Abstract
The cotton bollworm, Helicoverpa armigera is a highly polyphagous pest infesting a number of economically important crops, annually causing enormous crop losses. Management of this pest is challenging over the years due to various factors including development of resistance to wide spectrum of chemical insecticides. RNA interference (RNAi) has tremendous potential to combat insect pests. However, RNAi mediated silencing efficiency varies from gene to gene, hence successful RNAi mediated pest control requires selection of suitable target gene(s), which are effectively silenced by the exogenous delivery of cognate double-stranded RNA (dsRNA) through midgut. Therefore, we have evaluated the effect of two concentrations of dsRNA delivered through semi-synthetic diet in silencing five important genes, viz. glutathione-S-transferase, cytochrome P450 (both involved in detoxification of host allelochemicals); trypsin, chymotrypsin (both involved in digestion of proteins) and juvenile hormone acid methyl transferase (jhamt) (involved in larval metamorphosis). Extent of silencing was assessed by quantitative real-time PCR (qRT-PCR). Results revealed that above target genes were silenced variably, 20 μg dsRNA treatment having a more pronounced effect than 10 μg in reducing the transcript levels, larval, pupal weight, and pupation. Silencing of chymotrypsin had a more pronounced effect on larval and pupal weight compared to other target genes, while jhamt severely affected pupation. This study demonstrated that target genes have varied sensitivity to RNAi, chymotrypsin, and jhamt were shown to be suitable candidate genes that could be utilized for RNAi mediated management of H. armigera.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cotton bollworm, Helicoverpa armigera is one of the most widely distributed polyphagous pest causing significant crop loss in cotton, tomato, brinjal, potato, chickpea, pigeon pea, okra, chilies, maize, sorghum, groundnut, soybean, sunflower, etc., (Srivastava et al. 2005; El-Wakeil 2007). The intensive and widespread use of insecticides has resulted in high levels of insecticide resistance, rendering management of this pest a difficult task (Gross 2006; Nimbalkar et al. 2009). In this regard, the addition of powerful tool called RNAi to our arsenal, hailed as “Scientific breakthrough” in developing species-specific insecticides (Baum et al. 2007; Mao et al. 2007; Whyard et al. 2009). RNAi is characterized by a high degree of specificity, high molecular potency, and holds tremendous potential in insect pest management. RNAi of specific target gene could be achieved by administering cognate dsRNA (Griebler et al. 2008; Mao et al. 2011). However, efficacy of RNAi mainly governed by associating variables that include the susceptibility of the target gene, site of dsRNA accumulation, tissue where RNAi occurs, delivery method, developmental stage of target insect, susceptibility of test insect, presence or absence of RNA dependent RNA polymerase (RdRP) which is involved in silencing signal amplification, etc. (Gatehouse et al. 2004; Belles 2010). In this regard, the main challenge for successful and effective RNAi mediated pest management lies in the identification of suitable target gene(s) which are sensitive to RNAi, when the cognate dsRNA delivered through diet via midgut. Toward this, semi-synthetic diet mediated delivery of dsRNA is an attractive, simple, and inexpensive approach useful for finding of suitable target gene(s) for field level pest management and this approach has more relevance to field level insect pest management.
In the present study, we have evaluated RNAi mediated silencing effect of five target genes viz. glutathione-S-transferase (GST) and cytochrome P450 (cyp P450) (CYP9A14 isoform), trypsin, chymotrypsin, and juvenile hormone acid methyl transferase (jhamt) on larval and pupal growth. In this regard, GST and cyp P450 are involved in detoxification of the plant allelochemicals and they are expressed in the midgut (Mao et al. 2007; Rajurkar et al. 2003; Liu et al. 2006; War et al. 2013). While, trypsin and chymotrypsin are the two predominant dietary serine proteases expressed in the midgut that account for 95 % of proteolytic activity in Lepidoptera (Terra and Ferreira 1994; Liu et al. 2009). On the other hand, jhamt is a key enzyme involved in the biosynthesis of juvenile hormone (JH), expressed in neural tissue “corpora allata” (Shinoda and Itoyama 2003; Griebler et al. 2008). Here we emphasize that, above target genes play a crucial role in insects daily biological functions and there are no reports elsewhere on assessment of silencing of above target genes except Cyp P450 (Mao et al. 2007; Mao et al. 2011) in H. armigera by diet mediated dsRNA delivery. Sensitivity of the above target genes to RNAi was evaluated employing two concentrations of dsRNA (10 and 20 μg), delivered through semi-synthetic diet to the larvae. Our studies show that the above target genes had varied sensitivity to dietary RNAi which was evident in terms of reduction in transcript level, larval, pupal weight, and extent of pupation. The results of the present study have direct implications on the selection of suitable target gene/s for RNAi mediated management of H. armigera.
