Abstract
Throughout South America, the lonchaeid flies Dasiops spp. are important herbivores of passionfruit crops. However, little is known on the biology and ecology of these insects, resulting in inadequate pest management schemes. In this study, we describe Dasiops inedulis population dynamics in Colombian sweet passionfruit (SP; Passiflora ligularis Juss.) and elucidate biotic mortality factors at different fly developmental stages. From August 2009 to July 2010, D. inedulis and Dasiops spp. abundance was assessed through monthly McPhail bait trapping and collection of SP flower buds, flowers, and immature fruits. Mortality levels of D. inedulis were determined for early instar larvae by ovary dissection and for late-instar larvae or pupae by prey removal trials. Maximum infestation reached 80 % in fruits and flower buds, and bud infestation correlated with precipitation during the previous month. Two days after oviposition, 8.2 ± 2.3 (mean ± SD) Dasiops sp. eggs were found in SP ovaries and 4.4 ± 1.2 late-instar larvae were recovered from immature fruits at day 14. Upon larval drop on the orchard soil, 74.8 % larvae burrowed within the soil within 9 min, while 12.5 % larvae were attacked by ants. In-field mortality of young pupae amounted to 75.3 ± 7.0 %, with vertebrate predators likely causing 12.1 ± 6.0 % mortality. Late-instar larvae and pupae appear highly vulnerable to natural enemy action, with the ground-foraging predator community mainly composed of ants (80.37 %) and ground beetles (9.17 %). Our findings should help develop integrated pest management (IPM) tactics for SP crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In several parts of the Neotropics, different members of the genus Passiflora have gotten domesticated over time and their fruit is gradually finding its way into mainstream markets, with current world annual production levels amounting to >500,000 metric tons (Ocampo 2007). Sweet passionfruit (SP; Passiflora ligularis Juss.) is cultivated year-round by small-scale farmers in Colombia, Venezuela, and Ecuador (Asturizaga et al. 2006). Despite the importance of this crop in many rural areas, little or no research has been conducted on biology, ecology, and control of arthropod pests. Lack of reliable information on those aspects is actually reflected in rampant use of insecticides, with the majority of SP growers relying on weekly or bi-weekly insecticide sprays (Wyckhuys et al. 2011). Aside from constituting a substantial cost component for many resource-poor farmers, these practices are likely to affect the environment, consumer and farmer health alike.
A key herbivore in Colombian SP crops is Dasiops inedulis Steyskal, 1980 (Diptera: Lonchaeidae), while orchards are also occasionally infested by other lance flies such as Dasiops curubae, D. gracilis, D. yepezi, D. dentatus, and Neosilba batesi (Wyckhuys et al. 2012). All species severely impede the development of flower buds, flowers and fruits and cause their abortion due to their consumption of internal structures (Norrbom and McAlpine 1997). The species complex is dominated by D. inedulis, a fly that attacks SP buds, flowers, and immature fruits alike (Santos et al. 2009; Wyckhuys et al. 2012). Yield losses due to Dasiops spp. are thought to amount to 20–65 %, although economic studies wait to be conducted to confirm such figure (Armbrecht et al. 1986). As the pest spends a fair share of its life cycle within a fruit with hard endocarp, it is widely thought to be little susceptible to the action of natural enemies. However, only limited research has been conducted on biotic mortality factors of Dasiops spp. (Armbrecht et al. 1986; Uchôa-Fernandes et al. 2003; Aguiar-Menezes et al. 2004) and preliminary information exists regarding the role of biological control in SP crops (Santos et al. 2009).
For the superfamily Tephritoidea, which include lance flies, biological control research has mainly focused on parasitoids of fly larvae within the host fruit (Ovruski et al. 2000; Garcia and Ricalde 2013). Nevertheless, researchers are increasingly recognizing the role of natural enemies that act against exposed stages, such as adults, late-instar larvae, and pupae (Thomas 1993; Aluja et al. 2005; Orsini et al. 2007). Soil-foraging predators such as ants, staphylinid beetles, and spiders are important for regulating fruit fly densities (Bateman 1972), and inflict mortality on both fly pupae, teneral adults, and late-instar larvae (Wong and Wong 1988; Hodgson et al. 1998; Urbaneja et al. 2006; El Keroumi et al. 2010). For the Mexican fruit fly Anastrepha ludens, mortality due to epigeal predators can be as high as 94 % (Thomas 1995), while foliage-dwelling predators such as jumping spiders or vespids attack adult stages of several tephritoid flies (Hendrichs et al. 1994; Kaspi 2000; Wee and Tan 2005). Population dynamics of Tephritoidea is also greatly affected by abiotic factors, such as temperature or humidity (e.g., Aluja et al. 2005; Hulthen and Clarke 2006; Vayssieres et al. 2009). For Dasiops spp. in SP crops, predators of pupae, adults, or late-instar larvae still wait to be identified and no information is available on the abiotic mortality factors that impact this pest.
A comprehensive assessment of biotic and abiotic mortality factors of an insect pest in its respective cropping system should underpin rational pest management schemes (Van Driesche and Bellows 1996). In this study, we describe D. inedulis population fluctuations in small-scale SP crops in the Colombian Eastern highlands, and relate those with crop phenology and climatic conditions. In addition, we use periodic host dissection to determine the rates of mortality of egg and larval stages, and draw upon exclusion assays and direct observation to estimate the role of natural enemies in D. inedulis control.
