0001; and F 1,123 = 3,202.74, P < 0.0001), for the duration of immaturity inside the host: egg to third-instar; outside: until pupa, and pupa, respectively. There were significant effects of temperature on the longevity of the female and male and apparent parasitism (F 1,19 = 38.45, df = 1, P = 0.01; F 1,19 = 94.33, df = 1, P < 0.0001; and F 1,19 = 20.99, P = 0.0002, respectively).
The transition coefficients were calculated and are shown in Table 5.
Greenhouse
The functional response found for each field trial corresponds to type III (Table 6) that showed lower corrected Akaike information criterion (AICc) values (Motulsky and Christopoulos 2003) than did type II. This was performed in laboratory conditions (Garcia-Martin et al. 2008). The joint functional response was estimated and is shown in Fig. 3.
From the almost perfect fitting shown in Fig. 2, one may suspect that the model (or its components) was overfitted. In fact, this is not the case:
-
1.
Figure 2 is related to Table 3 and comes from the fitting of field data for five host stages [Section M&M, Evaluation of biological parameters of the host species, (b) Greenhouse trial]. The collected data correspond to four replications, 13 samplings, and five stages (total data points = 260), and the total fitted parameters are 15 (five stages and three parameters per stage), which is not considered overfitted. The appearance of overfitting may be due to the fact that Fig. 2 corresponds to mean values for the four replicates. For the fitting of parameters in Table 3, all data were used, not only mean values.
-
2.
Similarly, in Table 4 (there is no corresponding figure), there are nine total parameters for the parasitoid species (three stages and three parameters per stage) corresponding to data obtained in the laboratory [Section M&M, Evaluation of biological parameters of the host species, (b) Laboratory trial]. The minimum number of data points is 350 larvae inside the host, 172 larvae in the third-instar outside the host, 125 pupae, and 21 parasitoid adult couples for two temperature treatments (at least 1,336 data points). Therefore, there is no overfitting.
-
3.
Finally, Table 6 and Fig. 3 correspond to field data [Section M&M, Evaluation of biological parameters of the parasitoid species, (b) Greenhouse trial]. Three trials with five parasitoid densities and 12 replications per density were realized (total number of data points = 180), and there were two (Table 6) or three parameters (Fig. 3). Hence, no overfitting occurred in this case either.
Biological control in greenhouse
Validation of the model
In order to validate our model, we compared the percentage of parasitized larvae with the value calculated in the model using the same densities of host adults and released parasitoid adults for the sum of the stages, egg + larva + pupa of the model. Larval parasitism was 92.16 %, which is a high value. We carried out a partial validation of our model (Fig. 4). The validation is partial in the sense that we could use only a part of the life cycle data of the parasitoid without using those corresponding to the host species. Comparing the real data to those of the model, a rather good matching was found since the coefficient of determination, r 2, is 0.8139 (df = 8, P = 0.05). We emphasize that Montgomery (2010) proposed this type of validation pointing out that the closer this value is to one, the more valid is the model.
Model runs showing the dynamics of different stages of both species
For the proportion of host females, we have σ = 0.5. Tables 3 and 5 show the estimated parameters of the Weibull function. The parameters of α 5(τ) are as follows: ε = 49.87, φ = 1.21, and μ = 1,292.8. Based on the maximal possible time an individual can remain in a particular stage, we set the number of cohorts for both species to N = M = 40. For the parameters of functional response f 1 of Chelonus, we have T h = 0.0076 and \(\hat{\alpha }\) = 0.00828. In Fig. 5, we show the rate of development of different stages of host (Fig. 5a) and parasitoid (Fig. 5b) obtained by simulation. Furthermore, for the pest species, it is supposed that, from day 30 until day 60, entrance of adults to the greenhouse takes place at a rate of 50 adults per day. As for the parasitoid species, releases of adults (600 adults/100 m2, with sex ratio 1:1) on days 32 and 38 were considered.
One may think that 2–3 cohorts are sufficient. Nevertheless, we wanted to construct a more realistic model that can be applied to the S. exigua–Ch. oculator system in commercial greenhouse cultivars. Under these conditions, the cultivation cycle is summer through the following spring, and the infestation in the greenhouse occurs in the first 2–3 months of the cultivation cycle; afterward, the populations outside the greenhouse are very rare or absent, because the temperatures are higher inside than outside. The interior populations are important in terms of the crop damage.
