Abstract
Pecan scab (caused by Fusicladium effusum) limits the productivity of pecan in the southeastern USA. Alternatives to conventional fungicides should be biorational, of low environmental risk with a lower risk of fungicide resistance. Research showed that metabolites from the nematode symbiont Photorhabdus luminescens suppress pecan scab, but the bioactive molecules had not been identified. Extracts from P. luminescens were investigated using a bioactivity-directed fractionation approach to identify the constituent(s) responsible for the activity. High throughput antifungal bioautography assays against Colletotrichum gloeosporioides, C. fragariae, and C. acutatum were used to guide the fractionation. One of the metabolites was purified and identified as trans-cinnamic acid (TCA) using silica gel chromatography followed by semi-preparative high-performance liquid chromatography. In vitro tests confirmed toxicity of TCA to C. gloeosporioides, C. fragariae, and C. acutatum at 10 and 100 μg mL−1 using fungal bioautography inhibition screening plates. The antimycotic activity of TCA was tested in vitro against F. effusum. Zone of inhibition tests, and tests with TCA incorporated into agar showed TCA toxicity to F. effusum at concentration 148–200+ μg mL−1. Further tests incorporating TCA into liquid media demonstrated that TCA arrested all growth of F. effusum at a concentration even as low as 64 μg mL−1. Naturally occurring antimicrobial products might offer an alternative to disease control in crops, helping in minimizing the risk of fungicide resistance, while also minimizing any negative impact on the environment. Additional research is warranted to determine the potential to use TCA as a suppressive agent for pecan scab and other diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant pathogens are responsible for yield loss in many crops, including nut trees such as pecan (Carya illinoinensis (Wangenh.) K. Koch). Pecan scab, caused by the fungus Fusicladium effusum G. Winter, is the major disease that reduces the productivity and quality of pecan in the southeastern USA, although several other pathogens can also cause yield loss (Goff et al. 1996; Teviotdale et al. 2002). Some host resistance is available to reduce the impact of pecan scab, but there is a history of the resistance succumbing to new strains of the pathogen (Cole and Gossard 1956; Goff et al. 1996). Thus, conventional chemical fungicides have been widely used to control scab, often with more than ten sprays being applied to some crops in some seasons to ensure adequate control of the disease (Brenneman et al. 1998). As a result, the risk of fungicide resistance developing in these pathogens has been realized, and F. effusum is now resistant to at least two classes of fungicide (Reynolds et al. 1997; Stevenson 1999; Seyran et al. 2010). Furthermore, there is an increasing awareness of the environmental impact of conventional pesticides (National Research Council 1989).
Thus, there are incentives to try and develop alternatives to conventional fungicides, alternatives that are biorational, of low environmental risk, and that present a lower risk of fungicide resistance developing in the pathogen; a characteristic that will enhance durability of the chemistry. One option that has recently been explored is the use of naturally occurring antimicrobial compounds produced by Xenorhabdus spp. and Photorhabdus spp. of bacteria; these bacteria are symbionts of entomopathogenic nematodes in the genera Steinernema and Heterorhabditis, respectively (Kaya and Gaugler 1993; Gaugler 2002; Grewal et al. 2005). The bacteria can be cultured in media, and extracts of the cultures contain the bioactive, antimicrobial metabolites, which are active against a wide range of microbial pathogens of animals and plants including bacteria, fungi, and oomycetes (Akhurst 1982; McInerney et al. 1991; Chen et al. 1994; Li et al. 1995; Webster et al. 1995, 2002; Isaacson and Webster 2002; Shapiro-Ilan et al. 2009; Boeszoermenyi et al. 2009; Fang et al. 2011; San-Blas et al. 2012).
Shapiro-Ilan et al. (2009) demonstrated that soluble organic metabolites from X. bovienii Akhurst, X. nematophila Poinar and Thomas, and Photorhabdus luminescens Thomas and Poinar, and unidentified species of both Xenorhabdus and Photorhabdus suppressed growth of Glomerella cingulata (Stoneman) Spauld. & H. Schrenk, Phomopsis sp., Phytophthora cactorum (Lebert & Cohn) J. Schröt. and F. effusum, fungal pathogens on pecan, and Monilinia fructicola Winter on peach, showing that pathogens of scab were sensitive to extracts from several species both in vitro and in vivo.
