Abstract
Wood-boring insect pests, such as the invasive Asian longhorned beetle (ALB, Anoplophora glabripennis), are difficult to detect because larvae mine inside deciduous trees, logs or wood packing material. Currently, only visual survey methods are used, which are mostly unable to detect the presence of wood-boring insects. Bioacoustic detection, however, exploits sounds and vibrations produced by larvae during feeding and other movements inside the wood. Bioacoustic detection methods require mounting of the sensors, which can be complicated, time consuming and may even damage the surface of the tested material. Laser vibrometry avoids all these problems as vibrations produced by the larvae are detected via the laser beam. We used a portable digital laser vibrometer to detect the activity of mining ALB larvae within poplar logs. Three types of pulses were recorded: the broadband pulses lasting 1–2 ms were the most frequent, with frequency maxima between 8 and 13 kHz. Less frequent were the low and the high frequency pulses, covering frequency bands between 4 and 7, and 9 and 20 kHz, respectively. The signal-to-noise ratio across the whole frequency range (0–22 kHz) of the laser vibrometer was around 35 dB. We show that laser vibrometry can be successfully employed as a non-destructive diagnostic tool for detecting infestations by the wood-boring beetles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hidden insect infestations such as those of wood-boring beetle larvae inside trees, logs and wood packing material have always presented a challenge for pest managers, regulators and researchers. Substantial progress has been made over the last few decades towards developing new and improved methods for detecting and monitoring such pests in order to prevent their spreading outside their native regions and to help eradicate them or at least contain outbreaks (Walker 1996; Smith et al. 2009; Mankin et al. 2011).
One of the most destructive wood-borers to invade North America and Europe in recent decades is the Asian longhorned beetle (ALB), Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae) (Smith and Wu 2009). Following outbreaks in its native China in the 1980s, the first populations were established in N America and Europe in 1996 in New York, USA (Haack et al. 1997) and in 2001 in Braunau, Austria (Tomiczek et al. 2002), respectively. The hosts of ALB are healthy deciduous trees, most commonly poplars, Populus spp., maples, Acer spp., willows, Salix spp. and elms, Ulmus sp. (Hu et al. 2009; Haack et al. 2010). The adult beetles feed on leaves, twigs or tender bark; the females lay eggs into the bark, and the larval stages feed inside the trunk and branches, creating tunnels and galleries that weaken structural timber. The species has spread internationally, mainly inside wood packing material manufactured from infested trees (Haack et al. 2010).
Surveys to detect and delimit ALB infestations are currently carried out by visual inspection from the ground, using bucket trucks and tree climbers to look for oviposition sites and emergence holes, as well as sap and frass, since presently these are the only proven methods of ALB detection (Smith et al. 2009; Haack et al. 2010). Since no long-range pheromones have been reported for ALB, studies are now focused on establishing the role of plant volatiles (such as the volatile compounds released by the drought-stressed Acer negundo) and their use as potential lures for trapping programmes (Hu et al. 2009; Haack et al. 2010; Augustin et al. 2012). Another method of detection recently developed in Austria involves training sniffer dogs to find infested plants (Hoyer-Tomiczek and Sauseng 2013).
Since ALB is introduced into new localities mainly via wood packing material (Haack et al. 2010), there is a strong demand for a method that could enable quick and reliable detection of the larvae inside shipment crates, pallets, wooden reels, etc., in ports and airports as the main points of entry. The visual surveys of wooden cargo now used detect only emergence holes and adult beetles, and not the presence or absence of larvae hidden inside the wood. The aim is to avoid the very costly and time-consuming eradication programmes for infested areas, which are not always successful (Hu et al. 2009).
