Abstract
Spinetoram and spinosad have been evaluated against certain stored-product insect pests with success but there are no data available on the comparison of the efficacy of these two novel compounds in stored grains. Thus, laboratory bioassays were conducted to compare spinetoram and spinosad as grain protectants against Prostephanus truncatus (Horn) (Coleoptera: Bostrychidae) adults, Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) adults, Sitophilus oryzae (L.) (Coleoptera: Curculionidae) adults, and Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) adults and larvae. Factors such as treatment (1 ppm spinetoram, 1 ppm spinosad, 0.1 ppm spinetoram + 0.9 ppm spinosad, 0.5 ppm spinetoram + 0.5 ppm spinosad, and 0.9 ppm spinetoram + 0.1 ppm spinosad), exposure interval (1, 2, 7, and 14 days), temperature (20, 25, and 30 °C), and commodity (barley, maize, rye, and wheat) were evaluated. Progeny production was assessed after 60 days of exposure. Concerning temperatures, for P. truncatus adults, after 14 days of exposure, all adults were dead in treatments except of the case of spinosad alone at 20 °C. Offspring emergence was completely suppressed in all treatments at 20 and 25 °C. For R. dominica adults, after 7 days of exposure, the overall mortality ranged from 92.8 to 100 %. After 14 days of exposure, all adults were dead in all treatments of the combined use of spinetoram and spinosad at 25 and 30 °C. Progeny production was completely suppressed in all treatments at 30 °C. For S. oryzae adults, after 7 days of exposure, all S. oryzae were died at 25 and 30 °C in all treatments except in the case of spinosad alone. Offspring emergence was very low in all treatments and temperatures except in the case of spinosad alone at 30 °C. For T. confusum adults, after 1, 2, and 7 days of exposure, the overall mortality was low in all treatments and temperatures. Concerning commodities, for R. dominica adults, after 7 and 14 days of exposure, the overall mortality was >97 %. Offspring emergence was very low in all commodities. For S. oryzae adults, after 7 and 14 days of exposure, the overall mortality was increased exceeding 91 % except in the case of spinosad alone 7 days after exposure in barley. Progeny production was high in barley and rye in all treatments. For T. confusum adults, after 7 and 14 days, the overall mortality was low in barley, rye, and wheat. No offspring emergence was recorded in all treatments and commodities. For T. confusum larvae, after 14 days of exposure mortality was further increased, but did not reach 100 % for any of the combinations tested. The results of the present study suggest that the simultaneous application of spinetoram and spinosad was generally equally effective with the use of either spinosad or spinetoram alone. Furthermore, the increase of dose of either compound resulted in the same mortality levels. Thus, no benefits were achieved when spinetoram and spinosad were used simultaneously on grains, regardless of the proportion of each ingredient. These issues should be seriously considered when control measures against stored-product insects are designed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 20 years ago, several grain protectants, particularly organophosphorus (OP), and also carbamate and pyrethroid insecticides, were registered for application in raw grain commodities, such as wheat, rice, barley, and maize (Arthur 1996). Relatively recently, novel compounds with different modes of action than the OPs have been registered for direct use on grains, such as insect growth regulators (IGRs), which are not neurotoxic (Wijayaratne et al. 2012) and diatomaceous earths (DEs), which act through insect desiccation (Subramanyam and Roesli 2000). One of the novel compounds that have been evaluated with success for direct application on the grains is spinosad, which is based on fermentation products of the actinomycete Saccharopolyspora spinosa Mertz and Yao (Actinomycetales: Pseudonocardiaceae) (Thompson et al. 1997). These fermentation products are bacterial metabolites, which belong to a group known as “spinosyns,” while spinosad is based on spinosyns A and D (Hertlein et al. 2011). Laboratory and field trials with spinosad in many parts of the world clearly suggested that spinosad is quite effective against several major insect species (Thompson et al. 1997; Fang et al. 2002a, b; Fang and Subramanyam 2003; Toews and Subramanyam 2003; Toews et al. 2003; Subramanyam et al. 2007, 2012; Athanassiou et al. 2008a, b; Chintzoglou et al. 2008b; Getchell and Subramanyam 2008; Kavallieratos et al. 2010; Pozidi Metaxa and Athanassiou 2013). After a thorough experimentation, in 2013, spinosad became commercially available in the USA as a grain protectant with the name Sensat™.
More recently, a new member of the spinosyn group, spinetoram, has been commercially introduced in various crops (Sparks et al. 2008, 2012; Jones et al. 2010). Spinetoram is based on two secondary metabolites, spinosyn J and L, and has been proved very effective against a wide range of pests, in several crops, often more effective than spinosad (Sparks et al. 2008; Jones et al. 2010; Dripps et al. 2011; Yee and Alston 2012). On the contrary, according to Besard et al. (2010) spinetoram exhibits lower toxicity than spinosad on Bombus terrestris (L.) (Hymenoptera: Apidae), whereas both spinetoram and spinosad have been considered potentially hazardous to other pollinators, i.e., Megachile rotundata (F.) (Hymenoptera: Megachilidae) (Gradish et al. 2012). Since now, only six insect species, corresponding to certain local populations, have shown resistance to spinosad or spinetoram in the field, i.e., Bactrocera oleae (Rossi) (Diptera: Tephritidae) (spinosad), Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) (spinosad), Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) (spinosad), Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) (spinosad), Plutella xylostella (L.) (Lepidoptera: Plutellidae) (spinosad and spinetoram), and Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) (spinosad), whereas there are no reports for stored-product insects (Sparks et al. 2012). Moreover, initial evaluation tests clearly indicated that spinetoram was indeed a promising grain protectant, despite the fact that there is no commercially available formulation for this purpose (Vassilakos and Athanassiou 2012a, b, 2013). Despite the fact that against other pests there are several studies that compare the two spinosyn-based insecticides, there are no data available on the comparison of spinosad with spinetoram in stored grains. The purpose of this work is to make a direct comparison of these two compounds as grain protectants. In this effort, several mixtures of spinosad and spinetoram were also tested, in order to indicate the potential benefits for using both in one single formulation.