Materials and methods
Research was conducted in eight, randomly selected SP orchards within the municipality of Buena Vista (Boyacá, Colombia) (05°29′46N, 73°57′22W), at an altitude of 1,950–2,050 m above sea level. Field experiments were carried out from August 2009 to July 2010. Location and altitude of each orchard was recorded using a hand-held GPS unit (Garmin Etrex Vista Hcx, Bogotá, Colombia). Orchards were 2–4-years old and 1.5 ± 0.1 ha (mean ± SD) in size and were embedded within agro-landscapes composed of small-scale production of coffee, green bean, peas, pasture, sugarcane, and purple passionfruit. Planting density within the distinct orchards was identical, with separate plants established at 2.5 m distances. All SP varieties included in the study were selected and improved by local farmers. Orchards were exclusively managed by their owners using regional management practices. Orchard management basically comprised fertilization (5/year), pruning (3/year), and weeding (1/month). Although research was done in commercial orchards with comparatively low insecticide use, farmers did occasionally treat their crops for insect pests or plant diseases during the course of the experiment.
Population fluctuations of Dasiops sp.
On a monthly basis, we randomly collected 20 immature fruits and 30 flower buds and 20 flowers in each orchard. Samples were directly picked from the plant, and subsequently placed for 48 h in 7-cm high and two-diameter plastic cups to allow further larval development and (eventual) pupal formation. A slightly moistened paper towel was placed at the bottom of each cup, and cups were closed with a fine mesh. After the allotted time, we dissected each of the collected organs and determined the number of D. inedulis larvae per infested organ. Larvae were subsequently transferred to 4.5-cm diameter and 1.5-cm high ventilated Petri dishes with moistened vermiculite to allow pupation. Petri dishes and plastic cups were kept at 21 °C, 66 % RH and 12:12 L:D, until adult emergence (adapted from Uchôa-Fernandes and Zucchi 1999). We determined the number of larvae per organ, and computed infestation levels as the number of a given organ infested by at least one D. inedulis larva. For each sampling event and orchard, percent infestation was calculated by dividing the number of infested organs by the total number collected.
Every month, we randomly positioned two McPhail traps baited with hydrolyzed protein (Agrobiologicos-Safer, Medellín, Colombia) within each orchard. Traps were deployed for 15 days/month. After this time, traps were removed and the number of D. inedulis adults was counted. For each orchard and month, the average number of D. inedulis adults per trap and crop was computed. As distinct Dasiops spp. are easily confused by non-expert taxonomists, a (small) subset of fly specimens was sent for identification to Korytkowski, University of Panama, and voucher specimens were kept in the Museum Francisco Luis Gallego of the Universidad Nacional de Colombia, Medellín. The phenological stage of each crop was determined using a 1 × 1 m quadrant in which the number of flowers, flower buds, and immature fruit was counted. This procedure was repeated five times per sampling event and orchard.
During all field trials, temperature and humidity data were collected with iLog data loggers (Escort, Virginia, US), placed within an upturned white plastic cup as to avoid effect of direct sunlight. Rainfall data were obtained through a local weather station. Infestation levels and trap captures were related to rainfall and crop phenology measures of the current and previous month.
Assessment of in-field mortality factors
This research component consisted of four different sections, designed to quantify D. inedulis mortality under field conditions during each one of its developmental stages. Research activities were developed, based upon particularities of the D. inedulis life cycle (Armbrecht et al. 1986), with duration of the egg stage being 2–3 days, larval stage 4–9 days, pupal stage 10–17 days, and adult longevity 2–9 days. Eggs and early instar larvae complete their development within the developing SP flower buds, ovaries, and fruit, while late-instar larvae and pupae generally develop within the orchard soil.
First, we measured mortality rates of eggs and early instar larvae within developing SP ovaries and fruits. Within each orchard, we recorded Dasiops sp. oviposition events in SP flower ovaries and marked infested ovaries by attaching colored tape to the flower peduncle. Marked ovaries and subsequent fruits were collected at selected times past oviposition and taken to the laboratory for dissection. We dissected a share of initially marked ovaries at each of the following times: 2, 4, 6, 8, 12, and 14 days past oviposition. Upon dissection, we recorded the total number of live eggs or larvae within the organ. A minimum of 20 organs were dissected at each of the dissection dates.
Second, we measured mortality of late-instar larvae upon exiting SP organs. We collected fully developed larvae from naturally infested fruits in orchards that were not included in the experiment. Larvae were kept with fruit pulp within a sealed Petri dish with moistened filter paper and were used within 1–5 h of their collection. Larvae were not kept for later identification, but were assumed to be identical to species emerging from fruit samples (see above). We mimicked natural larval exit from the fruit by dropping a single larva each time from the same height as infested fruits (i.e., 1.7–2.0 m), with five repetitions per crop each 2 months. Upon arrival on the soil surface, we observed larval behavior and fate over the course of 40 min. We recorded the following behaviors: distance moved on soil surface, successful entry into the soil, death from climatic exposure, interaction with predators or parasitoids, and outcome of these interactions (see Aluja et al. 2005).