Although extracts of P. luminescens had good antimycotic activity against F. effusum, there has been no study to identify the major compounds responsible for the antimycotic activity. Identification of these bioactive compounds may facilitate optimization of potency in bacteria/metabolite production. Or, conceivably, these bioactive compounds could themselves be used as alternatives to standard fungicides. Thus, there is a need to both identify the bioactive compounds responsible, and to test these in a purified form to confirm efficacy and ensure that they might have a practical application to disease management, i.e., have low environmental risk, be sufficiently durable in the environment, and present a low risk of pathogen resistance developing.
The main objective of this study was to identify the major bioactive antimycotic compound produced by P. luminescens that is responsible for suppression of pecan scab.
Materials and methods
Culture of bacteria and extraction of metabolites
Bacteria of P. luminescens were isolated from the nematode host, Heterorhabditis bacteriophora Poinar, which was cultured in the last instar of Galleria mellonella L., as described by Kaya and Stock (1997). The bacteria were subsequently cultured on nutrient agar (NA) by streaking hemolymph from insects inoculated with the nematode (Kaya and Stock 1997).
The extraction of metabolites was performed following the procedures of Ng and Webster (1997). For purposes of metabolite extraction, a loopful of bacteria from the NA colony was transferred to 50 mL Tryptic Soy broth (DIFCO, Detroit, MI) in an Erlenmeyer flask. Cultures were incubated at 25 °C on a rotary shaker for 18–24 h when they were harvested by centrifuging at 10,000 rpm for 20 min. The supernatant from each flask was extracted thrice with ethyl acetate (Fisher Scientific, Fair Lawn, NJ) and the organic fractions dried with anhydrous ammonium sulfate (Sigma, St. Louis, MO), and concentrated using a rotary evaporator. The dried fraction was dissolved in acetone (Sigma, St. Louis, MO) and stored at 4 °C.
Culture of fungal plant pathogens
Pathogen production
Colonies of F. effusum were grown on potato dextrose agar (PDA) in Petri-plates placed in an incubator maintained at 27 °C for 3 weeks, from which conidia were harvested, and a suspension of 106 conidia mL−1 was prepared in sterile, distilled water for in vitro testing of the metabolite.
Isolates of Colletotrichum acutatum J.H. Simmonds, C. fragariae Brooks, and C. gloeosporioides (Penz.) Penz. and Sacc. were obtained from B. J. Smith, USDA, ARS, Small Fruit Research Station, Poplarville, MS. The three Colletotrichum species were isolated from strawberry (Fragaria × ananassa Duchesne).
Direct bioautography
Standardization of Colletotrichum inoculum
Standardizing the inoculum allows for meaningful comparison of growth inhibition between the different Colletotrichum species, test compounds, and replication of experiments in time. Conidia of each fungal species were harvested from 7–10-day-old cultures by flooding plates with 5 mL of sterile distilled water and dislodging conidia by softly brushing the colonies with an L-shaped glass rod. Conidial suspensions were filtered through sterile Miracloth (Calbiochem-Novabiochem Corp., La Jolla CA) to remove mycelia. Conidia concentrations were determined photometrically (Espinel-Ingroff and Kerkering 1991; Wedge and Kuhajek 1998) from a standard curve based on the percent of transmittance (%T) at 625 nm and using manual hemocytometer counts. Conidial stock suspensions were adjusted with sterile distilled water to a concentration of 1.0 × 106 conidia mL−1.
Bioautography technique
Bioautography provides a simple technique to visually follow antifungal components through the separation process. Matrix, one-dimensional, and two-dimensional bioautography protocols on silica gel thin-layer chromatography (TLC) plates using Colletotrichum spp. as the test organisms were used to identify the antifungal activity according to published bioautography methods (Homans and Fuchs 1970; Moore 1996; Wedge and Nagle 2000). Matrix bioautography was used to screen total amounts of the crude extract (10 and 100 μg).
Each was sprayed with a spore suspension (105 spores mL−1) of the fungus of interest and incubated in a moisture chamber at 26 °C with a 12-h photoperiod. Inhibition of fungal growth was evaluated at 3–4 days after treatment. Clear zones, devoid of fungal growth on the TLC plate, indicate the presence of the constituents of antifungal metabolites in each TLC plate (Vincent et al. 1999; Tellez et al. 2000).