Materials and methods
Commodities
The tested commodities were barley (var. Persephone), maize (variety Dias), rye (variety Danko), and hard wheat (variety Mexa), which are produced in Greece. All the tested commodities were free of infestation and pesticides. The moisture content of the tested commodities, as determined by a Dickey-John moisture meter (mini GAC plus, Dickey-John Europe S.A.S., Colombes, France), was 11.2, 11.4, 11.1, and 11.5 for barley, maize, rye, and wheat, respectively. Prior to treatment with insecticides, the tested commodities were dry sieved to remove impurities and dockage.
Insects
The insects used in the tests were reared at the Laboratory of Agricultural Entomology, Benaki Phytopathological Institute, Kifissia, Greece, at continuous darkness. The cultures, initially collected from Greek storage facilities, have been kept at Benaki Phytopathological Institute for more than 10 years. Prostephanus truncatus (Horn) (Coleoptera: Bostrychidae) was reared on whole maize at 25 °C and at 65 % relative humidity (RH). Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) and Sitophilus oryzae (L.) (Coleoptera: Curculionidae) were reared on whole wheat at 27 °C and at 65 % RH. Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) was reared on wheat flour including 5 % brewers’ yeast (w/w) at 27 °C and 60 % RH. In the experiments with T. confusum, both larvae and unsexed adults were used. In the experiments with P. truncatus, R. dominica, and S. oryzae, only unsexed adults were used. For T. confusum, 3rd–4th instar larvae were selected based on Park (1934), while all adults used were <2 weeks old.
Formulations
The insecticide formulations were spinetoram Delegate WG and spinosad Laser SC obtained from Dow AgroSciences (Indianapolis, IN, USA) and Dow Agrosciences Export S.A.S. (Lavrion, Attica, Greece), respectively. Delegate is a wettable granules formulation with 25 % spinetoram as active ingredient. Laser is a suspension concentrate formulation with 48 % spinosad as active ingredient.
Bioassays series 1
In this series of bioassays, spinetoram and spinosad were applied as solutions against P. truncatus adults in maize or against R. dominica, S. oryzae, and T. confusum adults in wheat by five insecticidal treatments: 1 ppm spinetoram, 1 ppm spinosad, 0.1 ppm spinetoram + 0.9 ppm spinosad, 0.5 ppm spinetoram + 0.5 ppm spinosad, and 0.9 ppm spinetoram + 0.1 ppm spinosad. Spraying was carried out on a tray, where 1 kg of maize or wheat was treated with 3 ml of an aqueous solution that contained the appropriate volume of Laser or Delegate corresponding to each insecticidal treatment. Spraying was carried out using an AG-4 airbrush (Mecafer S.A., Valence, France). Furthermore, additional 1 kg lots of maize or wheat, which served as controls, were sprayed with 3 ml of distilled water with an airbrush of the same type, which is kept for spraying of the controls only. After spraying with each insecticidal treatment, the airbrush was cleaned with acetone, and then the next insecticidal treatment was applied in the commodities. After spraying, all commodities with all treatments, the grain lots were placed into 5 l glass jars and were manually shaken for 10 min to achieve equal distribution of the insecticide in the entire grain mass. Different trays were used for each spraying. Three samples of 20 g were taken from each treated or untreated lot and put into small glass vials (7.5 cm in diameter, 12.5 in cm height) with a different scoop that was inside each jar. The quantity of 20 g was weighed with a Precisa XB3200D compact balance (Alpha Analytical Instruments, Gerakas, Greece) on a thin layer. New layer was used for each weighing. The closure of the vials had a 1.5 cm diameter hole in the middle, which was covered by gauze, to allow sufficient aeration inside the vial. Then, 20 adults of each species were separately placed inside each vial. The internal “neck” of the vials was covered by Fluon (Northern Products Inc., Woonsocket, RI, USA), to prevent insects from escaping. Following this, all vials were placed in incubators set at 25 °C. The tested levels of temperature were 20 (±0.1), 25 (±0.1), and 30 (±0.1) °C, while RH was maintained at 65 (±0.2) % during the entire experimental period of this bioassays series. Adult mortality was determined by prodding with a brush to detect movement under an Olympus stereomicroscope (SZX9, Bacacos S.A., Athens, Greece) after 1, 2, 7, and 14 days of exposure. The brush was carefully washed after the examination of each vial. Different brushes were used for each insecticidal treatment and untreated controls. It was also evaluated the progeny production of the tested adult species in the treated wheat or maize. Thus, after the 14 days mortality counts, all (alive or dead) treated parental individuals were discarded, as noted above, and the vials were again put inside the incubators for an additional period of 60 days. Afterward, the vials were opened again and the adult progeny was counted, following the procedure as noted above. In the cases of S. oryzae and R. dominica, all emerged offspring recorded were at the adult stage, given that the immature development of these species occurs in the internal part of the kernel. For T. confusum, adult and immature progeny were recorded and progeny production was expressed as number of individuals/vial. Adults, however, represented >88 % of the total number of progeny recorded. The entire procedure was repeated three times for each species by preparing new maize or wheat lots each time. Control mortality was very low (<2 %) and, therefore, no correction was considered necessary for the mortality data. Data were analyzed separately for each of the tested species according to the repeated measures analysis (Sall et al. 2001). The repeated factor was exposure interval, while mortality was the response variable. Temperature and insecticidal treatment were the main effects. The associated interaction of the main effects was incorporated in the analysis. Progeny production counts were subjected to two-way ANOVA, with temperature and insecticidal treatment as main effects. The associated interaction of the main effects was incorporated in the analysis. Progeny production in the untreated control vials was also included in the analysis. All analyses were conducted using the JMP 9 software (SAS Institute Inc. 2010). Means were separated by the Tukey–Kramer (HSD) test at 0.05 probability (Sokal and Rohlf 1995).