Third, we assessed in-field mortality of late-instar D. inedulis larvae or recently formed pupae on the soil surface. We collected fully developed larvae from naturally infested fruits and kept those within a sealed Petri dish with moistened filter paper until use in the assay. Within each orchard, we randomly deployed four traps, with each trap consisting of a 0.5-m2 wide and 5-cm deep excavation covered with a perforated plastic sheet. On top of the sheet, we placed a 3-cm thick layer of sieved soil (1.5-mm sieve opening). A total of ten larvae were placed on the loose soil and allowed to naturally submerge in the substrate. Traps were mounted every 2 months in each of the orchards. Of the four traps, two were covered with a 15-cm high screen mesh to exclude vertebrate predators, while the remaining two traps were left unprotected. After 48 h, we sieved the soil of each trap and counted the total number of D. inedulis larvae or pupae, number and identity of predators and noted the presence of pupae with eventual predation marks.
Fourth, we determined in-field mortality of D. inedulis pupae due to action of parasitoids. We collected fully developed larvae from naturally infested fruits, placed those within moist vermiculite and allowed them to pupate. One- to five-day-old pupae were used for further experiments. Within each orchard, we randomly deployed two traps, consisting of a 4.5-cm diameter, 1.5-cm high plastic Petri dish with moistened vermiculite, petroleum jelly applied to the outer walls to prevent entry of certain predators, such as ants, spiders, or ground beetles. In addition, Petri dishes were covered with a mesh screen (see above). Within each Petri dish, we placed 20 D. inedulis pupae within a vermiculite layer and covered these with 2–3 fallen SP flower buds (adapted from Guillén et al. 2002). After 48 h, we collected the pupae from each trap, placed them in vermiculite within 132-cm3 ventilated plastic cups and kept them at 19 °C, 64 % RH and 12:12 L:D at a field laboratory. Pupae were inspected on a daily basis for parasitoid emergence, until all pupae had successfully enclosed, for a maximum time of 45 days; twice the time it takes for adult or parasitoid emergence. Adults of D. inedulis were kept alive for 2–3 days to achieve their full coloration, and were subsequently killed and preserved in 70 % ethanol for further identification (Aguiar-Menezes et al. 2002). All the above assays were performed every 2 months for a total of six sampling events during the course of the study.
Identity of foliage- and ground-foraging predators
The foliage- and ground-foraging arthropod predator community in SP orchards was characterized and predation events on D. inedulis adults recorded. For the assessment of foliage predators, we randomly selected five 20 × 1 m transects within each orchard were randomly selected. Each transect walk was covered over a total time of 20 min, during which we visually recorded abundance and identity of predators. We also noted eventual predation events on D. inedulis adults, recording predator identity and fate of its prey. All predators were collected and voucher specimens deposited in the Museum Francisco Luis Gallego of the Universidad Nacional de Colombia, Medellín. All observations were done between 800 and 1,700 h.
To complement our assessment of late-instar larval and pupal mortality, pitfall trapping was conducted to assess the ground-foraging predator complex in SP orchards. More specifically, two pitfall traps were randomly positioned, consisting of a 250-cm3 beaker with a solution of water with detergent, covered with a 30 × 30 cm tile as protection against rain. After 48 h, traps were recovered and predators were collected and preserved in 70 % ethanol for future identification. In addition, the ground-foraging ant community was sampled using tuna fish bait. At two random locations within each orchard, 2 × 2 cm tuna pieces were deployed on a 10 × 15 cm piece of filter paper. After 30 min, all ants on or within 10 cm of bait samples were collected and preserved in 70 % alcohol for further identification (Wyckhuys and O’Neil 2006). Transect walks, tuna fish baiting, and pitfall trapping were conducted within each orchard on a 2-month basis.
Data analysis
Prior to analysis, all data were checked for homoscedasticity and normality, and data transformation was considered if needed. Both parametric and non-parametric tests were used in analyses; all of them are described in the text and tables below. For non-parametric analyses, Bonferroni corrections were used to account for multiple, pair-wise comparisons. To assess the effect of predator abundance on pest pressure, D. inedulis infestation level and adult abundance were compared between fields with low, medium, and high abundance of a given predator guild. All analyses were conducted using SPSS statistical software.
Results
Population fluctuations of D. inedulis
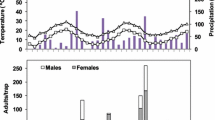
During the course of the experiment, we collected 2,670 flower buds, 937 flowers, and 1,288 fruits, from which 1,863 fly larvae emerged. In fruits, highest infestation levels were found in July and infestation was lowest in February, with respective levels of 55.00 ± 32.21 and 5.83 ± 9.17 % infested fruits (mean ± SD; Fig. 1). In flower buds, highest levels were recorded in July and lowest in March, at 22.50 ± 24.67 and 0 %, respectively. In flowers, D. inedulis infestation was only recorded during the month of September, with 2.50 ± 5.30 % infested organs. Infestation levels of flower buds and fruits significantly differed between months (repeated measures ANOVA, F 11,77 = 2.161, p = 0.025; F 11,54 = 3.315, p = 0.002, respectively). No D. inedulis larval parasitoids emerged from any of the collected flower buds, flowers, or fruits.