Materials and methods
Test insect
Neonate larvae of H. armigera were obtained from Bio Control Research Laboratories (BCRL), of Pest Control of India Ltd. (PCI), Bengaluru, India. The larvae were fed on chickpea based semi-synthetic diet under controlled conditions, viz. at 27 ± 2 °C, 65 ± 5 % relative humidity and 16:8 h of light and dark cycle (Gupta et al. 2004).
Double-stranded RNA (dsRNA) synthesis
We had earlier cloned the five target genes viz. GST (HM209431) cyp P450 (HM209438), trypsin (GU323797), chymotrypsin (HM209422), and jhamt (GU323798) from H. armigera (Asokan et al. 2012) and these clones were used for development of dsRNA. dsRNA region was selected using highly sensitive off-target search software “dsCheck” which has the ability to select the region having lesser similarity with Drosophila melanogaster, Caenorhabditis elegans, Arabidopsis thaliana, Oryza sativa, and Rattus norvegicus genome sequences (Naito et al. 2005). Further, selected dsRNA sequences were searched for short nearly perfect nucleotide matches against the other available sequences of H. armigera and with the genome of Homo sapiens, using NCBI-BLAST (National Center for Biotechnology Information—Basic Local Alignment Search Tool). Primers for dsRNA synthesis were tagged with T7 promoter sequence at 5′ end (Table 1). For non-target control, we used approximately 500 bp of dehydration responsive element binding protein 1A (dreb1A), a transcription factor from Arabidopsis thaliana (Liu et al. 1998).
Template for dsRNA synthesis was amplified by PCR in a total reaction volume of 50 μl comprising 37.2 μl PCR grade water, 5 μl 10X Taq buffer, 2.0 μl 25 mM MgCl2, 1.0 μl dNTP mix (10 mM), 1.0 μl each of forward and reverse primes (10 mM each) (Table 1), 2 μl 1:50 diluted respective plasmid clones as template, and 0.8 μl Taq Polymerase (5 U/μl) (Fermentas Life Sciences, USA), and thermal cycling was performed using following parameters: 95 °C for 3 min followed by 35 cycles of 94 °C for 30 s, 68 °C for 30 s, and 72 °C for 45 s and final extension at 72 °C for 5 min. PCR products were resolved in 1.2 % agarose gel and desired size band excised and eluted using Nucleospin Extract II kit (Macherey–Nagel, Germany). dsRNA was synthesized using 1 μg of eluted PCR product using MEGAscript® Kit (Ambion Life Technologies, USA) according to the manufacturer’s instructions. The concentration of the dsRNA was determined by using NanoDrop™ 1000 (Thermo scientific, USA) and integrity was analyzed by agarose gel electrophoresis (1.2 %).
Insect bioassay
Two independent experiments were performed to assess the sensitivity of the above target genes to RNAi using 10 and 20 μg of dsRNA. These concentrations were selected because as in a previous study the concentrations lower than that used here did not result in silencing (unpublished data). Chick pea based semi-synthetic diet was prepared with Diethyl pyrocarbonate (DEPC) treated water and 850 μl of diet was dispensed into each well of the bioassay plate and cooled to room temperature (28 °C). dsRNA stock was diluted with DEPC (0.1 %) treated water to yield 0.20 μg/μl (10 μg/50 μl), 0.40 μg/μl (20 μg/50 μl) of dsRNA. 50 μl of the above two dsRNA concentrations was applied on the surface of the semi-synthetic diet individually, allowed to imbibe and air dried. For non-target control and water control, same volume of dreb1A dsRNA and DEPC treated water without dsRNA was applied, respectively. There were 20 replications per each treatment i.e., cognate dsRNA treatment, non-target dsRNA (dreb1A) treatment, and water control. A single neonate larva was released per well and after every 48 h the larvae were shifted to the fresh diet containing fresh dsRNA of above mentioned concentrations and the feeding continued till pupation.