Bioassays series 2
In this series of bioassays, spinetoram and spinosad were applied as solutions against R. dominica adults, S. oryzae adults, and T. confusum adults and larvae in different grain commodities. Thus, lots of barley, maize, rye, and wheat were treated as described in bioassays series 1. The entire series of experiments was carried out at 25 (±0.1) °C and 65 (±0.2) % RH. Mortality counts were recorded as described above. Progeny production counts in the cases of R. dominica, S. oryzae, and T. confusum adults were recorded as described above. As previously mentioned, for S. oryzae and R. dominica, only adult progeny was found. For T. confusum, >83 % of the progeny found were at the adult stage. The entire procedure was repeated three times for each species (adults or larvae) by preparing new barley, maize, rye, or wheat lots each time. Control mortality was very low (<2 %) and, therefore, no correction was considered necessary for the mortality data. Data were analyzed separately for each of the tested species (adults or larvae) according to the repeated measures analysis (Sall et al. 2001). The repeated factor was exposure interval, while mortality was the response variable. Commodity and insecticidal treatment were the main effects. The associated interaction of the main effects was incorporated in the analysis. Progeny production counts were subjected to two-way ANOVA, with commodity and insecticidal treatment as main effects. The associated interaction of the main effects was incorporated in the analysis. Progeny production in the untreated control vials was also included in the analysis. All analyses were conducted using the JMP 9 software (SAS Institute Inc. 2010). Means were separated by the Tukey–Kramer (HSD) test at 0.05 probability (Sokal and Rohlf 1995).
Results
Bioassays series 1
Mortality and progeny of P. truncatus adults
Mortality of P. truncatus adults was significantly affected by the exposure interval (F 3,118 = 15,413.2; P < 0.01). Between exposure intervals, all main effects and associated interaction were significant (temperature: F 2,120 = 118.4; P < 0.01; treatment: F 4,120 = 14.0; P < 0.01; temperature × treatment: F 8,120 = 2.7; P < 0.01). Within exposure intervals, all main effects were significant (exposure × temperature: F 6,120 = 32.2; P < 0.01; exposure × treatment: F 12,120 = 4.7; P < 0.01). The associated interaction exposure × temperature × treatment was not significant (F 24,120 = 1.3; P = 0.13). After 1 day of exposure, mortality was low and it did not exceed 14.4 % at 30 °C when spinetoram was applied alone in wheat (Table 1). Generally, mortality increased with the increase of temperature. No significant differences were recorded among treatments in all tested temperatures. After 2 days of exposure, mortality was increased in all treatments and temperatures. Still, the mortality did not exceed 65.6 % at 25 °C when spinetoram and spinosad were applied at 0.5 ppm each in wheat. Significantly, more P. truncatus adults died at 30 °C in wheat treated with spinetoram alone in comparison to the other treatments. Seven days later, the overall mortality was further increased and ranged from 98.9 to 100 %. No significant differences were recorded either among temperatures or among treatments. After 14 days of exposure, all adults were dead in treatments except of the case of spinosad alone at 20 °C. Regarding progeny production, the main effect treatment was significant (F 5,161 = 41.4; P < 0.01), whereas the main effect temperature was not significant (F 2,161 = 1.9; P = 0.16). The associated interaction temperature × treatment was not significant (F 10,161 = 1.8; P = 0.06). Offspring emergence was completely suppressed at 20 and 25 °C and at 30 °C in combination treatments containing less than 0.9 ppm spinosad, and 0.2–0.3 progeny were produced at 30 °C in treatments containing at least 0.9 ppm spinosad or 1 ppm spinetoram. Control mortality increased with temperature from 36.7 to 80.0 %.