Average infestation levels (±SE) of flower buds and fruits, as related to Dasiops sp. adult abundance, in small-scale SP orchards in Buena Vista, Colombia during 2009–2010. Infestation levels are expressed as percentage of a given organ infested with at least one fly larva. Adult abundance is expressed as the number of fly adults captured in McPhail bait traps, over the course of 15 days, each month
No significant correlations were found between flower bud or fruit infestation levels, and the densities of flowers, buds, or fruit in a given orchard (Pearson’s, p > 0.05). However, flower bud and fruit infestation levels were highly correlated with flower density per orchard in the previous month (ρ = 0.316, p = 0.004; ρ = 0.371, p = 0.004, respectively). Also, the flower bud infestation level and the number of larvae per flower bud was negatively correlated with fruit density in the previous month (ρ = −0.257, p = 0.021; ρ = −0.257, p = 0.020, respectively). Flower bud infestation level was highly correlated with the amount of precipitation in the previous month (ρ = 0.709, p = 0.015).
For flower buds, an average of 0.22 ± 0.42 larvae was found per organ, while fruits were infested with 1.40 ± 1.71 larvae. Fruit infestation levels translated in 0.013 ± 0.016 larvae per gram. The average number of larvae per organ differed between months, to a marginal significant level for flower buds (F 11,77 = 1.803, p = 0.068) and significant degree for fruits (F 11,56 = 4.594, p < 0.001).
A total of 463 Dasiops sp. adults were captured in McPhail traps, with subsamples predominantly composed of D. inedulis. Adult abundance attained its highest level of 6.37 ± 6.13 flies per trap in May and lowest level of 1.00 ± 0.89 flies per trap in November (Fig. 1). Adult abundance marginally differed between months (repeated measures ANOVA, F 10,70 = 1.918, p = 0.057), and proved significantly different between orchards (F 7,70 = 3.185, p = 0.006). Adult abundance was not correlated with orchard-level density of any SP organ (Pearson’s, p > 0.05), and monthly Dasiops sp. abundance did neither correlate with current rainfall or precipitation levels during the previous month (Spearman Rank, p > 0.05). Finally, infestation level of SP fruits or flower buds was not correlated with current adult abundance or with abundance levels during the previous month (Spearman Rank, p > 0.05).
Rainfall levels affected SP phenology, and the flower bud density was correlated with precipitation during the previous month (Spearman Rank, ρ = 0.818, p = 0.002). Similarly, the number of fruits was negatively correlated with current rainfall and precipitation during the previous month (ρ = −0.608, p = 0.036; ρ = −0.773, p = 0.005, respectively).
Assessment of in-field mortality factors
During evaluation of mortality of early Dasiops sp. development stages, 724 individuals were found upon dissection of 45 infested floral ovaries and 83 immature fruits. Despite confirmed ovipositor intrusion, no fly eggs or larvae were found in a total of eight samples. Two days after oviposition, 8.24 ± 2.26 eggs were found per SP floral ovary. The number of immatures significantly differed between dissection times (ANOVA, F 5,117 = 7.432, p < 0.001), with an estimated survival rate of 53.5 % between 2-day-old eggs and 14-day-old larvae (Table 1).
For 239 D. inedulis larvae, we recorded fate after dropping on the orchard floor. Among those, 208 larvae successfully entered in the soil, 30 larvae were attacked by ants (i.e., Brachymyrmex sp., Pheidole biconstricta y Solenopsis sp.) and only one larva was killed by environmental exposure. Up to 74.8 % larvae entered the soil within the first 9 min (Fig. 2). Prior to soil entry, larvae moved for 2.35 ± 3.73 cm on the soil surface. For larvae that entered the soil, soil entry time was significantly higher in the superior temperature range (ANOVA, F 2,205 = 7.053, p = 0.001) and lower at highest humidity (F 2,205 = 4.958, p = 0.008). Soil entry time was 179.37 ± 165.89 s at the lowest temperatures (18–23 °C), while 380.87 ± 405.72 s in the highest temperature range (29–47 °C). Similarly, soil entry time was 349.71 ± 356.59 s in the lowest humidity range (<56 % RH) and 220.35 ± 211.12 s in the highest range (>65 %). In a similar way, soil entry time significantly differed between orchards (F 7,200 = 2.243, p = 0.032) and sampling events (F 5,202 = 2.708, p = 0.022). The frequency of larval entry in the soil significantly differed between 12 subsequent 3-min time slots (χ 2 = 689.20, p < 0.001), as did the frequency of larval attack by ants (χ 2 = 105.48, p < 0.001).
At times when larvae were attacked by ants, temperatures were marginally significantly lower than when larvae submerged in the soil (F 1,236 = 0.962, p = 0.068). The time until which larvae were attacked by ants (333.60 ± 144.71 s) did not differ from the time until entry in the soil (265.10 ± 270.09 s) (ANOVA, p > 0.05). Larvae attacked by ants moved significantly greater distances on the soil surface than those that entered the soil (F 1,236 = 18.745, p < 0.001). Finally, 52 % larval attack by ants occurred from the 6th to the 9th minute, with D. inedulis larvae attacked by workers of Brachymyrmex sp. and Solenopsis sp.