According to Wagner et al. (1985) (op. cit.): “The number of cohorts in a simulation is determined by the number of classes in the oviposition distribution. Unfortunately, no method has been developed relating class interval length to the accuracy of the final prediction for a general population. One way to ensure precision is to set the sampling interval of eggs, and thus the class interval of the oviposition distribution, equal to or less than the development time of eggs (e.g., oviposition to hatch) in the field.”
Taking into account the temperatures inside the greenhouse, and considering the data of Table 1, the duration of the egg stage is 2 days. For the indicated period, 40 cohorts (equivalent to 200 host population groups) have been considered. Furthermore, considering again the high inside temperatures, the durations of different host stages are reduced considerably in comparison with the outside conditions, generating great divergences between cohorts, justifying why, for a more precise model, it was necessary to consider such a high number of cohorts.
In case of the parasitoid, it was “only” necessary to consider 120 population groups to obtain a more realistic model under the conditions of greenhouse cultivars where usually two releases of parasitoid adults are realized, with a 7–10 day interval between releases. (We note that the question of improving the efficiency of natural enemies by optimal timing of releases will be the subject of a forthcoming paper.)
All data used in the model are available at Dryad Digital Repository: doi:10.5061/dryad.t3b5s.
Discussion
Biological parameters of the host species
The results obtained for the developmental stages of the host species, S. exigua (in days), are similar to those found by other authors (Butler 1966; Cayrol 1972; Fye and McAda 1972; Hogg and Gutierrez 1980; Sannino et al. 1986; Tisdale and Sappington 2001; Elvira et al. 2010). The slight differences might be attributable to the different food provided to larvae (Awmack and Leather 2002; Azidah and Sofian-Azirun 2006) or different origins of populations (Pashley 1986). Some differences were also found in the calculated developmental threshold temperatures with respect to those reported by other authors for the same species. The values threshold temperatures found for eggs (13.9 °C) are similar to those cited by El-Refai and Degheele (1988), but they are somewhat higher than those indicated by Cayrol (1972) and McNally (1983). In turn, for small larvae, temperatures (12.5 °C) are similar to those quoted by other authors (Cayrol 1972; Hogg and Gutierrez 1980; Ali and Gaylor 1992). The threshold temperature for the pupal stage (8.3 °C) is quite different from those mentioned by El-Refai and Degheele (1988) and Ali and Gaylor (1992) for this species. The differences may be owing to the different geographic locations of populations as demonstrated for this and other insect species (Honek 1996).
The duration of the adult stage of S. exigua in our assay is similar to that of Hogg and Gutierrez (1980), and both are somewhat higher than those cited for the species by Sannino et al. (1987). In addition, female fecundity is very similar to those reported by Hogg and Gutierrez (1980) and in both cases are higher than those quoted by other authors for this species (Sannino et al. 1987; Wakamura 1990).
Based on the above, the thermal requirements used in the mathematical model for the eggs, small and large larvae, and pupae are the average of the measured values (10 °C), corresponding to the characteristics for the species in our geographic area.
Biological parameters of the parasitoid species
The development time for the parasitoid species in the natural host (S. exigua; Table 1) is much shorter than that cited in the factitious hosts: E. kuehniella, Plodia interpunctella (Hübner) (Lep.: Pyralidae), or Cadra cautella (Walker) (Lep.: Pyralidae), which are the only data published until now for the species (Garcia-Martin et al. 2005; Ozkan 2006; Tunca et al. 2011). Two life history strategies exist among parasitoid wasps: idiobionts, where the host does not grow during the development of the parasitoid larvae and koinobionts, where the host continues to grow during the parasitoid development (Askew and Shaw 1986). In idiobionts, the female wasp uses its ovipositor to sting and kill or immobilize the host. The female’s progeny thus have to develop on a fixed amount of food. For this group, the influence of the host (size, species, age, etc.) on the biology of the parasitoid has been demonstrated (Thompson and Hagen 1999). In koinobionts, the host is not killed or immobilized and continues to grow after the female wasp oviposits. The progeny are thus not restricted to the original amount of food (Cabello et al. 2011a). Although the reason for this parasitoid group is less clear, the host may have an influence on the koinobiontic parasitoids that develop on the hosts and continue to feed and grow (Thompson and Hagen 1999).