Over the last two decades, research has been turned to the use of bioacoustic methods, which exploit communication as well as incidental sounds and vibrations generated by larvae during their feeding and other movements inside wood (Mankin et al. 2011). The bioacoustic methods that were previously tested on several wood-boring and stored grain pests include various types of condenser microphones, bimorphs, accelerometers and a number of piezoelectric transducers (Chesmore 2008; Mankin et al. 2011). However, the sensitivity and measuring accuracy of all these devices are strongly influenced by the nature of the sensor-substrate interface, since each sensor has to be mounted on or attached to the substrate surface. Mounting the sensor can influence measurements and, in some cases, damage the object under investigation. Ideally, a non-contact detection method, with zero loading of the test surface, would be desirable. Airborne-sound detecting sensors, such as microphones, raise additional issues. Substantial energy losses occur at the solid/gas interface, during the crossing of vibratory waves from the solid (wood) to the gaseous (air) media, before finally reaching the sensor. As a result, amplification is necessary, which results in background environmental noises being amplified, with the possibility of the insect sounds being masked (or, at least very least, the signal-to-noise ratio being decreased).
A laser Doppler vibrometer measures vibrations without loading the test surface, so that no interference with the substrate is involved before conducting the measurement. Because recording is carried out directly from the vibrating surface via the laser beam, there are no energy losses at the solid/gas interface.
We have therefore evaluated the portable digital laser vibrometer PDV-100 as a novel tool for detecting wood-boring beetle larvae inside logs and compared the results to those obtained with previously used and tested bioacoustic methods. Our data also represents a contribution to previously recorded ALB larval sounds (Mankin et al. 2008; Schofield 2011) for future reference.
Materials and methods
Wood samples
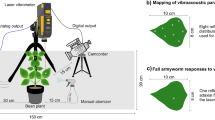
Samples of wood infested with larvae of A. glabripennis were obtained in October 2010 from an urban area in the town of Maser in Northern Italy. Here, large numbers of various deciduous trees with signs of infestation (oviposition pits and/or emergence holes) had been felled as part of an ongoing eradication programme in the Veneto region. The logs were transported to the Federal Forest Office, BFW in Vienna, Austria and kept in metal cages in the quarantine room at 21–24 °C and 40–55 % humidity. All cut surfaces were treated with Lac balsam wound sealant. Acoustic data were collected from four Populus sp. logs 45–90 cm long and 11–16 cm in diameter.
Recording procedure
Recordings were carried out in May 2011 in the quarantine room at the Federal Forest Office, BFW. Four to eight recording points were chosen on each log, and 4 mm2 rectangular pieces of reflective tape were attached to the bark at these points for optimum reflection of the laser beam. For recording, individual logs were taken out of the metal cage and placed on top of a table inside the quarantine room. The table had no special isolation from vibration. One to five 5-min recordings were made from each of the recording points using a digital portable laser vibrometer PDV-100 (Polytec GmbH, Waldbronn, Germany). The PDV-100 measures frequencies in the range from 0 to 22 kHz. The laser beam was pointed perpendicularly to the recording point from a distance of ca. 20 cm. Signals were digitized with a sample rate of 44.1 kHz and 16-bit depth and stored for further analysis in the form of WAV files directly onto the hard drive of a standard PC using Sound Blaster Extigy sound card (Creative Labs Inc., Milpitas, CA, USA) and Cool Edit Pro 2.0 software (Syntrillium, Phoenix, AZ, USA).
Signal processing and data analysis
Temporal and spectral characteristics of signals were measured using Raven 1.4 (Cornell Lab of Ornithology, Ithaca, NY, USA; Charif et al. 2010) (Fast Fourier Transform size 32.768, overlap 75 %, Blackman-Harris smoothing window) and Sound Forge software (Sonic Foundry Inc., Madison, WI, USA). The level of background noise was determined during periods in the recordings when no pulses were present. For spectrogram preparation, the recordings were band-pass filtered between 0.8 and 20 kHz.
Terminology
To avoid confusion, the terms sound and vibration—which have in the past taken on multiple meanings in the bioacoustic literature—are used in this paper as follows: sound is used in its broader sense for mechanical pressure waves propagating through the medium (gas, liquid or solid); and vibration is reserved for mechanical waves that are transmitted through solids.