Third, larval and early pupal survival significantly differed between covered and uncovered traps (F 1,94 = 7.918, p = 0.006), with 24.69 ± 20.35 % larvae surviving over a 48-h period in uncovered traps, compared to 36.67 ± 21.35 % larvae in covered traps. At the orchard level, significant differences were equally found between covered and uncovered traps (Pair-wise t = 3.484, n = 95, p = 0.001). No differences were found in larval survival according to either trap type between sampling events or orchards (ANOVA, p > 0.05). For both trap types, we recorded Dailodontus sp. (Coleoptera: Carabidae), and the ants P. biconstricta and Solenopsis sp. associated with D. inedulis larvae and pupae.
Fourth, assessments of D. inedulis pupal mortality due to parasitism yielded no parasitoids, from a total of 240 pupae that were deployed in SP orchards.
Identity of foliage- and ground-foraging predators
We recorded 93 foliage-foraging predators, of which social wasps, lacewings, and spiders were the most abundant (Table 2). Foliage-foraging predators were found in only 12.08 % of transects. Visual observations confirmed predation of D. inedulis by Polistes sp., Protopolybia sp. (for fly larvae within flower buds), and the presence of D. inedulis adults caught within spider webs. Through pitfall trapping, a total of 1,003 predators were collected, with relatively large numbers of ground beetles and ants (Table 3). The ant community was diverse and abundant, composed of representatives of 10 different genera. Tuna fish baiting showed an equally abundant ant community, primarily composed of Pheidole and Solenopsis spp. (Table 4). Abundance of ground-foraging predators, as determined through pitfall trapping or tuna fish baiting, did not differ between orchards or sampling events (ANOVA, p > 0.05). No correlations were found among the number of predators in pitfall traps or tuna baits, and survival rates of D. inedulis late-instar larvae or pupae (Pearson’s, p > 0.05). Finally, survival rates of D. inedulis larvae or pupae did not differ among orchards with either the absence or presence of ants, ground beetles, or spiders, as evidenced through pitfall trapping (ANOVA, p > 0.05).
Discussion
Our research characterized D. inedulis population fluctuations in SP crops over a 1-year period, and related adult abundance and infestation levels to SP crop phenology and precipitation patterns. As the vast majority of subsamples were of D. inedulis, our results indicate ecological particularities of this species. Nevertheless, we need to indicate that samples from McPhail traps and SP fruits contained significant numbers of other species such as D. dentatus and D. gracilis. As similar findings have been made in other crops, such as dragonfruit, and trap captures tend to be relatively low, the use of McPhail traps for monitoring of Dasiops spp. has been questioned (Quintero et al. 2012).
In accordance with previous studies, D. inedulis attained particularly high infestation levels in SP flower buds and immature fruits (Wyckhuys et al. 2011, 2012). Infestation levels up to 80 % for both fruits and flower buds show that D. inedulis is a key herbivore in the system; impacting SP yields to great extent. Low D. inedulis larval recovery from SP flowers starkly contrasts with previous work, where infestation levels of 6–50 % had been reported for some orchards (Wyckhuys et al. 2011, 2012). Considering large variability in D. inedulis floral infestation among orchards, sampling events and regions in those studies, it is likely that SP flower infestation only occurs under particular conditions that were largely absent during the course of our study.
For many tephritoid flies, population dynamics are tightly linked to host fruit phenology and climatic conditions (Celedonio-Hurtado et al. 1995; Vayssieres et al. 2009). In our study, D. inedulis adult captures were not correlated with precipitation levels or density of susceptible SP organs. However, infestation levels were related to flower and fruit density in the previous month, and the degree of flower bud infestation was strongly associated with rainfall levels in the previous month. As rainfall triggers SP flower bud development, farmers could use such events as a simple predictor of future D. inedulis infestation and make pest management decisions accordingly.
Captures of adult D. inedulis in McPhail traps were relatively low, with a maximum of 35 flies caught per trap over a 15-day period. McPhail traps, baited with food-based attractants, are used to monitor several pestiferous tephritoid flies and subsequent adult fly captures can guide pest management (e.g., Epsky et al. 2011). Possibly, Dasiops sp. adults emerge sexually mature and forage to limited extent for protein sources. In our study, monthly captures of D. inedulis adults did not correlate with current or future infestation levels of flower buds or fruits. Such is possibly due to rapid development of D. inedulis egg and larval stages (Armbrecht et al. 1986), which took up till 14 days under local climatic conditions. Hence, adult abundance at any given time will not be reflected in larval infestation levels 1 month later, and McPhail trap readings are more useful to direct immediate pest management interventions than to predict future pest pressure or to establish intervention thresholds. On the other hand, research on lance fly behavior and mating systems could pave the way for more efficient trapping systems and the eventual development of attractants.