Molecular validation of silencing
RNA extraction and cDNA synthesis
Total RNA was extracted from single larva of H. armigera on the 8th day of treatment using the RETROscript® Kit (Ambion life technologies, USA) according to the manufacturer’s instructions and DNA contamination was removed by treating with RNase-free DNase I (Fermentas Life Sciences, USA) and purified by phenol–chloroform (25:25) extraction (Sambrook and Russell 2001). Quality of RNA was determined by 1.2 % agarose gel electrophoresis and concentration was determined by using NanoDrop™ 1000 (Thermo Scientific, USA). Reverse transcription was performed in 20 μl reaction volume comprising 0.3 μl (60 U) reverse transcriptase, 1 μl dNTP mix (10 mM), 1 μl oligo-dT primer, and 1 μg total RNA, by following the manufacturer’s protocol (Fermentas Life Sciences, USA). After cDNA synthesis, it was diluted 10 folds with molecular biology grade water. In all reverse transcription assays RT negative controls containing all components except reverse transcriptase were included.
Quantitative real-time PCR (qRT-PCR)
The extent of target gene silencing was assessed by employing qRT-PCR (Light Cycler 480II-Roche Diagnostics Pvt. Ltd. Switzerland). 18S rRNA was used as an internal reference gene, which was identified as a suitable reference gene in a previous experiment (manuscript under communication). qRT-PCR reactions were carried out in triplicates for each sample in a total reaction volume of 20 μl comprising 10 μl SYBR® Green JumpStart™ Taq Ready-mix™ (Sigma-Aldrich, USA), 0.5 μl each of forward and reverse primers (5 μM each) (Table 1) and 5 μl diluted cDNA template were mixed with 4 μl nuclease free water and cycling was performed using following parameters: 94 °C for 4 min, followed by 40 cycles of 94 °C for 30 s, and 60 °C for 1 min. Melt curve analysis of amplicons was performed by continuous capturing of fluorescence while temperature increases from 60 to 95 °C with ramp rate of 0.11 °C/s.
Effect of silencing on larval, pupal weight, and pupation
Larval weight was recorded on the 8th day of treatment and the extent of pupation rate for all treated samples was recorded on 3rd day of all control larvae which pupated. Weight reduction of both larvae and pupae was calculated by taking the differences between the mean larval weights in treatment Vs mean larval weights in untreated (water) control.
Data analysis
The relative expression of target genes was calculated using 2−∆∆CT method (Livak and Schmittgen 2001). All qRT-PCR assays were designed according to the MIQE guidelines (Bustin et al. 2009). Effect of silencing on target gene expression was analyzed statistically by student’s t test with C q values of three replicates using GraphPadPrism v.5 (GraphPad Software, Inc., USA). Differences in weight of larvae and pupae among the treatments were analyzed by Tukey’s test of one-way ANOVA.
Results
Selection of dsRNA regions
The following regions were selected for dsRNA synthesis: 455 bp (from 87–541 bp), 442 bp (242–683 bp), 474 bp (96–569 bp), 470 bp (170–639 bp), and 421 bp (215–635 bp) for GST, cyp P450, trypsin, chymotrypsin, and jhamt, respectively. NCBI-BLAST analysis of the above selected regions with other genes of H. armigera and H. sapiens showed no significant homology.
Molecular validation of silencing
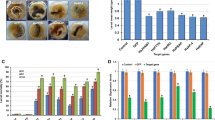
In qRT-PCR, the melt curve analysis of target genes, GST, cyp P450, trypsin, chymotrypsin, and jhamt and reference gene 18S rRNA amplicons exhibited a single melt peak, indicated specific amplification of respective gene. Further, resolving of qRT-PCR products on agarose gel reconfirmed the presence of single band and there was no-band in all negative controls. The present study showed that semi-synthetic diet mediated delivery of dsRNA triggered RNAi in H. armigera. We observed a drastic reduction in the mRNA levels of target genes in both concentrations. Silencing of GST caused 13.73 and 21.36 % reduction in the transcript level in 10 and 20 μg treatments, respectively. Similarly, silencing of cyp P450 caused 48.03 and 65.52 % reduction in the transcript level in 10 and 20 μg, respectively. Silencing of trypsin caused 19.78 and 48.46 % reduction in the transcript level in 10 and 20 μg, respectively, while silencing of chymotrypsin caused 56.23 and 94.55 % reduction in the transcript level in 10 and 20 μg, respectively. While, silencing of jhamt caused 44.15 and 88.46 % reduction in the transcript level in 10 and 20 μg, respectively (Fig. 1a, b).