Bioassay-directed purification and identification of TCA from the ethyl acetate extract of P. luminescens culture media
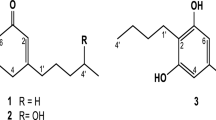
A 680-mg aliquot of the ethyl acetate extract was dissolved in chloroform. The chloroform extractables were separated using a Biotage Silica, 100 g, 40 + M cartridge (40–63 μm, 60 Å, 40 × 150 mm) running at 32 mL min−1 using a hexane:ethyl acetate (EtOAc) gradient beginning with 100:0 to 50:50 over 1,728 mL followed by 50:50 to 0:100 over 576 mL, and finishing with a 500 mL methanol wash. Portions of 22-mL volume were collected in 16 × 150 mm test tubes. Seven fractions (A to G) were generated on the basis of TLC similarities, and re-evaluation of activity was determined using bioautography. The most active fraction E was subjected to further purification using a reversed-phase C-8 HPLC column (Zorbax, 9.4 mm × 250 mm, 5 μm) running a linear gradient from 50/50 (H2O/methanol both containing 0.25 % trifluoroacetic acid) to 28/72 (H2O/methanol both containing 0.25 % trifluoroacetic acid) providing a pure extract of the metabolite of interest. The metabolite of interest was identified as trans-cinnamic acid (TCA; Fig. 1). As described in the previous section, matrix bioautography was used to screen toxicity of total amounts of TCA (10 and 100 μg).
Analytic instrumentation used in identification
Identification of TCA was confirmed using 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and a comparison of chemical shift data with commercially available TCA (Acros, Sigma-Aldrich, St. Louis, MO). 1H and 13C NMR spectra were recorded on a Varian Unity Inova AS600 spectrometer (Varian, Palo Alto, CA). Electron impact mass spectrometry spectra were recorded on a Varian CP-3800 gas chromatograph coupled to a Varian Saturn 2000 mass spectrometer (Varian, Inc., Palo Alto, CA). Column chromatography was performed using a Biotage Isolera One Flash Purification System (Biotage, Uppsala, Sweden). High-performance liquid chromatography (HPLC) was performed using an Agilent 1200 HPLC system (Agilent, Palo Alto, CA).
In vitro tests of TCA toxicity against F. effusum
Agar-based media tests
Two different Petri plate tests were undertaken. The first was a zone of inhibition test (Shapiro-Ilan et al. 2009). The conidial suspension of F. effusum was uniformly spread over the surface of PDA on a Petri plate, and a 1-cm-diameter filter paper disk, into which 20 μL acetone containing TCA (99 % purity, Sigma, St. Louis, MO) with 20, 200, and 2000 μg had been pipetted, was placed in the center of the plate. In addition, there was a water control and a treatment of crude metabolite (2,000 μg) dissolved in acetone. Control plates received filter paper disks with only the acetone. The treatments and control were replicated four times, and the experiment repeated twice. After 6 and 10 days, the zone of inhibition was measured taking the average of the two diameters perpendicular to each other.
In the second series of experiments, the TCA was incorporated into the PDA at 0, 74, 148, 741, and 1482 μg mL−1. The TCA was dissolved in ethanol, with 5.8 mL of ethanol added per 500 mL of PDA media. Control treatments included PDA with no amendment and PDA amended with ethanol alone. A well in the center of the agar in the plate was made using a transfer tube to remove a plug of agar. The conidia suspension (0.1 mL) of F. effusum was added to the well in the center of the agar. The diameter of the resulting colony was measured (mm) at 7, 14, and 21 days after inoculating the plate. The experiment was repeated once. There were three replicate plates of each treatment in each experiment.
Liquid culture tests
Erlenmeyer flasks containing 50 mL potato dextrose broth were amended with TCA at 0, 64, 127, 635, and 1270 μg mL−1, and 0.1 μL of F. effusum conidia suspension was added to each flask. The TCA was dissolved in ethanol, with 0.58 mL ethanol being added to each flask to obtain the required concentrations. In addition to the TCA treatments, there was a nontreated control and a control with only ethanol. After 21 days, the experiment was terminated, and the cultures were vacuum filtered through No. 4 filter paper (Whatman, Maidstone, UK). The filter paper was dried in an oven at 70 °C for 24 h before vacuum filtering the culture broth. After vacuum filtering, the filter paper was again dried in an oven for 24 h at 70 °C and re-weighed.