The aforementioned effect of shorter development time in S. exigua can be explained by the amount of food available; therefore, the maximum weight of the last-instar larvae is 55 mg (unpublished data) unlike weights presented by E. kuehniella (31 mg) (Kallenborn and Mosbacher 1983) or P. interpunctella (16 mg) (Silhacek and Miller 1972). An increase in developmental time due to amount of food was shown in koinobiont solitary parasitoids (Harvey et al. 1995). This is motivated by host quality, and the koinobiont parasitoids are crucially dependent on the host growth rate after parasitism and on the final size of the host when it is destroyed by the parasitoid. When attacking nutritionally suboptimal hosts, such as early instars, the host may grow too slowly for the parasitoid to maximize size and minimize development time (Harvey 2005).
Moreover, in the adult, longevities found for males and females (Table 1) are unaffected by the rearing host; the values found are similar to previous studies that consider the same species, but in the host E. kuehniella (Garcia-Martin et al. 2005).
The longevities of females and males (Table 4) are not affected by the rearing host at their immature stages since their longevities are very similar to those found in a different host by Garcia-Martin et al. (2005). In contrast, the fecundity of the females is influenced by the rearing host at their immature stages; therefore, the fecundity values (Table 4) are 5–6 times higher than those reported by Garcia-Martin et al. (2005) for the host E. kuehniella. Similar effects for fecundity were published for other Chelonus species (Legner and Thompson 1977) and also in other hymenopteran parasitoids (Harvey and Thompson 1995; Stoepler et al. 2011).
The minimum development threshold temperatures could not be determined from the measured data. The only value quoted for this species is 12.5 °C, but in a different host species (Tunca et al. 2011). As noted above, the development rate is affected by the host species, and this in turn influences, according to the method of calculation, the threshold temperatures; this issue has not been considered in the literature. Therefore, for the simplicity of the model, we have considered the same development threshold temperature of the host species. We did not consider this as causing serious error in the model, since for other species of the genus, for example, Ch. texanus, Cresson (Hym.: Braconidae) in the same natural host, S. exigua, Butler (1966) found that the rate of development of the parasitoid was no different than that of the host.
Using this temperature (10 °C), the average development time (expressed in ADD) was estimated (Table 4) for the parasitoid species Ch. oculator reared in the host S. exigua.
Dynamic model
Over 500 mathematical models have been applied to establish the phenology of pest species in relation to the biological time expressed in ADD (Nietschke et al. 2007), many of which considered the age structure (e.g., Curry et al. 1978; Osawa et al. 1983; Hudes and Shoemaker 1988; Munholland and Dennis 1992) for their applications to pest control (Nietschke et al. 2007). However, far fewer (50) are applicable in biological control (Barlow 2004). Some refers to host–parasitoid models that are already applied. Concerning host–parasitoid systems, this may be due, in part, to the fact that the host and parasitoid populations with discrete generations frequently show imperfect phenological synchronization, resulting in some hosts experiencing reduced or even no risk of parasitism (Godfray et al. 1994).
Although our model building approach is general, a novelty of our model is the use of an improved Holling type III functional response found in an earlier paper by some of the authors (Cabello et al. 2007). In fact, a statistical analysis demonstrates that (at least under the considered temperature conditions) this functional response fits the data better than the Holling type II functional response. To our knowledge, no multistage dynamic model has been built with this improved functional response.
In general terms, similar well-fitted dynamic models can be used in biological pest control for several purposes: (1) They may facilitate the selection of an adequate control agent. (2) The model simulations help determine the most efficient release strategy, and in particular, the optimal timing of the release(s) of the agent for biological control in greenhouse crops. (3) Such models can be extended to include economic aspects of biological control for the anticipated analysis of cost efficiency of biological control. Such developments concerning the S. exigua–Ch. oculator system may be the subject of further research.