Relative quantification of target gene expression by employing qRT-PCR. a Target gene expression levels in 10 μg cognate dsRNA and non-target (dreb1A) dsRNA treatments. b Target gene expression levels in 20 μg dsRNA and non-target (dreb1A) dsRNA treatments. Error bars indicate standard error of the triplicate samples. *P = 0.05, **P = 0.01 analyzed by student’s t test
Effect of silencing on larval and pupal weight
The target genes exhibited considerable variations in the extent of silencing and it had also manifested in a reduction of larval and pupal weight. Silencing of GST had manifested 25.32 and 31.73 % reduction in larval weight in 10 and 20 μg, respectively. Cyp P450 dsRNA treatment had manifested 44.65 and 48.16 % reduction in larval weight in 10 and 20 μg, respectively. Silencing of trypsin had manifested 29.74 and 57.71 % reduction in larval weight in 10 and 20 μg, respectively. While silencing of chymotrypsin had a more pronounced effect on larval weight and a reduction of 41.20 and 70.21 % was observed in 10 and 20 μg, respectively. Similarly, silencing of jhamt had manifested 37.07 and 44.49 % reduction in larval weight in 10 and 20 μg, respectively. An unrelated, non-insect gene, the dreb1A dsRNA application had no effect on weight of larvae and which was on par with untreated control. The absolute values of weight reduction were depicted in Fig. 2 (Fig. 2a–d).
Effect of two concentrations of dsRNA on larval weight gain. a Photograph of 10 μg dsRNA fed larvae. b Mean larval weight in 10 μg dsRNA treatment. c Photograph of 20 μg dsRNA fed larvae. d Mean larval weight in 20 μg dsRNA treatment. The error bar represents the standard error of the all replicates in each treatment. Significance at *P = 0.05, analyzed by one-way ANOVA
Target gene silencing had also affected the pupal weight and observation showed reduction of pupal weight was correlated with larval weight, but the extent of reduction differed considerably (Fig 3a, b). Silencing of GST had manifested 8.36 and 10.59 % reduction in pupal weight in 10 and 20 μg, respectively. Cyp P450 dsRNA treatment had manifested 26.15 and 27.41 % reduction in pupal weight in 10 and 20 μg, respectively. Silencing of trypsin had manifested 8.54 and 25.89 % reduction in pupal weight in 10 and 20 μg, respectively. While silencing of chymotrypsin had a more pronounced effect on pupal weight to a tune of 19.09 and 33.76 % reduction in 10 and 20 μg, respectively. Silencing of jhamt had manifested 18.89 and 29.25 % reduction in pupal weight in 10 and 20 μg, respectively.
Silencing effect on the rate of pupation
Silencing of jhamt caused weight reduction of larvae as well as a drastic reduction in pupation rate. In case of jhamt, 20 μg cognate dsRNA treatment severely affected the pupation ~60 % compared to control and it was lesser in other cases (Fig. 4). Whereas, 10 μg dsRNA treatment affected the pupation 15 and 10 % in case of jhamt and cyp P450, respectively, and silencing of the remaining genes didn’t affect the pupation.
Discussion
Double-stranded RNA mediated inhibition of specific gene expression by the process known as RNAi has been reported in many insect species including Coleoptera, Lepidoptera, Diptera, Hemiptera, Hymenoptera, Isoptera, and Orthoptera (Huvenne and Smagghe 2010; Asokan et al. 2013). Successful application of RNAi for field level pest management lies in the identification of suitable target gene(s) and the method of delivery. Accomplishment of RNAi through feeding is a more attractive approach than the other methods such as microinjection, soaking, droplet feeding, etc., as it is non-invasive and further opens the possibility to develop dsRNA expressing transgenic plants or development of products which may be applied similar to conventional insecticides for field level pest control (Bettencourt et al. 2002; Mao et al. 2011; Terenius et al. 2011; Hunter et al. 2012). In spite of its potential benefits, exploitation of this method is hampered by several intricacies such as dsRNA degradation in the midgut, uptake and transportation of dsRNA to target tissues, etc. (Terenius et al. 2011; Liu et al. 2013). Some of the previous studies were unsuccessful when dsRNA was administered through diet. For example, silencing of aminopeptidase-N gene specific to larval midgut did not show silencing after dsRNA feeding in Spodoptera litura, although injection of same dsRNA into the hemocoel caused silencing of aminopeptidase-N (Rajagopal et al. 2002). However, our study demonstrated that the diet-delivered dsRNA was successful in inducing silencing of various target genes. Similarly, studies on the Fall Armyworm, Spodoptera frugiperda and Light brown apple moth, Epiphyas postvittana have shown that silencing of target genes was successful upon administration of dsRNA through feeding (Griebler et al. 2008; Turner et al. 2006). Therefore, accomplishment of silencing through semi-synthetic diet-delivered dsRNA is relevant to the field level pest management point of view by the way of deploying transgenic plants, baits or other foods (Hunter et al. 2010), or topical sprays (Pridgeon et al. 2008; El-Shesheny et al. 2013).