A sustained D. inedulis adult abundance throughout the sampling period, even at times with virtual absence of susceptible SP host organs (e.g., February–April; Table 5), may signal the presence of alternative host plants or crops. Purple passionfruit is widely grown in the study region and is an alternative host of Dasiops spp. such as D. inedulis, but its phenology is similarly linked to precipitation as SP. Certain Dasiops species attack flowers and fruits of wild and ornamental Passiflora spp. (Norrbom and McAlpine 1997), while others are also associated with other plant genera and families, such as dragonfruit or hog plums (Uchôa-Fernandes et al. 2002; Delgado et al. 2010; Garcia and Norrbom 2011). A thorough assessment of other D. inedulis host plants in local agro-landscapes can help advance regional pest management initiatives and provide the basis for trap crop systems, among others (e.g., Hokkanen 1991; Shelton and Badenes-Perez 2006; Lu et al. 2009).
Aside from SP host phenology, precipitation and the presence of alternative hosts, D. inedulis population abundance is likely determined by farmers’ use of insecticides. Farmers’ pest management practices in experimental orchards and surrounding SP and purple passionfruit crops possibly affected local D. inedulis population dynamics. Over 90 % of Colombian passionfruit growers rely on calendar-based insecticide applications, and SP growers apply pesticides on a 2–3 week frequency (Wyckhuys et al. 2011). As most Colombian horticulture producers use pesticide mixtures and apply products below recommended doses (Bojaca et al. 2010), it is not easy to estimate their effect on D. inedulis populations. Also, as the bulk of locally used insecticides have contact action and D. inedulis spend a large share of its life cycle within thick-skinned SP fruits or within the soil, their efficacy should be scrutinized.
More so, insecticide application frequency positively relates to D. inedulis fruit infestation level, eventually hinting that pesticide overuse triggers pest outbreaks in these crops (Wyckhuys et al. 2011). Overhead sprays of insecticides could greatly impact resident natural enemies such as Theridiidae, Salticidae, Formicidae, Coccinellidae, or Miridae (Theiling and Croft 1988; Santos et al. 2007), and could explain why our work only yielded foliage-foraging predators in as little as 12 % of transect walks. Also, among the foliage-foraging predator community, highly mobile organisms, such as Vespidae, and pesticide-tolerant species, such as Chrysopa spp., attained high abundance (e.g., Santos et al. 2007). Farmers’ pervasive use of pesticides can also explain the complete absence of pupal, larval, or egg parasitoids, although such could equally be due to the particularly firm exocarp of Passiflora fruits providing enemy-free space for Dasiops spp. With recurrent insecticide use likely having a devastating impact on resident natural enemy communities, and a questionable effect on D. inedulis, current pest management may do more harm than good. To further assess the effects of insecticides on the resident natural enemy community, parasitoid abundance could be monitored in unsprayed orchards.
Periodic dissection of SP fruits revealed considerable variability in D. inedulis egg clutch size, and substantial degrees of egg and larval mortality. Female flies laid an average of 8.24 eggs, of which only 53 % developed into late-instar larvae over a 14-day period. Variability in clutch size and subsequent immature development relates to several parameters of the host fruit (Leyva et al. 1991; Diaz-Fleischer and Aluja 2003b). Also, plasticity in oviposition strategies for a given tephritoid fly species could lead to important variability in clutch size, and can depend upon phenology, density, and ephemerality of its host (Aluja et al. 2001; Diaz-Fleischer and Aluja 2003a). In the meantime, certain tephritoid flies exhibit great intraspecific variability in pre-imaginal survivorship rates, with some species <50 % combined egg and larval survival (Duyck and Quilici 2002; Goncalves et al. 2012). Although there is preliminary but valuable data on D. inedulis life history in yellow passionfruit (Armbrecht et al. 1986), information is lacking on physiology or ovipositional strategies of Dasiops spp. or lance flies in general.
Our mimicking of larval drop showed predation levels and soil entry times similar to those of Ceratitis capitata or Anastrepha spp. (Eskafi and Kolbe 1990; Aluja et al. 2005). For example, Aluja et al. (2005) showed that 90 % of Anastrepha sp. larvae entered the soil within 10 min, and different species of ants attacked larvae within 5 min of exit from fruit. Similarly, ants were important predators of wandering larvae in other studies (Eskafi and Kolbe 1990; Hennessey 1997). Although we mimicked larval drop during the day time, D. inedulis may adopt diel shifts in its behavior to evade predators (Eskafi and Kolbe 1990; Hendrichs et al. 1991). Followup research should determine the periodicity of emergence of D. inedulis larvae from SP fruits and its coincidence with activity patterns of ants and other ground-foraging predators.
For tephritoid flies with relatively long pupation times, ground-foraging predators can play a central role in regulating fly densities (Bateman 1972; Monzo et al. 2009). In our study, pupal survival rate was between 24.6 and 36.7 % over a 48 h period, which is lower than some other studies (Wong and Wong 1988; Hodgson et al. 1998; Urbaneja et al. 2006). This may be related to our use of sieved soil, from which pupae tend to disappear at high rates (Hodgson et al. 1998). Pupal mortality was 12.1 % higher in covered traps, which could signal the role of vertebrate predators such as mice or birds (Bigler et al. 1986; Thomas 1993). Surprisingly, pupal survival rates did not relate to abundance of the ground-foraging predator complex, as determined through tuna fish baiting or pitfall trapping. Ants, the dominant predator group in either pitfall traps as on tuna fish baits, may not be the sole natural enemy of D. inedulis late-instar larvae and pupae. Other arthropod families, such as lycosid spiders, earwigs, or ground beetles, could equally include very effective predators of Dipteran pupae (Monzo et al. 2009, 2011), but regression analysis may not reveal their effect given their much lower abundance. Molecular gut content analysis could be a very useful tool to help identify those important natural enemies (see Monzo et al. 2011).