Efficiency of biological control
The genus Chelonus has received little attention as a natural or biological control agent of pest species, especially, compared to other species of Lepidopteran parasitoids (e.g., Trichogramma, Cotesia). Nine Chelonus species have been used in the control of Lepidopteran pests, worldwide, through classical biological control (introduction into a new geographic area). In most cases, the parasitoid was not established, or information on it does not exist, but in three cases, when they became established, they colonized new geographic areas and achieved satisfactory control of the pest (Greathead 1976; Clausen 1978; Nechols et al. 1995; Neuenschwander et al. 2003).
Furthermore, the use of the Chelonus species through augmentation has been minor (Elzen and King 1999; Etzel and Legner 1999). In this regard, four species were tested: Ch. eleaphilus, Silvestri (Hym.: Braconidae) for the control of Prays oleae Hübner (Lep.: Praydidae) had a very high level of parasitism (92.0 %) (Stavraki 1970; Stinner 1977) and Chelonus sp. p. curvimaculatus, at a release rate of 0.3 females/m2, for control of the Pectinophora gossypiella (Saunders) (Lep.: Gelechiidae) with a good rate of parasitism (69.9 %; Legner and Medved 1979). In contrast, low levels of parasitism were detected in two cases. In Ch. inanitus (L.) (Hym.: Braconidae), the rate of 0.1 females/m2 achieved a parasitism of 23.6 % for S. littoralis in cotton crops (Rechav 1976) and for Ch. heliopae, a high release rate (10 adults/m2) presented a very low level of parasitism of Spodoptera litura (F.) (Lep.: Noctuidae) (8.8 %) on a cauliflower crop (Patel et al. 1979).
The parasitism found in our study (92.16 %) indicates that Ch. oculator is a good biological control agent of S. exigua in the greenhouse pepper crop. In fact, the species is used for the control of S. exigua in southern Spain.
The total area that can be covered by biological pest control using the presented application in Spanish greenhouses will be over 25,000 Ha in the next growing season of 2013–14, representing 90 % of the total area. This implies that the utilization of a large amount of natural enemies through the application of different techniques is beneficial. Therefore, to understand the efficiency and cost-effectiveness of this method, it is important to understand how, when and in what doses natural enemies of pests should be released? Despite the great experience of technicians and farmers, this work should not be left to them (Cabello et al. 2011b) alone. Therefore, we consider that similar research works must be carried out to improve and optimize biological control of pests in greenhouses.
We emphasize that, although our multistage dynamic model is fitted only to the data of S. exigua and Ch. oculator, the model can be easily adapted to other pairs of interacting species involved in biological or integrated pest control with either parasitoid or predator agents. Indeed, the possibility of such extensions of our model rests on the following pillars necessary for the adequate description of stage-specific interactions between the two insect populations: (a) It accounts for the specific developmental temperature threshold. (b) The population densities are structured by developmental stages and by cohorts within each stage. (c) Transition rates depend on biological time according to the Weibull cumulative distribution function generally accepted in modeling of insect development (Wagner et al. 1984). (d) The stage-dependent interspecific interaction is described by a functional response fitted to the data on the concrete host–parasitoid or prey–predator interaction. Of course, for the model fitting (estimation of the model parameters), trials analogous to ours should be carried out, combined with data available in the literature. Thus, an immediate adaptation of our model to similar important koinobiont parasitoids–host pairs can be obtained, such as Aphidius spp.-aphids, Dacnusa sibirica Telenga-leaf miners, Eretmocerus mundus Mercet-withefly, and Encarsia formosa Gahan-withefly; and to idiobiont parasitoids such as: Diglyphus isaea (Walker)-leaf miners and Trichogramma achaeae Nagaraja & Nagarkatti-Tuta absoluta (Meyrick), see Vila and Cabello (2014). Analogously to our model, corresponding multistage dynamic models can be built for predator–prey interactions, as well.
Author contributions
ZV, JG and TC conceived and designed research, AT and JEB conducted the experiments under the direction of TC; ZS programmed the model and made simulation runs, MG was in charge of data analysis, model fitting and validation. ZV and TC wrote the manuscript. All authors read and approved the manuscript.