Interestingly, diet-delivered cognate dsRNA caused silencing of both midgut and non-midgut genes in respective treatments. Although, the previous report showed that feeding of dsRNA did not cause the non-midgut gene silencing in Bombyx mori (Tomoyasu et al. 2008). Therefore, achievement of silencing of non-midgut gene through diet-delivered dsRNA is of prime importance as there is no report of RdRP in Helicoverpa species, which enhances the systemic spread of silencing signal to non-midgut tissue (Belles 2010). In the absence of RdRP, the midgut is the primary organ that directly contacts the dsRNA, when dsRNA was administered through diet; the dsRNA concentration becomes diluted over a distance traveled from the entry site to target organs leading to decline in dsRNA effect (Minakuchi et al. 2008; Tian et al. 2009). However, this study presumed that non-midgut gene, jhamt silencing through diet-delivered dsRNA could be induced due to lower abundance of jhamt transcripts as confirmed by qRT-PCR showed quantification cycle (C q ) ranging from 32–35 cycles in control samples compared to other target genes ranging from 16–25 C q ,. Thus far, many studies are focused mainly on the genes which are specifically expressed in the midgut (Rajagopal et al. 2002; Mao et al. 2007; Mao et al. 2011), and very few reports are available on non-midgut gene silencing through diet-delivered dsRNA (Li et al. 2011), but no report on jhamt from other researchers. Hence results of this study may provoke the other researchers by utilizing non-midgut genes as potential targets for field level pest management of H. armigera and this strategy could also be translated in the management of other insect pests.
The extent of silencing varied between various target genes and we observed a greater reduction in chymotrypsin followed by jhamt, cyp P450, trypsin, and GST. It has also been accepted that the inhibition of target gene expression is dependent on dsRNA concentration. The extent of silencing of the GST was less pronounced as compared to other target genes and these variations might be attributed due to number factors that include quantum of available transcripts, tissue where the target gene is expressed, its highly regulated nature in the test insect etc. Results of this study indicate that GST was less sensitive to RNAi as compared to other target genes used in this experiment.
Silencing of target genes adversely affected the weight gain of the larvae compared to control group which was taken as 100 % weight gain. Similarly, weight gain of larvae in different treatments varied significantly. Whereas, silencing of cyp P450 severely affected the larval weight gain compared to other target genes in 10 μg dsRNA treatment, followed by chymotrypsin, jhamt, trypsin, and GST. Silencing of chymotrypsin severely affected the weight gain of larvae compared to other target genes in the 20 μg dsRNA treatment, followed by trypsin, cyp P450, jhamt, and GST. These variations in weight gain might be due to biological role of target gene(s) in growth, development, detoxification, etc. In this regard, crop plants suffer attacks by insect herbivores; hence to protect themselves, plants produce secondary metabolites which are toxic to insect herbivores. To counter this, insect triggers the secondary metabolite detoxification genes such as GST, cyp P450, and esterase which help insects to protect themselves (Rajurkar et al. 2003; Mao et al. 2007). Hence the expression of cyp P450 and GST was found necessary for Cotton bollworm to actively detoxify the secondary metabolites present in chickpea based semi-synthetic diet. Silencing of GST or cyp P450 presumably resulted in accumulation of allelochemicals in the insect body which eventually caused the weight reduction of larvae. Our results support Mao et al. (2007), that feeding of transgenic plants expressing cyp P450 dsRNA reduced the weight of H. armigera larvae. On the other hand, serine proteases that include trypsin and chymotrypsin play various roles such as food digestion, immune defense, and zymogen activation, in insects (Broehan et al. 2010; Mohammadi et al. 2010). Therefore, RNAi mediated silencing of trypsin and chymotrypsin may lead to the impairment of the above functions, which eventually cause the weight reduction or mortality and we observed weight reduction of larvae. In contrast, injection of TcCTLP-dsRNA (chymotrypsin like serine protease) did not affect the weight of the larvae in red flour beetle, Tribolium castaneum (Broehan et al. 2010). Hence, the observed variations of larval weight were attributed due to RNAi which impaired the target gene(s) biological function essential for growth and survival. Similarly, in the Western corn rootworm, Diabrotica virgifera virgifera, silencing was performed for 290 genes, out of which only 125 caused larval mortality/reduction in growth rate, while other genes were less effective (Baum et al. 2007). The above experiments revealed that all target genes are not equally sensitive to RNAi in terms the resultant debilitation in insect.