Results
Results presented in this paper were obtained by analysing the recordings from four logs of Populus sp., infested by A. glabripennis. The total duration of recordings was 3 h and 30 min (log 1: 70 min, log 2: 40 min, log 3: 50 min and log 4: 50 min). All recordings were made using a portable digital laser vibrometer.
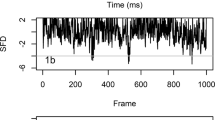
Three types of vibratory pulses, differing in their frequency spectra, were recorded in each of the four logs: low frequency pulses (LF), high frequency pulses (HF) and broadband pulses (Fig. 1). Of these, the broadband pulses are the most frequently occurring type in all of the recordings. The frequency spectra of the LF pulses were between 4 and 7 Hz, while those of the HF pulses were between 9 and 20 kHz (with peak intensities at 12.5, 15 and 17 kHz). The frequency spectra of the broadband pulses were between 3 and 20 kHz, with the energy maximum between 8 and 13 kHz. The duration of these pulses was around 1.5 ms, those of LF pulses and HF pulses 1.9 ± 0.7 ms (n = 8) and 1.1 ± 0.4 ms (n = 8), and of the broadband pulses 1.6 ± 0.7 ms (n = 50).
The summed frequency spectrum of 150 broadband pulses reveals frequency peaks at 3, 5.3, 8.6 and 10.6 kHz (Fig. 2, grey). The spectrum exhibits a high signal-to-noise ratio across the whole measuring frequency range of the laser vibrometer (35 dB at 3 kHz and 25 dB at 10.6 kHz). The noise level is more or less uniform except for a high peak in the low frequency region (below 500 Hz) (Fig. 2, black shading).
The vibratory pulses occasionally merged into short trains lasting up to 10 ms, but more often they occurred in bursts of 10–20, 9.4 ± 7.2 ms (n = 40) apart. The average duration of such bursts was 177 ± 77 ms. Pulses assigned to larval activity displayed no fixed temporal patterns.
The velocity of broadband vibrations ranged from 0.01 to 0.96 mm/s (Fig. 3). The average values ranged from 0.04 ± 0.01 mm/s (n = 7), as measured on log 3/point 4, to 0.28 ± 24 mm/s (n = 30) on log 1/point 1.
Discussion
Classes of different sized pulses were observed throughout the recordings (Fig. 4), which points to the presence of several different larvae inside the log. The larvae may either differ in size or be positioned at different locations relative to the recording point. It was confirmed at a later date that the adult A. glabripennis emerged from these and other logs gathered at the same time at the same location, but the logs were not infested artificially and the exact number of the larvae inside the logs is therefore unknown.
Sounds showing no fixed temporal pattern were attributed to side effects of eating/locomotion. Such vibrations have been proposed to serve for securing resources for larvae when the food is scarce and for avoiding competition by signalling their presence to each other so that they each stay in their part of the tree (Kočárek 2009).
Based on the properties of the broadband pulse—short duration and impulsive nature—and previous results on insect bioacoustics (Chesmore 2008), we speculate that, as the most frequently recorded sound, the broadband pulse is caused by larvae biting the wood fibres. The low frequency (LF) and high frequency (HF) pulses, similar in duration, may be also caused by biting or by some movements unrelated to feeding.
Although relative values of vibration intensity (in dB) are adequate when discussing the results of a single study, it is important to provide absolute values, either as velocity, acceleration or displacement, in order to make possible comparisons between different methods and different studies. The amplitude of recorded pulses was between 0.01 and 0.96 mm/s, which, for example, is in the range of substrate-borne vibratory communication signals of stink bugs and leafhoppers recorded from green plants (Čokl et al. 2007; Eriksson et al. 2011).