References
Aarvik L (1981) The migrant moth Spodoptera exigua (Hübner) (Lepidoptera, Noctuidae) recorded in Norway. Fauna Nor D 28:90–92
Ali A, Gaylor MJ (1992) Effects of temperature and larval diet on development of the beet armyworm (Lep.: Noctuidae). Environ Entomol 21:780–786
Alto BW, Kesavaraju B, Juliano SA, Lounibos LP (2009) Stage-dependent predation on competitors: consequences for the outcome of a mosquito invasion. J Anim Ecol 78:928–936
Askew RR, Shaw MR (1986) Parasitoid communities: their size, structure, and development. In: Waage J, Greathead D (eds) Insect parasitoids. Academic Press, London, pp 225–264
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Azidah AA, Sofian-Azirun M (2006) Life history of Spodoptera exigua (Lep.: Noctuidae) on various host plants. Bull Entomol Res 96:613–618
Barbeau MA, Caswell H (1999) A matrix model for short-term dynamics of seeded populations of sea scallops. Ecol Appl 9:266–287
Barlow ND (2004) Models in biological control: a field guide. In: Hawkins BA, Cornell HV (eds) Theoretical approaches to biological control. Cambridge University Press, Cambridge, pp 43–68
Belda J (1994) Biología, ecología y control de Spodoptera exigua (Lep.: Noctuidae) en cultivo de pimiento en invernadero. Dissertation, University of Granada, Spain
Birt A, Feldman RM, Cairns DM, Coulson RN, Tchakerian M, Xi W, Guldin JM (2009) Stage-structured matrix models for organisms with non-geometric development times. Ecology 90:57–68
Bommarco R (2001) Using matrix models to explore the influence of temperature on population growth of arthropod pests. Agric For Entomol 3:275–283
Butler GD (1966) Development of beet armyworm and its parasite Chelonus texanus in relation to temperature. J Econ Entomol 59:1324–1327
Cabello T (1988) Natural enemies of noctuid pests in alfalfa, corn, cotton and soybean crops in Southern Spain. J Appl Entomol 108:80–88
Cabello T (2009) Control biológico de Noctuidos y otros Lepidópteros. In: Urbaneja A, Jacas J (eds) Control biológico de Plagas. Phytoma, Valencia, pp 279–306
Cabello T, Cañero R (1994) Technical efficiency of plant-protection in Spanish greenhouses. Crop Prot 13:153–159
Cabello T, Rodriguez H, Vargas P (1984a) Development, longevity and fecundity of Sopodoptera littoralis (Boisd.) (Lep.: Noctuidae) reared on eight artificial diets. J Appl Entomol 97:494–499
Cabello T, Rodriguez H, Vargas P (1984b) Utilización de una dieta artificial simple en la cría de Heliothis armigera Hb., Spodoptera littoralis Boisd. y Trigonophora meticulosa (Lep.: Noctuidae). Anales del Instituto Nacional de Investigaciones Agrarias. Serie Agricola 27:101–107
Cabello T, Gámez M, Varga Z (2007) An improvement of the Holling type III functional response in entomophagous species model. J Biol Syst 15:515–524
Cabello T, Gámez M, Torres T, Garay J (2011a) Possible effects of inter-specific competition on the coexistence of two parasitoid species: Trichogramma brassicae Bezdenko and Chelonus oculator (F.) (Hym.: Trichogrammatidae, Braconidae). Community Ecol 12:78–88
Cabello T, Gámez M, Garay J, Varga Z (2011b) Lucha biológica y modelos matemáticos cuándo y cómo hacer las sueltas de enemigos naturales. Vida Rural 336:24–29
Castañera MB, Aparicio JP, Gurtler RE (2003) A stage-structured stochastic model of the population dynamics of Triatoma infestans, the main vector of Chagas disease. Ecol Model 162:33–53
Cayrol RA (1972) Famille des Noctuidae. In: Balachowsky AS (ed) Entomologie Appliquée a L’Agriculture. Lépidoptères. II (2), Masson et Cie., Paris, pp 1255–1520
Choi WI, Ryoo MI (2003) A matrix model for predicting seasonal fluctuations in field populations of Paronychiurus kimi (Collembola: Onychiruidae). Ecol Model 162:259–265
Chown SL, Nicolson SW (2004) Insect physiological ecology: mechanisms and patterns. Oxford Univ Press, New York
Clausen CP (ed) (1978) Introduced parasites and predators of arthropod pests and weeds: a world review. USDA, Washington