On several occasions, we observed Polistes and Protopolybia spp. preying upon fly larvae within SP flower buds. In the experimental orchards, flower buds with chewing holes were commonly found, but those could also have been caused by nectar-robbing wasp or bee species (e.g., Nicolson 2007). Possibly, social wasps can be equally effective predators of adult D. inedulis, and are even attracted to pheromones of some tephritoid fly species (Hendrichs et al. 1994). Occasionally, D. inedulis adults were found in spider webs in the SP canopy. The particularly low numbers of foliage-foraging predators could hint low levels of biological control of the adult stage of D. inedulis in SP orchards.
Despite drawbacks of having been conducted in commercial orchards, this study provides valuable information to develop D. inedulis management tools. Linkages between infestation levels, SP crop phenology and precipitation could readily be translated in mass trapping schemes, targeted insecticide application and orchard sanitation. In the meantime, our assessment of biotic mortality factors identifies tangible potential for increased levels of D. inedulis biological control, targeted to the susceptible pupal and adult stage. As current pest management tactics interfere with a diverse (vertebrate and arthropod) natural enemy community, a more rational use of insecticides could increase the abundance and action of these agents and possibly lead to vast reductions in D. inedulis pest pressure. Integrated pest management and organic approaches greatly benefit biological control in other fruit production systems, such as olive, citrus, or stone fruits (Santos et al. 2007; Jacas and Urbaneja 2010; Simon et al. 2010). These pest management approaches wait to be validated in Colombian SP crops, as in a multitude of other horticultural crops in the region.
References
Aguiar-Menezes EL, Menezes EB, Cassino PCR, Soares MA (2002) Passion fruit, pp. 361–390. In: Pena JE, Sharp JL, Wyoski M (eds) Tropical fruit pests and pollinators: biology, economic importance, natural enemies and control. CABI Publishing, Wallingford
Aguiar-Menezes EL, Nascimento RJ, Menezes EB (2004) Diversity of fly species (Diptera: Tephritoidea) from Passiflora spp. and their hymenopterous parasitoids in two municipalities of Southeastern Brazil. Neotrop Entomol 33:113–116
Aluja M, Diaz-Fleischer F, Papaj DR, Lagunes G, Sivinski J (2001) Effects of age, diet, female density, and the host resource on egg load in Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae). J Insect Physiol 47:975–988
Aluja M, Sivinski J, Rull J, Hodgson PJ (2005) Behavior and predation of fruit fly larvae (Anastrepha spp.) (Diptera: Tephritidae) after exiting fruit in four types of habitats in tropical Veracruz, Mexico. Environ Entomol 34:1507–1516
Armbrecht I, Chacon P, Rojas M (1986) Biologia de la mosca de los botones florales del maracuya Dasiops inedulis (Diptera: Lonchaeidae) en el Valle del Cauca. Rev Colomb Entomol 12:1
Asturizaga AS, Øllgaard B, Balslev H (2006) Frutos comestibles, pp. 329–346. In: Moraes RM, Øllgaard B, Kvist LP, Borchsenius F, Balslev H (eds) Botánica económica de los Andes Centrales. Universidad Mayor de San Andrés, La Paz
Bateman MA (1972) The ecology of fruit flies. Annu Rev Entomol 17:18–49
Bigler F, Neuenschwander P, Delucchi V, Michelakis S (1986) Natural enemies of preimaginal stages of Dacus oleae Gmel. (Dipt., Tephritidae) in Western Crete. II. Impact on olive fly populations. Boll Lab Entomol Agrar Filippo Silvestri 43:79–96
Bojaca CR, Wyckhuys KAG, Gil R, Jimenez J, Schrevens E (2010) Sustainability aspects of vegetable production in the peri-urban environment of Bogotá, Colombia. Int J Sustain Dev World Ecol 17:487–498
Celedonio-Hurtado H, Aluja M, Liedo P (1995) Adult population fluctuations of Anastrepha species (Diptera: Tephritidae) in tropical orchard habitats of Chiapas, Mexico. Environ Entomol 24:861–869
Delgado A, Kondo T, Lopez K, Quintero EM, Manrique MB, Medina JA (2010) Biologia y algunos datos morfologicos de la mosca del boton floral de la pitaya amarilla, Dasiops saltans (Townsend) (Diptera: Lonchaeidae) en el Valle del Cauca, Colombia. B Mus Entomol Univ Val 11:1–10
Diaz-Fleischer F, Aluja M (2003a) Behavioural plasticity in relation to egg and time limitation: the case of two fly species in the genus Anastrepha (Diptera: Tephritidae). Oikos 100:125–133
Diaz-Fleischer F, Aluja M (2003b) Clutch size in frugivorous insects as a function of host firmness: the case of the tephritid fly Anastrepha ludens. Ecol Entomol 28:268–277