Accelerometers, piezoelectric sensors and other acoustic devices that have been tested for detecting wood-boring beetle larvae are usually mounted by inserting a nail or some other metal rod into the substrate as a waveguide. This inevitably damages the object under investigation, which is especially undesirable in the case of high-value bonsai trees, which are often attacked by wood-boring beetles. It also means that the measuring device necessarily has to be in close contact with the object under investigation. In contrast, the laser vibrometer, in addition to being a non-destructive method, offers the possibility of operating at up to 30-m distance (PDV-100 Portable Digital Vibrometer Data sheet 2012). Ultrasound imaging is another non-destructive and non-contact acoustic method that has been tested for detecting larvae of wood-boring beetles. However, it proved not to be feasible with the current technology (Fleming et al. 2005).
Since the present study was done on infested logs in the laboratory, additional studies in the field are needed to assess the impact of environmental background noise (wind, traffic) on detection of the beetle larvae. The energy peaks of wind induced vibrations in broadleaf trees, however, are well outside the frequency range of larval feeding noises, mostly below 10 Hz (de Langre 2008; Rodriguez et al. 2009; Schönborn et al. 2009). Most frequency spectra of traffic noise display a prominent peak in the range of 700–1,300 Hz (Sandberg 2003), which also falls outside the spectra of beetle larval sounds. Although the amplitude of noises in urban environment may be relatively high (Mankin et al. 2008), their frequency spectra indicate that they should not interfere with detection of the beetle larvae.
Vibrations emitted by the ALB larvae have been recorded using a piezoelectric sensor mounted on a steel waveguide inserted into the wood (Mankin et al. 2008; Schofield 2011). Most of the recorded vibrations exhibited energies between 1.5 and 4 kHz, much lower than that in the present study. Since the spectral profiles were shown not to depend significantly on either the tree species, tissue type (cambium, heartwood or sapwood), temperature, moisture level or instar stage (Mankin et al. 2008), the considerable differences in spectra can be attributed to the different properties of the measuring devices. Due to the outstanding linearity and accuracy of measurement, recording larval sounds using the digital laser vibrometer should improve discrimination of larval sounds from the background noise and from non-insect sounds caused by various processes inside the wood. A larger number of data points for the signal processing algorithms should also improve their detectability.
Laser vibrometry has here been employed successfully as a novel non-contact and non-destructive diagnostic tool for detecting wood-boring beetle larvae; the technique proved to be highly sensitive over a broad frequency range, with a high signal-to-noise ratio.
References
Augustin S, Boonham N, De Kogel WJ, Donner P, Faccoli M, Lees DC, Marini L, Mori N, Toffolo EP, Quilici S, Roques A, Yart A, Battisti A (2012) A review of pest surveillance techniques for detecting quarantine pests in Europe. EPPO Bull 42(3):515–551
Chesmore D (2008) Automated bioacoustic identification of insects for phytosanitary and ecological applications. In: Frommolt K-H, Bardeli R, Clausen M (eds) Computational bioacoustics for assessing biodiversity, Proceedings of the international expert meeting on IT-based detection of bioacoustical patterns. Federal Agency for Nature Conservation, Bonn, pp 59–72
Čokl A, Zorović M, Millar JG (2007) Vibrational communication along plants by the stink bugs Nezara viridula and Murgantia histrionica. Behav Process 75:40–54
de Langre E (2008) Effects of wind on plants. Annu Rev Fluid Mech 40:141–168
Eriksson A, Anfora G, Lucchi A, Virant-Doberlet M, Mazzoni V (2011) Inter-plant vibrational communication in a leafhopper insect. PLoS One 6(5):e19692. doi:10.1371/journal.pone.0019692