Data analysis
All data from the zone of inhibition tests and the concentration tests in solid and liquid media were analyzed to determine treatment effects using an analysis of variance. Where a significant effect (α = 0.05) was found, the differences between the means were explored using the Student–Newman–Keul’s test (P = 0.05). All data were analyzed using SAS software (SAS Institute Inc., Cary, NC).
Results
The ethyl acetate crude extract of P. luminescens culture medium was fungistatic to two of the three species of Colletotrichum using the fungal bioautography inhibition screening plate technique (Table 1). Only C. acutatum showed no zone of inhibition at either amount tested. At the lower amount (10 μg), the extract was fungistatic to only C. gloeosporioides, but at the higher amount (100 μg), it was fungistatic to both C. gloeosporioides and C. fragariae.
One of the biologically active compounds was identified as TCA. The fungal bioautography inhibition screening plates test showed that TCA prevented growth of all three species of Colletotrichum at both 10 and 100 μg (Table 2). The greater the quantity of TCA on the plate, the larger the zone of inhibition, with a 4–5-fold increase in the diameter of the inhibition zone at 100 μg as compared to 10 μg.
There was no effect of the solvent (acetone) or water alone on F. effusum, but there was a significant effect (F value = 166, df = 5, 64, P < 0.0001 for the 6-day assessment and F value = 139, df = 5, 63, P < 0.0001 for the 10-day assessment) on growth of F. effusum on Petri-plates of PDA when filter paper disks infused with TCA were placed in the center of the plate (Fig. 2a, b). The characteristics of the zone of inhibition were similar when measured at 6 or 10 days after plate inoculation, and the Student–Newman–Keul’s means separation confirmed that concentrations of TCA ≥0.2 mg were toxic to F. effusum in these zone of inhibition tests. At day 10, the crude extract (2,000 μg) produced a zone of inhibition, a third that of the TCA at 2,000 μg, and threefold that of the TCA at 200 μg.
The inhibition of growth of Fusicladium effusum on potato dextrose agar due to trans-cinnamic acid (TCA) at different concentrations. Filter paper disks infused with the TCA solution were placed in the center of a Petri plate on which a spore suspension of conidia of F. effusum was spread. The zone of inhibition was measured after 6 (a) and 10 (b) days. Standard error bars are indicated. Means with different letters are significantly different according to Student–Newman–Keul’s means separation (P = 0.05)
On PDA amended with different concentrations of TCA, there was inhibition of growth of F. effusum (Fig. 3a–c). At 7 days after seeding the plate, no growth of F. effusum could be seen on the amended plates, although there was appreciable growth on the control plates and on those amended with the solvent, ethanol (F value = 706, df = 5, 24, P value = <0.0001). By 14 days after seeding, growth was measurable on both the 74 and 148 μg mL−1 concentrations of TCA, but less than the controls, and none on plates amended with TCA at concentrations >148 μg mL−1 (F value = 240, df = 5, 24, P value = <0.0001). By 21 days after seeding, the growth on the 74 and 148 μg mL−1 concentrations of TCA had increased, but there was no evidence of growth on any plates with concentrations of TCA >148 μg mL−1 (F value = 358, df = 5, 24, P value = <0.0001). Nonetheless, the TCA at concentrations of 74 and 148 μg mL−1 were significantly less compared with the control on all days.
In PDB liquid culture, there was no growth of F. effusum at any of the concentrations of TCA tested (Fig. 4). There was no difference between the control and the ethanol solvent used to dissolve the TCA (F value = 64, df = 5, 18, P value = <0.0001), and the lowest concentration of TCA (64 μg mL−1) was completely inhibitory to growth of the fungus.
Effect of trans-cinnamic acid (TCA) concentration on the dry weight of mycelium of Fusicladium effusum grown in potato dextrose broth amended with different concentrations of TCA after 21 days incubation. Means with different letters are significantly different according to Student–Newman–Keul’s means separation (P = 0.05)
Discussion
A bioactive ingredient of extracts of P. luminescens was found to be TCA, a small molecule compound which is known to have antibiotic properties (Si et al. 2006; Wong et al. 2008; Chen et al. 2011; Hakkim et al. 2012), hence its use as a possible preservative in food (for which patents exists [e.g., US patent doc numbers 6036986; 6042861]). Furthermore, TCA has previously been identified as a necessary precursor in the biosynthesis of the antibiotic stilbene in Photorhabdus (Williams et al. 2005; Eleftherianos et al. 2007; Chalabaev et al. 2008). The results here demonstrate antimycotic activity of TCA against at least two genera of important fungal plant pathogens (Colletotrichum and Fusicladium), the latter being the major disease of pecan in the southeastern USA.