Further, the weight reduction of pupae in different treatments showed a correlation with reduction of larval weight in respective treatments being greater in the 20 μg treatment. Hence, the detrimental effects on larvae were passed to pupae or the pupae were unable to recover from the impaired metabolism in the larval stage. Interestingly, silencing of jhamt severely affected the pupation rate compared to other target genes. In this regard, jhamt is a key enzyme in the maturation of juvenile hormone (JH), whereas, JH play fundamental roles in many aspects of postembryonic life, including development, metamorphosis, reproduction (fecundity), yolk protein uptake, oogenesis, migration, diapauses, innate immunity, fat body development, extension of life span, regulation of zinc-finger transcription which is involved in neurogenesis, and foraging behavior, etc. (Yamamoto et al. 2013). The previous studies revealed that jhamt expression levels were highly correlated to the rates of JH biosynthesis, suggesting that jhamt is playing an important role in regulating JH synthesis (Griebler et al. 2008; Li et al. 2013). Therefore, alteration of jhamt will lead to the change in the JH titer in hemolymph that eventually affects the above physiological functions. In this study, silencing of jhamt in the larval stage affected approximately 60 % pupation and larvae underwent incomplete metamorphosis i.e., showed a kind of larval-pupal intermediate phenomenon and growth is arrested and this is presumably by inhibition of JH biosynthesis. Our results are comparable to Daimon et al. (2012) reported that JH deficient mutant of the silkmoth, Bombyx mori showed a larval-pupal intermediate and eventually died. Contrastingly, silencing one of the isoforms of jhamt (TcMT3) caused precocious larval-pupal metamorphosis in T. castaneum, but the silencing of other isoforms (TcMT1 and TcMT2) did not cause precocious larval-pupal metamorphosis (Minakuchi et al. 2008). However, mutants of JH didn’t show precocious metamorphosis in Drosophila melanogaster (Wilson et al. 2006). In another study, knockout of JH severely affected the fecundity, oogenesis, impaired the fat body development in Drosophila and also altered the expression of nearly 100 downstream genes (Yamamoto et al. 2013). Therefore, the above observations reveal that RNAi mediated silencing of jhamt has a dramatic effect on insects especially in the metamorphosis of larva to pupa and other biological functions and targeting the correct isoform of jhamt, severely affects the larval-pupal metamorphosis. Hence, the observed larval-pupal intermediated phenomenon is presumably due to biological intended effects of JH reduction and not due to non-specific off-target effects. Thus we propose that, this gene should be one of the choices for future prospective insect pest management.
Conclusion
Degradation of the specific target gene transcript by RNAi leads to impairment of specific function and affects growth and survivability of the insect. Therefore, development of a reliable RNAi based approach for the management of H. armigera provides a starting point for novel species-specific insecticides development. In this regard, identification, and validation of potential target genes are crucial for exploitation of RNAi as a novel insect pest management strategy. Results revealed that semi-synthetic diet-delivered dsRNA successfully triggered silencing of the genes tested. Interestingly, semi-synthetic diet-delivered dsRNA caused systemic silencing of non-midgut gene, jhamt. The sensitivity of the above target genes to RNAi varied considerably with digestion related genes severely affected compared to metamorphosis and detoxification related genes. Apart from these, dsRNA concentration was the determining factor governing RNAi efficiency, where 20 μg dsRNA concentration was more effective in silencing of the target genes as compared to 10 μg. The method described in the present study provides a means to identify and validate potential targets, and selection of appropriate dsRNA concentration to achieve optimal silencing of target genes. Further, indicate the feasibility of utilizing non-midgut genes as potential targets for field level pest management of H. armigera. Therefore, the selected target genes could be utilized for effective control of H. armigera and the approach described here could be useful for controlling other destructive pests.