Mortality and progeny of R. dominica adults
Mortality of R. dominica adults was significantly affected by the exposure interval (F 3,118 = 1,600.5; P < 0.01). Between exposure intervals, all main effects were significant (temperature: F 2,120 = 171.5; P < 0.01; treatment: F 4,120 = 3.5; P < 0.01). The associated interaction temperature × treatment was not significant (F 8,120 = 0.9; P = 0.49). Within exposure intervals, all main effects were significant (exposure × temperature: F 6,120 = 55.5; P < 0.01; exposure × treatment: F 12,120 = 2.0; P = 0.02), whereas the associated interaction exposure × temperature × treatment was not significant (F 24,120 = 1.4; P = 0.09). After 1 day of exposure, no adult mortality was recorded in all treatments at 20 °C (Table 2). The highest mortality was recorded at the case of 0.5 ppm spinetoram + 0.5 ppm spinosad at 30 °C. After 2 days of exposure, mortality was further increased. No significant differences were recorded among treatments in all tested temperatures. At 20 °C, mortality remained low and it did not exceed 40 % in wheat treated with spinetoram alone. In contrast, at 25 and 30 °C, the overall mortality ranged from 67.8 to 91.1 %. Seven days later, the overall mortality became high and ranged from 92.8 to 100 %. No significant differences were recorded either among temperatures or among treatments. After 14 days of exposure, all adults were dead in all treatments of the combined use of spinetoram and spinosad at 25 and 30 °C. Regarding progeny production, all main effects and associated interaction were significant (temperature: F 2,161 = 15.0; P < 0.01; treatment: F 5,161 = 27.3; P < 0.01; temperature × treatment: F 10,161 = 15.1; P < 0.01). Progeny production did not exceed 0.2 adults/vial at 20 and 25 °C, and it was completely suppressed at 30 °C. Control mortality increased from 12 adults/vial at the two lower temperatures to 115.6 at 30 °C adults/vial.
Mortality and progeny of S. oryzae adults
Mortality of S. oryzae adults was significantly affected by the exposure interval (F 3,118 = 20,655.9; P < 0.01). Between exposure intervals, the main effect temperature was significant (F 2,120 = 744.8; P < 0.01), whereas the main effect treatment was not significant (F 4,120 = 1.8; P = 0.13). The associated interaction temperature × treatment was significant (F 8,120 = 2.1; P = 0.04). Within exposure intervals, the main effect exposure × temperature was significant (F 6,120 = 285.5; P < 0.01), whereas the main effect exposure × treatment was not significant (F 12,120 = 0.6; P = 0.81). The associated interaction exposure × temperature × treatment was not significant (F 24,120 = 0.9; P = 0.58). After 1 day of exposure, no mortality was recorded at 20 and 25 °C in all treatments except in the case of spinosad alone where it was not exceeded 0.6 % (Table 3). At 30 °C, mortality remained low and it did not exceed 14.4 % in wheat treated with spinetoram alone. After 2 days of exposure, mortality remained very low at 20 °C, whereas it ranged from 50.6 to 76.7 % at 25 and 30 °C. Seven days later, mortality did not exceed 65 % at 20 °C when spinetoram and spinosad were applied at 0.5 ppm each in wheat. In contrast, all S. oryzae were died at 25 and 30 °C in all treatments except in the case of spinosad alone where mortality was 93.9 %. After 14 days of exposure, the overall mortality was high in all treatments and temperatures and ranged from 93.9 to 100 %. Regarding progeny production, all main effects and associated interaction were significant (temperature: F 2,161 = 16.8; P < 0.01; treatment: F 5,161 = 49.0; P < 0.01; temperature × treatment: F 10,161 = 16.0; P < 0.01). Offspring emergence was <1 adult/vial in all treatments except in the case of spinosad alone at 30 °C where it was 1.9 adults/vial. Control progeny production increased with temperature from 14.3 to 168.9 adults/vial.
Mortality and progeny of T. confusum adults
Mortality of T. confusum adults was significantly affected by the exposure interval (F 3,118 = 441.9; P < 0.01). Between exposure intervals, all main effects were significant (temperature: F 2,120 = 43.7; P < 0.01; treatment: F 4,120 = 8.9; P < 0.01), whereas the associated interaction temperature × treatment was not significant (F 8,120 = 1.1; P = 0.38). Within exposure intervals, all main effects were significant (exposure × temperature: F 6,120 = 21.0; P < 0.01; exposure × treatment: F 12,120 = 3.1; P < 0.01). The associated interaction exposure × temperature × treatment was not significant (F 24,120 = 0.7; P = 0.84). After 1, 2, and 7 days of exposure, the overall mortality was low in all treatments and temperatures and ranged from 0.0 to 20.0 % (Table 4). Fourteen days later, mortality was further increased but it remained < 59 %. Generally, mortality was increased with the increase of temperature. Regarding progeny production, all main effects and associated interaction were not significant (temperature: F 2,161 = 7.4; P = 0.17; treatment: F 5,161 = 0.9; P = 0.52; temperature × treatment: F 10,161 = 0.8; P = 0.61). No data for T. confusum progeny production are reported because an average of less than one progeny was produced in the controls.