The antimicrobial activity of metabolites produced by Photorhabdus spp. has been demonstrated before (Paul et al. 1981; Isaacson and Webster 2002; Webster et al. 2002), and the antimycotic activity was characterized as being based on exo- and endochitinases, as well as other proteinaceous moieties and small molecules. In this study, we discovered that, in P. luminescens, one of these small molecules is TCA, and the preliminary in vitro studies using fungal bioautography inhibition screening showed that TCA produced by P. luminescens was active at concentrations of ~100 μg mL−1 against both C. fragariae and C. gloeosporioides. However, C. acutatum was not sensitive at any of the concentrations tested, and the reason for this is unknown, but warrants further testing with more fungal pathogens.
Based on these observations, it would appear that the level of toxicity of raw extracts from P. luminescens was probably in large part due to the TCA component within, although other factors in the extract might also be involved in the antimicrobial activity (Paul et al. 1981; McInerney et al. 1991; Shapiro-Ilan et al. 2009). Other components of the extracts that are antimycotic remain to be identified (indeed other components were also antimycotic, but did not have as strong an antimicrobial effect and were not identified—data not presented).
The potency of TCA as an antimycotic is comparable with other observations of the effect of TCA on other microbes including human pathogens (Si et al. 2006; Hakkim et al. 2012), where concentrations in the range of 100–500 μg mL−1 provided a maximum zone of inhibition. TCA extracted from Serrano chili was previously found to be an antibiotic to bacterial plant pathogens at concentrations of 500 μg mL−1 (Acero-Ortega et al. 2003). The various solid and liquid media experiments in this study demonstrate that TCA is antimycotic over a range of concentrations similar to those previously reported, although sensitivity appeared to be a little lower, in the 50–100+ μg mL−1 range. The reason for the liquid culture assay resulting in greater antimycotic activity is not known, but no growth was observed in this test, even at TCA concentration of 64 μg mL−1. A greater sensitivity in liquid cultures similar to that in certain antifungal molecules has been noted before (Azevedo and Cassio 2010; Shreaz et al. 2011).
Interestingly, TCA is an important compound in the induced resistance response in plants (Métraux 2002), although it is not known if it can induce resistance per se. It can also build up in soils as an allelopathic compound released from plant roots, and cause autotoxicity and increased root disease (Chen et al. 2011), yet exogenously applied TCA can also reduce the effects of drought stress in some plant species (Ye et al. 2004, 2006; Sun et al. 2012). Thus, it has diverse effects on plant hosts and pathogens, and application of TCA to protect plants from disease might have some unexpected consequences.
If TCA (or other antimycotic metabolites from these bacteria) is to be considered as a potential biorational plant protection chemical, it will be necessary to address several other issues. In particular, further in vitro and in vivo laboratory, greenhouse, and field tests on the efficacy of pathogen and disease suppression need to be established, possible phytotoxicity issues will need to be ascertained, and for any practical formulations, field longevity and environmental impact characterized. Finally, there is the cost of production and application, which would need to be considered before utilizing TCA, or any other antimycotic from bacteria as a potential biorational control agent for pecan scab or other crop diseases.