References
Asokan R, Nagesha SN, Manamohan M, Krishnakumar NK, Mahadevaswamy HM, Prakash MN, Sharath Chandra G, Rebijith KB, Ellango R (2012) Common siRNAs for various target genes of the fruit borer, Helicoverpa armigera Hubner (Lepidoptera: Noctuidae). Curr Sci 102:1692–1699
Asokan R, Sharath Chandra G, Manamohan M, Krishna Kumar NK (2013) Effect of diet delivered various concentrations of double-stranded RNA in silencing a midgut and a non-midgut gene of Helicoverpa armigera. Bull Entomological Res 103:555–563
Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M, Vaughn T, Roberts J (2007) Control of coleopteran insect pests through RNA interference. Nat Biotechnol 25:1322–1326
Belles X (2010) Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu Rev Entomol 55:111–128
Bettencourt R, Terenius O, Faye I (2002) Hemolin gene silencing by ds-RNA injected into Cecropia pupae is lethal to next generation embryos. Insect Mol Biol 11:267–271
Broehan G, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S, Merzendorfer H (2010) Chymotrypsin-like peptidases from Tribolium castaneum: a role in molting revealed by RNA interference. Insect Biochem Mol Biol 40:274–283
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipleym GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines-minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Daimon T, Kozaki T, Niwa R, Kobayashi I, Furuta K, Namiki T, Uchino K, Banno Y, Katsuma S, Tamura T, Mita K, Sezutsu H, Nakayama M, Itoyama K, Shimada T, Shinoda T (2012) Precocious metamorphosis in the Juvenile Hormone-Deficient mutant of the silkworm. Bombyx Mori Plos Genet 8(3):e1002486. doi:10.1371/journal.pgen.1002486
El-Shesheny I, Hajeri S, El-Hawary I, Gowda S, Killiny N (2013) Silencing abnormal wing disc gene of the Asian citrus psyllid, Diaphorina citri disrupts adult wing development and increases nymph mortality. PLoS ONE 8(5):e65392
El-Wakeil NE (2007) Evaluation of efficiency of Trichogramma evanescens reared on different factitious hosts to control Helicoverpa armigera. J Pest Sci 80:29–34
Gatehouse HS, Gatehouse LN, Malone LA, Hodges S, Tregidga E, Todd J (2004) Amylase activity in honey bee hypopharyngeal glands reduced by RNA interference. J Apic Res 43:9–13
Griebler M, Westerlund SA, Hoffmann KH, Meyering-Vos M (2008) RNA interference with the allatoregulating neuropeptide genes from the fall armyworm Spodoptera frugiperda and its effects on the JH titer. J Insect Physiol 54:997–1007
Gross J (2006) New challenges in pest science. J Pest Sci 79:1–2
Gupta GP, Birah A, Rani S (2004) Development of artificial diet for mass rearing of American bollworm, Helicoverpa armigera. Indian J Agric Sci 74:548–551
Hunter W, Ellis J, vanEngelsdorp D, Hayes J, Westervelt D et al (2010) Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog 6(12):e1001160
Hunter WB, Glick E, Paldi N, Bextine BR (2012) Advances in RNA interference: dsRNA treatment in trees and grapevines for insect pest population suppression. Southwest Entomol 37:85–87
Huvenne H, Smagghe G (2010) Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56:227–235
Li X, Zhang M, Zhang H (2011) RNA interference of four genes in adult Bactrocera dorsalis by feeding their dsRNAs. PLoS ONE 6(3):e17788
Li W, Huang ZY, Liu F, Li Z, Yan L et al (2013) Molecular cloning and characterization of juvenile hormone acid methyltransferase in the honey bee, Apis mellifera, and its differential expression during caste differentiation. PLoS ONE 8(7):e68544
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Shinozaki KY, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low- temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Liu X, Liang P, Gao X, Shi X (2006) Induction of the cytochrome P450 activity by plant allelochemicals in the cotton bollworm, Helicoverpa armigera (Hübner). Pestic Biochem Physiol 84:127–134
Liu Y, Sui YP, Wang JX, Zhao XF (2009) Characterization of the trypsin-like protease (Ha-TLP2) constitutively expressed in the integument of the cotton bollworm, Helicoverpa armigera. Arch Insect Biochem Physiol 72:74–87
Liu J, Smagghe G, Swevers L (2013) Transcriptional response of BmToll9-1 and RNAi machinery genes to exogenous dsRNA in the midgut of Bombyx mori. J Insect Physiol 59:646–654
Livak KL, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant- mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 25:1307–1313
Mao YB, Tao XY, Xue XY, Wang LJ, Chen XY (2011) Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res 20:665–673
Minakuchi C, Namiki T, Yoshiyama M, Shinoda T (2008) RNAi-mediated knockdown of juvenile hormone acid O-methyl transferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J 275:2919–2931