Bioassays series 2
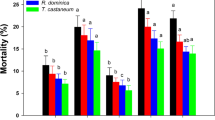
Mortality and progeny of R. dominica adults
Mortality of R. dominica adults was significantly affected by the exposure interval (F 3,158 = 742.3; P < 0.01). Between exposure intervals, the main effect commodity was significant (F 3,160 = 5.3; P < 0.01), whereas the main effect treatment was not significant (F 4,160 = 2.4; P = 0.06). The associated interaction commodity × treatment was not significant (F 12,160 = 0.6; P = 0.80). Within exposure intervals, the main effect exposure × commodity was significant (F 9,160 = 5.6; P < 0.01), whereas the main effect exposure × treatment was not significant (F 12,160 = 1.1; P = 0.40). The associated interaction exposure × commodity × treatment was not significant (F 36,160 = 0.9; P = 0.70). After 1 day of exposure, significant differences were noted among commodities, while the overall mortality ranged from 11.1 to 45.0 % (Table 5). No significant differences were noted among treatments in any of the tested commodities. After 2 days of exposures, the mortality was further increased in all commodities. No significant differences were recorded either among treatments or commodities. Seven and 14 days later, the overall mortality was >97 %, but complete mortality in all treatments was recorded only in wheat. Regarding progeny production, all main effects and associated interaction were significant (commodity: F 3,215 = 4.9; P < 0.01; treatment: F 5,215 = 24.5; P < 0.01; commodity × treatment: F 15,215 = 4.8; P < 0.01). Offspring emergence was ≤0.4 adults/vial in all commodities, but it was completely suppressed only in wheat in all treatments. Progeny production in controls was recorded as follows: 14.4 adults/vial in maize, 20.0 adults/vial in wheat, 67.8 adults/vial in rye, and 120.0 adults/vial in barley.
Mortality and progeny of S. oryzae adults
Mortality of S. oryzae adults was significantly affected by the exposure interval (F 3,158 = 1,200.1; P < 0.01). Between exposure intervals, all main effects were significant (commodity: F 3,160 = 26.6; P < 0.01; treatment: F 4,160 = 3.5; P < 0.01), whereas the associated interaction commodity × treatment was not significant (F 12,160 = 1.1; P = 0.41). Within exposure intervals, all main effects were significant (exposure × commodity: F 9,160 = 13.6; P < 0.01; exposure × treatment: F 12,160 = 2.2; P < 0.01). The associated interaction exposure × commodity × treatment was not significant (F 36,160 = 1.2; P = 0.26). After 1 day of exposure, no significant differences were noted among treatments, while the overall mortality did not exceed 37.2 % (Table 6). After 2 days of exposure, significant differences were recorded among treatments and commodities, but despite the mortality was increased in all commodities, it still remained < 78 %. Seven and 14 days later, the overall mortality was further increased exceeding 91 % except in the case of spinosad alone 7 days after exposure in barley. Regarding progeny production, all main effects and associated interaction were significant (commodity: F 3,215 = 4.2; P = 0.01; treatment: F 5,215 = 48.0; P < 0.01; commodity × treatment: F 15,215 = 1.8; P = 0.03). Progeny production was as high as 21.3 and 30.1 adults/vial in barley and rye, respectively, but never exceeded 0.3 or 2.4 adults/vial in wheat and maize, respectively (Table 7). In contrast, offspring emergence was very low in the case of wheat for all treatments.
Mortality and progeny of T. confusum adults
Mortality of T. confusum adults was significantly affected by the exposure interval (F 3,158 = 587.7; P < 0.01). Between exposure intervals, all main effects and associated interaction were significant (commodity: F 3,160 = 69.9; P < 0.01; treatment: F 4,160 = 10.8; P < 0.01; commodity × treatment: F 12,160 = 5.2; P < 0.01). Within exposure intervals, all main effects and associated interaction were significant (exposure × commodity: F 9,160 = 27.6; P < 0.01; exposure × treatment: F 12,160 = 12.6; P < 0.01; exposure × commodity × treatment: F 36,160 = 4.1; P < 0.01). After 1 day of exposure, no mortality was recorded in maize and rye in all tested treatments (Table 8). In barley and wheat, very low mortality was recorded only in the case of 0.5 ppm spinetoram + 0.9 spinosad and 0.5 ppm spinetoram + 0.5 spinosad, respectively. After 2 days of exposure, the overall mortality was slightly increased and it did not exceed 10 %. Seven and 14 days later, significant differences were recorded among treatments and commodities, but still the overall mortality was low in barley, rye, and wheat and it did not exceed 67.8 %. In maize, however, mortality reached 96.7 % when it was treated with 0.5 ppm spinetoram + 0.5 ppm spinosad. Regarding progeny production, the main effect treatment was significant (F 5,215 = 7.2; P < 0.01), whereas the main effect commodity was not significant (F 3,215 = 1.9; P = 0.12). The associated interaction commodity × treatment was significant (F 15,215 = 2.0; P = 0.02). No offspring emerged in any treatment. Control progeny production ranged from 0 individuals/vial in rye to 2.1 individuals/vial in maize.