Conway GR, Glass NR, Wilcox J (1970) Fitting nonlinear models to biological data by Marquardt’s algorithm. Ecology 51:503–507
Curry GL, Feldman RM, Smith KC (1978) A stochastic model of a temperature-dependent population. Theor Popul Biol 13:197–213
Daumal J, Voegele J, Brun P (1975) Les Trichogrammes. Unite de production massive et quotidienne d’un hóte de substitution E. kuehniella Zell. (Lep.: Pyralidae). Ann Zool Ecol Anim 7:45–59
El-Refai SA, Degheele D (1988) Development time of the beet armyworm, Spodoptera exigua (Hübner) and the tobaceo budworm, Heliothis virescens (F.) (Lep.: Noctuidae) in function of temperature. Meded Fac Landbouww Rijksuniv 53
Elvira S, Gorria N, Muñoz D, Trevor W, Caballero P (2010) A simplified low-cost diet for rearing Spodoptera exigua (Lep.: Noctuidae) and its effect on S. exigua Nucleopolyhedrovirus production. J Econ Entomol 103:17–24
Elzen GW, King EG (1999) Chapter 11: periodic release and manipulation of natural enemies. In: Bellows TS, Fisher TW, Caltagirone LE, Dahlsten DL, Gordh G, Huffaker CB (eds) Handbook of biological control: principles and applications of biological control. Academy Press, San Diego, pp 253–270
Etzel LK, Legner EF (1999) Chapter 7: culture and colonization. In: Bellows TS, Fisher TW, Caltagirone LE, Dahlsten DL, Gordh G, Huffaker CB (eds) Handbook of biological control: principles and applications of biological control. Academy Press, San Diego, pp 125–197
Fye RE, McAda WC (1972) Laboratory studies on the development, longevity and fecundity of six lepidopterous pests of cotton in Arizona. USDA Tech Bull 1454
Garcia-Martin M, Gámez M, Cabello T (2005) Estudio de respuesta funcional en sistemas parasitoide-huésped: aplicación en lucha biológica contra plagas. University of Almeria, Spain
Garcia-Martin M, Gámez M, Cabello T (2008) Functional response of Chelonus oculator according to temperature. Community Ecol 9:45–51
Gauld I, Bolton B (1996) The hymenoptera. Oxford University Press, Oxford
Godfray HCJ, Hassell MP, Holt RD (1994) The population dynamic consequences of phenological asynchrony between parasitoids and their hosts. J Anim Ecol 63:1–10
Greathead DJ (ed) (1976) A review of biological control in western and southern Europe. CAB, Farnham
Hairston NG (1985) Obtaining life table data from cohort analyses: a critique of current methods. Limnol Oceanogr 30:886–893
Harvey JA (2005) Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomol Exp Appl 117:1–13
Harvey JA, Thompson DJ (1995) Developmental interactions between the solitary endoparasitoid Venturia canescens (Hym.: Ichneumonidae), and two of its hosts, Plodia interpunctella and Corcyra cephalonica (Lep.: Pyralidae). Eur J Entomol 92:427–435
Harvey JA, Harvey IF, Thompson DJ (1995) The effect of host nutrition on growth and development of the parasitoid wasp Venturia canescens. Entomol Exp Appl 75:213–220
Hassell M (1978) The dynamics of arthropod predator-prey systems. Princeton University Press, Princeton
Hogg DB, Gutierrez AP (1980) A model of the flight phenology of the beet armyworm (Lepidoptera: Noctuidae) in central California. Hilgardia 48:1–36
Honek A (1996) Geographical variation in thermal requirements for insect development. Eur J Entomol 96:303–312
Hudes ES, Shoemaker CA (1988) Inferential method for modeling insect phenology and its application to the spruce budworm (Lep.: Tortricidae). Environ Entomol 17:97–108
Ikemoto T, Takai K (2000) A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ Entomol 29:671–682
Ims RA, Andreassen HP (2005) Density-dependent dispersal and spatial population dynamics. Proc Biol Sci 272:913–918
Kallenborn HG, Mosbacher GC (1983) The ecdysteroid titres during the last-larval instar of Ephestia kuehniella Z. (Lep.: Pyralidae). J Insect Physiol 29:749–753