Mohammadi D, Abad RFP, Rashidi MR, Mohammadi SA (2010) Activity and some properties of Helicoverpa armigera Hubner and Spodoptera exigua Hubner (Lepidoptera:Noctuidae) midgut protease. Munis Entomol Zool 5:697–706
Naito Y, Yamuda T, Mastumiya T, Kumiko UT, Saigo K, Morishita S (2005) dsCheck: highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res 33:W589–W591
Nimbalkar RK, Shinde SS, Tawar DS, Muley SP (2009) Response of cotton bollworm Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) to different insecticides in Maharashtra, India. World J Agric Sci 5:250–255
Pridgeon JW, Zhao L, Becnel JJ, Strickman DA, Clark GG, Linthicum KJ (2008) Topically applied AaeIAP1 double-stranded RNA kills female adults of Aedes aegypti. J Med Entomol 45:414–420
Rajagopal R, Sivakumar S, Agrawal N, Malhotra P, Bhatnagar RK (2002) Silencing of midgut aminopeptidase N of Spodoptera litura by double-stranded RNA establishes its role as Bacillus thuringiensis toxin receptor. J Biol Chem 277:46849–46851
Rajurkar RB, Khan ZH, Gujar GT (2003) Studies on levels of glutathione S-transferase, its isolation and purification from Helicoverpa armigera. Curr Sci 85:9
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shinoda T, Itoyama K (2003) Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. PNAS 100:11986–11991
Srivastava CP, Ahmad R, Ujagir R, Das SB (2005) Helicoverpa armigera management in pulses-present scenario and future strategies. Recent Advances in Helicoverpa armigera Management. Indian Society of Pulses Research and Development, Kanpur, pp 265–286
Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, Albrechtsen M, An C, Aymeric JL, Barthel A, Bebas P, Bitram K, Bravo A, Chevalier FC, Collinge DP, Crava CM, Maagd RA, Duvic B, Erlandson M, Faye I, Felfoldi G, Fujiwara H, Futahashi R, Gandhe AS, Gatehouse HS, Gatehouse LN, Giebultowicz JM, Gomez I, Grimmelikhuijzen CJP, Groot AT, Hauser F, Heckel DG, Hededus DD, Hrycaj S, Huang L, Hull JJ, Latrou K, Iga M, Kanost MR, Kotwica J, Li C, Li J, Liu J, Lundmark M, Matsumoto S, Meyering-Vos M, Millichap PJ, Monteiro A, Mrinal N, Niimi T, Nowara D, Ohnishi A, Oostra V, Ozaki K, Papakonstantionou M, Popadic A, Rajam MV, Saenko S, Simpson RM, Soberon M, Strand MR, Tomita S, Toprak U, Wang P, Wee CW, Whyard S, Zhang W, Nagaraju J, Ffrench-Constant RH, Herrero S, Gordon K, Swevers L, Smagghe G (2011) RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol 57:231–245
Terra WR, Ferreira C (1994) Insect digestive enzymes: properties, compartmentalization and function. Comp Biochem Physiol 109:1–62
Tian H, Peng H, Yao Q, Chen H, Xie Q, Tang B, Zhang W (2009) Developmental control of a Lepidopteran pest, Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 4:e6225
Tomoyasu Y, Miller SC, Tomita S, Schoppmeier M, Grossmann D, Bucher G (2008) Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Genome Boil 9:R10
Turner CT, Davy MW, Macdiarmid RM, Plummer KM, Birch NP, Newcomb RD (2006) RNA interference in the light brown apple moth, Epiphyyas postvittana (Walker) induced by double-stranded RNA feeding. Insect Biochem Mol Biol 15:383–391
War AR, Paulraj MG, Hussain B, Buhroo AA, Ignacimuthu S, Sharma HC (2013) Effect of plant secondary metabolites on legume pod borer, Helicoverpa armigera. J Pest Sci 86:390–408
Whyard S, Singh AD, Wong S (2009) Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Mol Biol 39:824–832
Wilson TG, Yerushalmi Y, Donnell DM, Restifo LL (2006) Interaction between hormonal signaling pathways in Drosophila melanogaster as revealed by genetic interaction between methoprene-tolerant and broad-complex. Genetics 172:253–264
Yamamoto R, Bai H, Dolezal AG, Amdam G, Tatar M (2013) Juvenile hormone regulation of Drosophila aging. BMC Biol 11:85
Acknowledgments
The authors are grateful to ICAR, New Delhi for funding the subproject “Potential of RNAi in insect pest management: A model in silencing genes specific to tomato fruit borer, Helicoverpa armigera Hubner (Noctuidae: Lepidoptera)” under NAIP. We express our sincere thanks to the National Director and the National Co-ordinator (Component 4), NAIP, New Delhi for their support. We also thank our Director, IIHR, Bengaluru for encouragement and facilities. The authors thankfully acknowledge the Pest Control of India (PCI) for providing H. armigera larva.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by C. Stauffer.
Rights and permissions
About this article
Cite this article
Asokan, R., Sharath Chandra, G., Manamohan, M. et al. Response of various target genes to diet-delivered dsRNA mediated RNA interference in the cotton bollworm, Helicoverpa armigera . J Pest Sci 87, 163–172 (2014). https://doi.org/10.1007/s10340-013-0541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-013-0541-7