Mortality of T. confusum larvae
Mortality of T. confusum larvae was significantly affected by the exposure interval (F 3,158 = 5,429.1; P < 0.01). Between exposure intervals, all main effects were significant (commodity: F 3,160 = 8.7; P < 0.01; treatment: F 4,160 = 2.5; P = 0.05), whereas the associated interaction commodity × treatment was not significant (F 12,160 = 1.3; P = 0.20). Within exposure intervals, all main effects and associated interaction were significant (exposure × commodity: F 9,160 = 7.4; P < 0.01; exposure × treatment: F 12,160 = 3.5; P < 0.01; exposure × commodity × treatment: F 36,160 = 3.3; P < 0.01). After 1 day of exposure, the overall mortality was low and it did not exceed 7.8 % (Table 9). After 2 days of exposure, the overall mortality was slightly increased and ranged from 7.2 to 17.2 %. Seven days later, mortality was increased in all commodities. Significant differences were noted among treatments in wheat. Still, the overall mortality did not exceed 84.4 %. After 14 days of exposure, mortality was further increased, but did not reach 100 % for any of the combinations tested.
Discussion
There are several mixtures of insecticides in the market, which are registered either as grain protectants or for treatment of surfaces in storage and processing facilities. However, the simultaneous use of two compounds instead of one, does not always provide better results, as compared to the use of one single active ingredient. For instance, in a recent study, Temprid®, which is based on beta-cyfluthrin and imidacloprid, was proved equally effective with Tempo®, which contains only beta-cyfluthrin, against adults of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) and Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) (Athanassiou et al. 2013). On the other hand, the commercial combination of chlorpyriphos-methyl with deltamethrin was very effective against major stored-product psocids, Lepinotus reticulatus Enderlein (Psocoptera: Trogiidae), Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae), and Liposcelis entomophila (Enderlein) (Psocoptera: Liposcelididae), in wheat, rice, and maize, respectively (Athanassiou et al. 2009). In the present work, we examined if the simultaneous use of four spinosyns, instead of two, would have some benefits in grain protection against major stored-product insect species. Our results clearly indicated that both spinosad and spinetoram have the same efficacy levels for the control of the species tested here. Nevertheless, there were several cases where spinetoram was more effective than spinosad, under short exposure intervals. For example, after 2 days of exposure, spinetoram was significantly more effective than spinosad in all temperatures tested against P. truncatus. Concerning R. dominica and T. confusum spinetoram performed better than spinosad but without significant differences, while it was more effective at 20 and 25 °C on S. oryzae without or with significant differences, respectively. Similarly, about T. confusum adults, spinetoram was significantly more effective in maize, after 7 days of exposure. Finally, regarding progeny production, S. oryzae emergence was higher in barley, maize, and rye treated with spinosad than with spinetoram but without significant differences. In a recent study, Vassilakos and Athanassiou (2012a, b) concluded that spinetoram was equally and in some cases more effective than spinosad against major stored-product beetle species. Our results stand in accordance with this observation. However, it is more representative to compare insecticides directly than comparing data that come from different studies in which each insecticide is tested individually.
One of the most important issues in the current study is testing the mixtures of both insecticides. In light of the present findings, despite the fact that in some cases, the use of both ingredients together performed better than spinosad or spinetoram alone, the simultaneous application of spinosad and spinetoram was generally equally effective with the use of either spinosad or spinetoram alone. In addition, in this combination, the increase of the dose of either compound resulted in the same mortality levels. Thus, nothing is gained by the simultaneous use of spinosad and spinetoram on grains, regardless of the proportion of each ingredient in this mixture. It is well established that both insecticides have the same mode of action targeting the nicotinic and gamma aminobutyric acid (GABA) receptors (Salgado 1998; Orr et al. 2009; Dripps et al. 2011; Hertlein et al. 2011; Sparks et al. 2012). Apparently, since both active ingredients interact with the same receptors, is it postulated that additive effect is unlikely to occur and also that resistance development to spinosad will automatically trigger the resistance development to spinetoram (Dripps et al. 2011). However, spinosyns are known to have secondary mode of actions, distinct from currently known insecticidal sites, which merits additional investigation (Orr et al. 2009). Practically, by definition, insecticide mixtures should include compounds with different mode of action, in order to ensure multiple site binding. For example, Lord (2001) first reported that DEs synergize the insecticidal effect of the entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales), for the control of R. dominica, O. surinamensis, and Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae). In this study, the author suggested that DEs inactivate the epicuticular lipids that play an inhibitory role in B. bassiana conidial attachment and germination. Similarly, Chintzoglou et al. (2008a) found that the combination of DE with spinosad caused higher mortality of T. confusum than either compounds alone.
Temperature highly affected mortality in most of the cases tested. Generally, the increase of temperature increased mortality for all species and all insecticide combinations, especially at the shorter exposure intervals. This fact should be considered as a direct consequence of the increased metabolic activities of the insects at elevated temperatures, which is related with increased stress after the contact with the toxic agent. In a previous study, Athanassiou et al. (2008a, b) found that spinosad was generally more effective at high temperatures for both dry (dust) and liquid formulations against R. dominica and S. oryzae, respectively. Recently, Vassilakos and Athanassiou et al. (2013) found that temperature increased the efficacy of spinetoram against S. oryzae, while its efficacy against R. dominica and T. confusum was not much affected by changes in temperature.