References
Acero-Ortega C, Dorantes-Alvarez L, Jaramillo-Flores ME, Hernandez-Sanchez H, Lopez-Malo A (2003) Effect of Chilli (Capsicum annum L.) extracts and derived compounds on growth of Erwinia carotovora subsp. carotovora (Jones) Bergey, Harrison, Breed Hammer and Huntoon. Rev Mex Fitopatol 21:233–236
Akhurst RJ (1982) Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol 128:3061–3065
Azevedo MM, Cassio F (2010) Effects of metals on growth and sporulation of aquatic fungi. Drug Chem Toxicol 33:269–278
Boeszoermenyi E, Ersek T, Fodor A, Fodor AM, Foeldes LSz, Hevesi M, Hogan JS, Katona Z, Klein MG, Kormany A, Pekar S, Szentirmai A, Sztaricskai F, Taylor RAJ (2009) Isolation and activity of Xenorhabdus antimicrobial compounds against the plant pathogens Erwinia amylovora and Phytophthora nicotianae. J Appl Microbiol 107:746–759
Brenneman TB, Bertrand PF, Mullinix B (1998) Spray advisories for pecan scab: recent developments in Georgia. In: The pecan industry: current situation and future challenges, 3rd national pecan workshop proceedings, pp 7–13
Chalabaev S, Turlin E, Bay S, Ganneau C, Brito-Fravallo E, Charles J-F, Danchin A, Biville F (2008) Cinnamic acid, an autoinducer of its own biosynthesis, is processed via hca enzymes in Photorhabdus luminescens. Appl Environ Microbiol 74:1717–1725
Chen G, Dunphy G, Webster J (1994) Antifungal activity of two Xenorhabdus species and Photorhabdus luminescens bacteria associated with the nematodes Steinernema species and Heterorhabditis megidis. Biol Control 4:157–162
Chen YL, Huang ST, Sun FM, Chiang YL, Chiang CJ, Tsai CM, Weng CJ (2011) Transformation of cinnamic acid from trans- to cis-form raises a notable bactericidal and synergistic activity against multiple-drug resistant Mycobacterium tuberculosis. Eur J Pharm Sci 43:188–194
Cole RJ, Gossard AC (1956) Stuart pecan found to be susceptible to scab in Mississippi. Plant Dis Rep 40:156
Eleftherianos I, Boundy S, Joyce S, Aslam S, Marshall J, Cox R, Simpson T, Clarke D, French-Constant R, Reynolds S (2007) An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc Natl Acad Sci USA 104:2419–2424
Espinel-Ingroff A, Kerkering TM (1991) Spectrophotometric method of inoculum preparation for the in vitro susceptibility testing of filamentous fungi. J Clin Microbiol 29:393–394
Fang XL, Li ZZ, Wang YH, Zhang X (2011) In vitro and in vivo antimicrobial activity of Xenorhabdus bovienii YL002 against Phytophthora capsici and Botrytis cinerea. J Appl Microbiol 111:145–154
Gaugler R (ed) (2002) Entomopathogenic nematology. CABI Publishing, Wallingford
Goff WD, McVay JR, Gazaway WS (1996) Pecan production in the southeast. Alabama Cooperative Extension System Circular ANR-459, University, Auburn, p 222
Grewal PS, Ehlers R-U, Shapiro-Ilan DI (eds) (2005) Nematodes as biocontrol agents. CABI Publishing, Wallingford
Hakkim FL, Mathiraj, Essa MM, Arivazhagan G, Guizani N, Song H (2012) Evaluation of food protective property of five natural products using fresh-cut apple slice model. Pak J Biol Sci 15:10–18
Homans AL, Fuchs A (1970) Direct bioautography on thin-layer chromatograms as a method for detecting fungitoxic substances. J Chromatogr 51(2):327–329
Isaacson PJ, Webster JM (2002) Antimicrobial activity of Xenorhabdus sp. Rio Enterobacteriaceae) symbiont of the entomopathogenic nematode Steinernema riobrave (Rhabditidae: Steinernematidae). J Invertebr Pathol 79:146–153
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Annu Rev Entomol 38:181–206
Kaya HK, Stock SP (1997) Techniques in nematology. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, San Diego, pp 281–324
Li J, Chen G, Webster JM (1995) Antimicrobial metabolites from a bacterial symbiont. J Nat Prod 58:1081–1086
McInerney BV, Gregson RP, Lacey MJ, Akhurst RJ, Lyons GR, Rhodes SH, Smith DR, Engelhardt LM, White AH (1991) Biologically active metabolites from Xenorhabdus spp. Part 1. Dithiolopyrrolone derivatives with antibiotic activity. J Nat Prod 54:774–784
Métraux J-P (2002) Recent breakthroughs in the study of salicylic acid biosynthesis. Trends Plant Sci 7:332–334
Moore RE (1996) Cyclic peptides and depsipeptides from cyanobacteria: a review. J Ind Microbiol 16:134–143
National Research Council (1989) Alternative agriculture. National Academy Press, Washington, DC