Legner EF, Medved RA (1979) Influence of parasitic hymenoptera on the regulation of pink bollworm Pectinophora gossypiella on cotton in the lower Colorado Desert. Environ Entomol 8:922–923
Legner EF, Thompson SN (1977) Effects of the parental host on host selection, reproductive potential, survival and fecundity of the egg-larval parasitoid Chelonus sp. near curvimaculatus, reared on Pectinophora gossypiella and Phthorimaea operculella. Entomophaga 22:75–84
Logan JD, Wolesensky W (2007) An index to measure the effects of temperature change on trophic interactions. J Theor Biol 246:366–376
Logan JD, Wolesensky W, Joern A (2006) Temperature-dependent phenology and predation in arthropod systems. Ecol Model 196:471–482
Manly BFJ (1990) Stage-structured populations: sampling, analysis and simulation. Chapman & Hall, London
McNally PS (1983) Monitoring beet armyworm: traps and heat units help second-guess this pest. Am Veg Grower March: 12
Mills N (1997) Techniques to evaluate the efficacy of natural enemies. In: Dent DR, Walton MP (eds) Methods in ecological and agricultural entomology. CAB Int, Wallingford, pp 271–291
Montgomery DC (2010) Design and analysis of experiments, minitab manual. Wiley, New York
Motulsky H, Christopoulos A (2003) Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. GraphPad Software Inc, San Diego
Munholland PL, Dennis B (1992) Biological aspects of a stochastic model for insect life history data. Environ Entomol 21:1229–1238
Murdoch WW, Briggs CJ, Nisbet RM (1997) Dynamical effects of size- and state-dependent selection by insect parasitoids. J Anim Ecol 66:542–555
Nechols JR, Andres LA, Beardsley JW, Goeden RD, Jackson CG (eds) (1995) Biological control in the western United States. University of California, Oakland
Neuenschwander P, Borgemeister C, Langewald J (eds) (2003) Biological control in IPM systems in Africa. CABI Publishing, Wallingford
Nietschke BS, Magarey RD, Borchert DM, Calvin DD, Jones E (2007) A developmental database to support insect phenology models. Crop Prot 26:1444–1448
Osawa K, Shoemaker CA, Stedinger JR (1983) A stochastic-model of balsam fir bud phenology utilizing maximum likelihood parameter estimation. For Sci 29:478–490
Ozkan C (2006) Laboratory rearing of the solitary egg-larval parasitoid, Chelonus oculator Panzer (Hym.: Braconidae) on a newly recorded factitious host Plodia interpunctella (Hübner) (Lep.: Pyralidae). J Pest Sci 79:27–28
Ozkan C, Tunca H (2005) A new laboratory host record, Ephestia cautella Walker (Lep.: Pyralidae) for an egg-larval Parasitoid, Chelonus oculator Panzer (Hym.: Braconidae) and a possible rearing method of the parasitoid on the new host. Int J Zool Res 1:70–73
Pashley DP (1986) Host-associated genetic differentiation in fall armyworm (Lep.: Noctuidae): a sibling species complex. Ann Entomol Soc Am 79:898–904
Patel RC, Yadav DN, Saramma PU (1979) Impact of mass releases of Chelonus heliopae and Telenomus remus against Spodoptera litura. J Entomol Res 3:53–56
Prasad RP, Roitberg BD, Henderson DE (2002) The effect of rearing temperature on parasitism by Trichogramma sibericum Sorkina at ambient temperatures. Biol Control 25:110–115
Rechav Y (1976) Biological and ecological studies of the parasitoid Chelonus inanitus (L.) (Hym.: Braconidae) in Israel. II. Releases of adults in a cotton field. J Entomol Soc S Afr 39:83–85
Rigler FH, Cooley JM (1974) The use of field data to derive population statistics of multivoltine copepods. Limnol Oceanogr 19:636–655
Sannino L, Balbiani A, Aviguano M (1986) Spodoptera littoralis (Boisduval) and Spodoptera exigua (Hub.) (Lep.: Noctuidae) on tobacco in Italy. Ann Ist Sper Tab 12:65–68
Sannino L, Balbiani A, Espinosa B (1987) Osservazioni morfo-biologiche su alcune specie del genera Spodoptera (Lep.: Noctuidae) e rapporti di parassitismo con la coltura del tabacco in Italia. Informatore Fitopatol 11/87:29–40