Progeny production was successfully suppressed in comparison with the untreated grains. Despite the fact that progeny production was not 100 % suppressed in all cases, it was always lower than that in the control vials and usually did not exceed 1 individual/vial. T. confusum was an exception in this pattern, given that, as a secondary colonizer, it was expected that progeny production would be low, even in the untreated grains. Furthermore, parental survival was not expected to increase progeny production in the treated grains.
Regarding the differences among grains, mortality was similar among the different commodities for exposure intervals >2 days, again with the exception of T. confusum adults. For this species, mortality was generally higher in maize than in the other three grains. Vassilakos et al. (2012) found that spinetoram was very effective in maize and wheat against P. truncatus and R. dominica, respectively. However, Athanassiou et al. (2008b) reported that S. oryzae survival and progeny production were lower in wheat than in maize, rice, or barley treated with spinosad. Surprisingly, offspring emergence of S. oryzae was high in barley and rye, but not in wheat. This could be attributed to higher reproduction rates in these commodities. This trend was expressed more vigorously in the case of rye when it was treated with either spinosad alone or with the high proportion of spinosad in the mixture (0.9 ppm). This finding indicates that S. oryzae spinetoram may be more effective to spinosad in this commodity.
Regarding the susceptibility of the species tested, the two Bostrychidae, P. truncatus and R. dominica, were highly susceptible to all combinations examined. On the other hand, T. confusum was the least susceptible species. These observations are in agreement with previous studies that examined spinosad or spinetoram alone (Athanassiou et al. 2008a, b; Chintzoglou et al. 2008b; Hertlein et al. 2011; Vassilakos et al. 2012). Hence, under “real world” conditions, where several species coexist, the efficacy of either spinosad or spinetoram should be regarded in conjunction with other insecticides that demonstrate a different mode of action.
References
Arthur FH (1996) Grain protectants: current status and prospects for the future. J Stored Prod Res 32:293–302
Athanassiou CG, Kavallieratos NG, Yiatilis AE, Vayias BJ, Mavrotas CS, Tomanović Ž (2008a) Influence of temperature and humidity on the efficacy of spinosad against four stored grain beetle species. J Insect Sci 8:60
Athanassiou CG, Kavallieratos NG, Chintzoglou GJ, Peteinatos GG, Boukouvala MC, Petrou SS, Panoussakis EC (2008b) Effect of temperature and commodity on insecticidal efficacy of spinosad dust against Sitophilus oryzae (Coleoptera: Curculionidae) and Rhyzopertha dominica (Coleoptera: Bostrychidae). J Econ Entomol 101:976–981
Athanassiou CG, Athur FH, Throne JE (2009) Efficacy of grain protectants against four psocid species on maize, rice and wheat. Pest Manag Sci 65:1140–1146
Athanassiou CG, Kavallieratos NG, Arthur FH, Throne JE (2013) Efficacy of a combination of beta-cyfluthrin and imidacloprid and beta-cyfluthrin alone for control of stored-product insects on concrete. J Econ Entomol 106:1064–1070
Besard L, Mommaerts V, Abdu Alla G, Smagghe G (2010) Lethal and sublethal side-effect assessment supports a more benign profile of spinetoram compared with spinosad in the bumblebee Bombus terrestris. Pest Manag Sci 67:541–547
Chintzoglou GJ, Athanassiou CG, Arthur FH (2008a) Insecticidal effect of spinosad dust, in combination with diatomaceous earth, against two stored-grain beetle species. J Stored Prod Res 44:347–353
Chintzoglou GJ, Athanassiou CG, Markoglou AN, Kavallieratos NG (2008b) Influence of commodity on the effect of spinosad dust against Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) and Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Int J Pest Manag 54:277–285
Dripps JE, Boucher RE, Chloridis A, Cleveland CB, DeAmicis CV, Gomez LE, Paroonagian DL, Pavan LA, Sparks TC, Watson GB (2011) The spinosyn insecticides. In: Lopez O, Fernandez Bolanos JG (eds) Trends in insect control. Royal Society of Chemistry, Cambridge, pp 163–212
Fang L, Subramanyam Bh (2003) Activity of spinosad against adults of Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) is not affected by wheat temperature and moisture. J Kansas Entomol Soc 75:529–532
Fang L, Subramanyam Bh, Arthur FH (2002a) Effectiveness of spinosad on four classes of wheat against five stored product insects. J Econ Entomol 95:640–650
Fang L, Subramanyam Bh, Dolder S (2002b) Persistence and efficacy of spinosad residues in farm stored wheat. J Econ Entomol 95:1102–1109
Getchell AI, Subramanyam Bh (2008) Immediate and delayed mortality of Rhyzopertha dominica (Coleoptera: Bostrichidae) and Sitophilus oryzae (Coleoptera: Curculionidae) adults exposed to spinosad-treated commodities. J Econ Entomol 101:1022–1027
Gradish AE, Scott Dupree CD, Cutler GC (2012) Susceptibility of Megachile rotundata to insecticides used in wild blueberry production in Atlantic Canada. J Pest Sci 85:133–140