Ng KK, Webster JM (1997) Antimycotic activity of Xenorhabdus bovienii (Enterobacteriaceae) metabolites against Phytophthora infestans on potato plants. Can J Plant Pathol 19:125–132
Paul VJ, Frautschy S, Fenical W, Nealson KH (1981) Antibiotics in microbial ecology. J Chem Ecol 7:589–597
Reynolds KL, Brenneman TB, Bertrand PF (1997) Sensitivity of Cladosporium caryigenum to propiconazole and febuconazole. Plant Dis 81:163–166
San-Blas E, Carrillo Z, Parra Y (2012) Effect of Xenorhabdus and Photorhabdus bacteria and their exudates on Moniliophthora roreri. Arch Phytopathol Plant Prot 45:1950–1967
Seyran M, Brenneman TB, Stevenson KL (2010) A rapid method to monitor fungicide sensitivity in the pecan scab pathogen, Fusicladium effusum. Crop Prot 29:1257–1263
Shapiro-Ilan DI, Reilly CC, Hotchkiss MW (2009) Suppressive effects of metabolites from Photorhabdus and Xenorhabdus spp. on phytopathogens of peach and pecan. Arch Phytopathol Plant Prot 42:715–728
Shreaz S, Sheikh RA, Bhatia R, Neelofar K, Imran S, Hashmi AA, Manzoor N, Basir SF, Khan LA (2011) Antifungal activity of a-methyl transcinnamaldehyde, its ligand and metal complexes: promising growth and ergosterol inhibitors. Biometals 24:923–933
Si W, Gong J, Tsao R, Zhou T, Yu H, Poppe C, Johnson R, Du Z (2006) Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J Appl Microbiol 100:296–305
Stevenson KL (1999) Fungicide resistance management in pecans. In: McCraw BE, Dean EH, Wood BW (eds) The pecan industry: current situation and future challenges, third national pecan workshop proceedings, USDA Agricultural Research Service, 1998–2004, pp 1–6
Sun W-J, Nie Y-X, Gao Y, Dai A-H, Bai J-G (2012) Exogenous cinnamic acid regulates antioxidant enzyme activity and reduces lipid peroxidation in drought stressed cucumber. Acta Physiol Plant 34:641–655
Tellez MR, Dayan FE, Schrader KK, Wedge DE, Duke SO (2000) Composition and some biological activities of the essential oil of Callicarpa americana (L.). J Agric Food Chem 48:3008–3012
Teviotdale BL, Michailides TJ, Pscheidt JW (eds) (2002) Compendium of nut crop diseases in temperate zones. The American Phytopathological Society, St. Paul
Vincent A, Dayan FE, Maas JL, Wedge DE (1999) Detection and isolation of antifungal compounds in strawberry inhibitory to Colletotrichum fragariae. Adv Strawb Res 18:28–36
Webster JM, Chen G, Li J (1995) Novel fungicidal properties of metabolites, culture broth, stilbene derivatives and indole derivatives produced by the bacteria Xenorhabdus and Photorhabdus spp. Patent no. WO9503695
Webster JM, Chen G, Hu K, Li J (2002) Bacterial metabolites. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 99–114
Wedge DE, Kuhajek JM (1998) A microbioassay for fungicide discovery. SAAS Bull Biochem Biotechnol 11:1–7
Wedge DE, Nagle DG (2000) A new 2D-TLC bioautography method for the discovery of novel antifungal agents to control plant pathogens. J Nat Prod 63:1050–1054
Williams JS, Thomas M, Clarke DJ (2005) The gene stlA encodes a phenylalanine ammonia-lyase that is involved in the production of a stilbene antibiotic in Photorhabdus luminescens TT01. Microbiology 151:2543–2550
Wong SY, Grant IR, Friedman M, Elliott CT, Situ C (2008) Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl Environ Microbiol 74:5986–5990
Ye SF, Yu JQ, Peng YH, Zheng JH, Zou LY (2004) Incidence of Fusarium wilt in Cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates. Plant Soil 263:143–150
Ye SF, Zhou YH, Sun Y, Zou LY, Yu JQ (2006) Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ Exp Bot 56:255–262
Acknowledgments
The authors appreciate the technical assistance of Stacy Byrd, Kathy Halat, Jatayah Sheed, Stephanie de Vos, and Peir Wangnar, with regard to helping with the assays and recording data. The authors thank the Georgia Agricultural Commodity Commission for the partial funding of the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Brownbridge
Rights and permissions
About this article
Cite this article
Bock, C.H., Shapiro-Ilan, D.I., Wedge, D.E. et al. Identification of the antifungal compound, trans-cinnamic acid, produced by Photorhabdus luminescens, a potential biopesticide against pecan scab. J Pest Sci 87, 155–162 (2014). https://doi.org/10.1007/s10340-013-0519-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-013-0519-5