Schmidt K, Hoppmann D, Holst H, Berkelmann-Löhnertz B (2003) Identifying weather-related covariates controlling grape berry moth dynamics. EPPO Bull 33:517–524
Silhacek DL, Miller GL (1972) Growth and development of the Indian meal moth, Plodia interpunctella (Lep.: Phycitidae), under laboratory mass-rearing conditions. Ann Entomol Soc Am 65:1084–1087
Son Y, Lewis EE (2005) Modelling temperature-dependent development and survival of Otiorhynchus sulcatus (Col.: Curculionidae). Agric For Entomol 7:201–209
Söndgerath D, Müller-Pietralla W (1996) A model for the development of the cabbage root fly (Delia radicum L.) based on the extended Leslie model. Ecol Model 91:67–76
Southwood R, Henderson PA (2000) Ecological methods. Blackwell Publishing Ltd, Oxford
Stavraki HG (1970) Données préliminaires sur les lâchers de Chelonus eleaphilus (Hym. Braconidae) contre Prays oleae (Lep. Hyponomeutidae) dans les oliveraies de Kessariani et Thebes en 1968. Ann Institut Phytopathol Benaki 9:281–287
Stinner RE (1977) Efficacy of inundative releases. Annu Rev Entomol 22:515–531
Stoepler TM, Lill JT, Murphy SM (2011) Cascading effects of host size and host plant species on parasitoid resource allocation. Ecol Entomol 36:724–735
Thompson SN, Hagen KS (1999) Nutrition of entomophagous insect and other arthropods. In: Bellows TS, Fisher TW, Caltagirone LE, Dahlsten DL, Gordh G, Huffaker CB (eds) The handbook of biological control. Academic Press, New York, pp 594–652
Tisdale RA, Sappington TW (2001) Realized and potential fecundity, egg fertility, and longevity of laboratory-reared female beet armyworm (Lep.: Noctuidae) under different adult diet regimes. Ann Entomol Soc Am 94:415–419
Tunca H, Ozkan C, Kilinçer N (2011) Temperature dependent development of the egg-larval parasitoid Chelonus oculator on the factitious host, Ephestia cautella. Turk J Agric For 34:421–428
van der Blom J (2010) Applied entomology in Spanish greenhouse horticulture. Proc Neth Entomol Soc Meet 21:9–17
van Lenteren JC (2007) Biological control for insect pests in greenhouses: an unexpected success. In: Vicent C, Goettel MS, Lazarovits G (eds) Biological control: a global perspective. CAB Int, Wallingford, pp 105–117
Vila E, Cabello T (2014) Biosystems engineering applied to greenhouse pest control, 99–128. In: Torres I, Guevara R (eds) Biosystems engineering: biofactories for food production in the XXI Century. Springer, Berlin, pp 99–128
Wagner TL, Wu HI, Sharpe PJH, Coulson RN (1984) Modeling distributions of insect development time: a literature review and application of the Weibull function. Ann Entomol Soc Am 77:475–487
Wagner TL, Wu HI, Feldman RM, Sharpe PJH, Coulson RN (1985) Multiple-cohort approach for simulating development of insect populations under variable temperatures. Ann Entomol Soc Am 78:691–704
Wakamura S (1990) Reproduction of the beet armyworm, Spodoptera exigua (Hübner) (Lep.: Noctuidae), and influence of delayed mating. Jpn J Appl Entomol Zool 34:43–48
Zalom FG, Goodell PB, Wilson LT, Barnett WW, Bentley WJ (1983) Degree-days: the calculation and use of heat units in pest management. University of California, Davis
Acknowledgments
The present research has been supported in part by the Hungarian Scientific Research Fund OTKA (K81279) and by the Excellence Project Programme of the Ministry of Economy, Innovation and Science of the Andalusian Regional Government, supported by FEDER Funds (P09-AGR-5000). We are also grateful to the anonymous referees and editor for their very helpful suggestions and careful reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Lavandero.
Rights and permissions
About this article
Cite this article
Garay, J., Sebestyén, Z., Varga, Z. et al. A new multistage dynamic model for biological control exemplified by the host–parasitoid system Spodoptera exigua–Chelonus oculator . J Pest Sci 88, 343–358 (2015). https://doi.org/10.1007/s10340-014-0609-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0609-z