Hertlein MB, Thompson GD, Subramanyam Bh, Athanassiou CG (2011) Spinosad: a new natural product for stored grain protection. J Stored Prod Res 47:131–146
Jones MM, Robertson JL, Weinzierl RA (2010) Susceptibility of oriental fruit moth (Lepidoptera: Tortricidae) larvae to selected reduced-risk insecticides. J Econ Entomol 103:1815–1820
Kavallieratos NG, Athanassiou CG, Vayias BJ, Kotzamanidis S, Synodis SD (2010) Efficacy and adherence ratio of diatomaceous earth and spinosad in three wheat varieties against three stored-product insect pests. J Stored Prod Res 46:73–80
Lord JC (2001) Desiccant dusts synergize the effect of Beauveria bassiana (Hyphomycetes: Moniliales) on stored-grain beetles. J Econ Entomol 94:367–372
Orr N, Shaffner AJ, Richey K, Crouse GB (2009) Novel mode of action of spinosad: receptor binding studies demonstrating lack of interaction with known insecticidal targets. Pestic Biochem Physiol 95:1–5
Park T (1934) Observations on the general biology of the flour beetle, Tribolium confusum. Q Rev Biol 9:36–54
Pozidi Metaxa E, Athanassiou CG (2013) Comparison of spinosad with three traditional grain protectants against Prostephanus truncatus (Horn) and Ephestia kuehniella (Zeller) at different temperatures. J Pest Sci 86:203–210
Salgado VL (1998) Studies on the mode of action of spinosad: the internal effective concentration, and the concentration dependence of neural excitation. Pestic Biochem Physiol 60:103–110
Sall J, Lehman A, Creighton L (2001) JMP start statistics. A guide to statistics and data analysis using JMP and JMP IN software. Duxbury Press, Belmont
SAS Institute Inc (2010) Using JMP 9. SAS Institute Inc., Cary
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman WH and Company, New York
Sparks TC, Crouse GD, Dripps JE, Anzeveno P, Martynow J, DeAmicis CV, Gifford J (2008) Neural network-based QSAR and insecticide discovery: spinetoram. J Comput Aided Mol Des 22:393–401
Sparks TC, Dripps JE, Watson GB, Paroonagian D (2012) Resistance and cross-resistance to the spinosyns—a review and analysis. Pest Biochem Physiol 102:1–10
Subramanyam Bh, Roesli R (2000) Inert dusts. In: Subramanyam Bh, Hagstrum DW (eds) Alternatives to pesticides in stored-product IPM. Kluwer Academic Publishers, Dordrecht, pp 321–380
Subramanyam Bh, Toews MD, Ileleji KE, Maier DE, Thompson GD, Pitts TJ (2007) Evaluation of spinosad as a grain protectant on three Kansas farms. Crop Prot 26:1021–1030
Subramanyam Bh, Hartzer M, Boina DR (2012) Performance of pre-commercial formulations of spinosad against five stored-product insect species on four stored commodities. J Pest Sci 85:331–339
Thompson GD, Michel KH, Yao RC, Mynderse JS, Mosburg CT, Worden TV, Chio EH, Sparks TC, Hutchins SH (1997) The discovery of Saccharopolyspora spinosa and a new class of insect control products. Down Earth 52:1–5
Toews MD, Subramanyam Bh (2003) Contribution of contact toxicity and wheat condition to mortality of stored product insects exposed to spinosad. Pest Manag Sci 59:538–544
Toews MD, Subramanyam Bh, Rowan JM (2003) Knockdown and mortality of adults of eight species of stored product beetles exposed to four surfaces treated with spinosad. J Econ Entomol 96:1967–1973
Vassilakos TN, Athanassiou CG (2012a) Effect of short exposures to spinetoram against three stored-product beetle species. J Econ Entomol 105:1088–1094
Vassilakos TN, Athanassiou CG (2012b) Effect of uneven distribution of spinetoram-treated wheat and rice on mortality and progeny production of Rhyzopertha dominica (F.), Sitophilus oryzae (L.) and Tribolium confusum Jacquelin du Val. J Stored Prod Res 50:73–80
Vassilakos TN, Athanassiou CG (2013) Effect of temperature and relative humidity on the efficacy of spinetoram for the control of three stored product beetle species. J Stored Prod Res 55:73–77
Vassilakos TN, Athanassiou CG, Saglam O, Chloridis AS, Dripps JE (2012) Insecticidal effect of spinetoram against six major stored grain insect species. J Stored Prod Res 51:69–73
Wijayaratne LKW, Fields P, Arthur F (2012) Residual efficacy of methoprene for control of Tribolium castaneum (Coleoptera: Tenebrionidae) larvae at different temperatures on varnished wood, concrete and wheat. J Econ Entomol 105:718–725
Yee WL, Alston DG (2012) Behavioral responses, rate of mortality, and oviposition of western cherry fruit fly exposed to malathion, zeta-cypermethrin, and spinetoram. J Pest Sci 85:141–151
Acknowledgments
This study was partially supported by the project “Evaluation of the insecticidal efficacy of spinosad, fipronil and abamectin against stored-products insect pests” (Benaki Phytopathological Institute).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Athanassiou, C.G., Kavallieratos, N.G. Evaluation of spinetoram and spinosad for control of Prostephanus truncatus, Rhyzopertha dominica, Sitophilus oryzae, and Tribolium confusum on stored grains under laboratory tests. J Pest Sci 87, 469–483 (2014). https://doi.org/10.1007/s10340-014-0563-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0563-9