Fleming MR, Agrawal DK, Bhardwaj MC, Bauer LS, Janowiak JJ, Miller DL, Shield JE, Hoover K, Rustum R (2005) Noncontact ultrasound detection of exotic insects in wood packing materials. For Prod J 55(6):33–37
Haack RA, Law KR, Mastro VC, Ossenbruggen HS, Raimo BJ (1997) New York’s battle with the Asian long-horned beetle. J For 95:1–15
Haack RA, Hérard F, Sun J, Turgeon JJ (2010) Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: a worldwide perspective. Annu Rev Entomol 55:521–546
Hoyer-Tomiczek U, Sauseng G (2013) Sniffer dogs to find Anoplophora spp. infested plants. J Ent Acarol Res 45(s1):10–12
Hu J, Angeli S, Schuetz S, Luo Y, Hajek AE (2009) Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agric For Entomol 11:359–375
Kočárek P (2009) Sound production and chorusing behaviour in larvae of Icosium tomentosum. Cent Eur J Biol 4(3):422–426
Mankin RW, Smith MT, Tropp JM, Atkinson EB, Jong DY (2008) Detection of Anoplophora glabripennis (Coleoptera: Cerambycidae) larvae in different host trees and tissues by automated analyses of sound-impulse frequency and temporal patterns. J Econ Entomol 101:838–849
Mankin RW, Hagstrum DW, Smith MT, Roda AL, Kairo MTK (2011) Perspective and promise: a century of insect acoustic detection and monitoring. Am Entomol 57(1):30–44
PDV-100 Portable Digital Vibrometer Datasheet: Polytec (2012) High Resolution Digital Velocity Measurement. http://www.polytec.com/us/products/vibration-sensors/single-point-vibrometers/complete-systems/pdv-100-portable-digital-vibrometer/. Accessed 17 Dec 2013
Rodriguez M, Moulia B, de Langre E (2009) Experimental investigations of a walnut tree multimodal dynamics. In: Mayer H, Schindler D (eds) Proceedings of the 2nd international conference wind effects on trees. Albert-Ludwigs-University of Freiburg, Germany, pp 95–100
Sandberg U (2003) The multi-coincidence peak around 1,000 Hz in tyre/road noise spectra. In: Proceedings of Euronoise 2003: 5th European Conference on Noise Control, Naples, Italy
Schofield J (2011) Real-time acoustic identification of invasive wood-boring beetles. PhD thesis, University of York
Schönborn J, Schindler D, Mayer H (2009) Measuring vibrations of a single, solitary broadleaf tree. In: Mayer H, Schindler D (eds) Proceedings of the 2nd international conference wind effects on trees. Albert-Ludwigs-University of Freiburg, Germany, pp 135–139
Smith MT, Wu J (2009) Asian longhorned beetle: renewed threat to north-eastern USA and implications worldwide. Int Pest Control 50(6):311–316
Smith MT, Turgeon JJ, Groot P, Gasman B (2009) Asian longhorned beetle Anoplophora glabripennis (Motschulsky): lessons learned and opportunities to improve the process of eradication and management. Am Entomol 55(1):21–25
Tomiczek C, Krehan H, Menschhorn P (2002) Dangerous Asiatic longicorn beetle found in Austria: new danger for our trees? Allg Forst Z Waldwirtsch Umweltvorsorge 57:52–54
Walker TJ (1996) Acoustic methods of monitoring and manipulating insect pests and their natural enemies. In: Rosen D, Bennett FD, Capinera JL (eds) Pest management in the subtropics: integrated pest management: a Florida perspective. Intercept, Andover, pp 245–257
Acknowledgments
The authors wish to thank Martin Brandstetter of the Austrian Federal Forest Office (BFW) for providing infested wood samples. This work was supported financially by the EU Seventh Research Framework Program (FP7) project Q-DETECT [Developing quarantine pest detection methods for use by national plant protection organizations (NPPO) and inspection services] (contract no. 245047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Stauffer.
Rights and permissions
About this article
Cite this article
Zorović, M., Čokl, A. Laser vibrometry as a diagnostic tool for detecting wood-boring beetle larvae. J Pest Sci 88, 107–112 (2015). https://doi.org/10.1007/s10340-014-0567-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0567-5