Duyck PF, Quilici S (2002) Survival and development of different life stages of three Ceratitis spp. (Diptera: Tephritidae) reared at five constant temperatures. Bull Entomol Res 92:461–469
El Keroumi A, Naamani K, Dahbi A, Luque I, Carvajal A, Cerda X, Boulay R (2010) Effect of ant predation and abiotic factors on the mortality of medfly larvae, Ceratitis capitata, in the Argan forest of Western Morocco. Biocontrol Sci Technol 20:751–762
Epsky ND, Kendra PE, Pena J, Heath RR (2011) Comparison of synthetic food-based lures and liquid protein baits for capture of Anastrepha suspensa (Diptera: Tephritidae) adults. Fla Entomol 94:180–185
Eskafi FM, Kolbe MM (1990) Predation on larval and pupal Ceratitis capitata (Diptera: Tephritidae) by the ant Solenopsis geminata (Hymenoptera: Formicidae) and other predators in Guatemala. Environ Entomol 19:148–153
Garcia FR, Norrbom AL (2011) Tephritoid flies (Diptera, Tephritoidea) and their plant hosts from the state of Santa Catarina in Southern Brazil. Fla Entomol 94:151–157
Garcia FR, Ricalde MP (2013) Augmentative biological control using parasitoids for fruit fly management in Brazil. Insects 4:55–70
Goncalves FM, Rodrigues PC, Pereira JA, Thistlewood H, Torres LM (2012) Natural mortality of immature stages of Bactrocera oleae (Diptera: Tephritidae) in traditional olive groves from north-eastern Portugal. Biocontrol Sci Technol 22:837–854
Guillén L, Aluja M, Equihua M, Sivinski J (2002) Performance of two fruit fly (Diptera: Tephritidae) pupal parasitoids (Coptera haywardi [Hymenoptera: Diapriidae] and Pachycrepoideus vindemiae [Hymenoptera: Pteromalidae]) under different environmental soil conditions. Biol Control 23:219–227
Hendrichs J, Katsoyannos BI, Papaj DR, Prokopy RJ (1991) Sex differences in movement between natural feeding and mating sites and tradeoffs between food consumption, mating success and predator evasion in Mediterranean fruit flies (Diptera: Tephritidae). Oecologia 86:223–231
Hendrichs J, Katsoyannos BI, Wornoayporn V, Hendrichs MA (1994) Odour-mediated foraging by yellowjacket wasps (Hymenoptera: Vespidae): predation on leks of pheromone-calling Mediterranean fruit fly males (Diptera: Tephritidae). Oecologia 99:88–94
Hennessey MK (1997) Predation on wandering larvae and pupae of Caribbean fruit fly (Diptera: Tephritidae) in guava and carambola grove soils. J Agric Entomol 14:129–138
Hodgson PJ, Sivinski J, Quintero G, Martin A (1998) Depth of pupation and survival of fruit fly (Anastrepha spp.: Tephritidae) pupae in a range of agricultural habitats. Environ Entomol 27:1310–1314
Hokkanen H (1991) Trap cropping in pest management. Annu Rev Entomol 36:119–138
Hulthen AD, Clarke AR (2006) The influence of soil type and moisture on pupal survival of Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Aust J Entomol 45:16–19
Jacas JA, Urbaneja A (2010) Biological control in citrus in Spain: from classical to conservation biological control, pp. 61–72. In: Ciancio A, Mukerji KG (eds) Integrated management of arthropod pests and insect borne diseases. Springer, Dordrecht
Kaspi R (2000) Attraction of female Chiracanthium mildei (Araneae: Clubionidae) to olfactory cues from male Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae). Biocontrol 45:463–468
Leyva JL, Browning HW, Gilstrap FE (1991) Development of Anastrepha ludens (Diptera: Tephritidae) in several host fruit. Environ Entomol 20:1160–1165
Lu YH, Wu KM, Wyckhuys KAG, Guo YY (2009) Potential of mungbean, Vigna radiatus as a trap crop for managing Apolygus lucorum (Hemiptera: Miridae) on Bt cotton. Crop Prot 28:77–81
Monzo C, Molla O, Castañera P, Urbaneja A (2009) Activity-density of Pardosa cribata in Spanish citrus orchards and its predatory capacity on Ceratitis capitata and Myzus persicae. Biocontrol 54:393–402
Monzo C, Sabater-Munoz B, Urbaneja A, Castañera P (2011) The ground beetle Pseudophonus rufipes revealed as predator of Ceratitis capitata in citrus orchards. Biol Control 56:17–21
Nicolson SW (2007) Nectar consumers, pp. 289–342. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht
Norrbom AL, McAlpine JF (1997) A revision of the Neotropical species of Dasiops rondani (Diptera: Lonchaeidae) attacking Passiflora (Passifloraceae). Mem Entomol Soc Wash 18:189–211
Ocampo JA (2007) Study of the diversity of the genus Passiflora L. (Passifloraceae) and its distribution in Colombia. PhD Thesis, Centre International d’Etudes Superieures en Sciences Agronomiques, Montpellier
Orsini MM, Daane KM, Sime KR, Nelson EH (2007) Mortality of olive fruit fly pupae in California. Biocontrol Sci Technol 17:797–807
Ovruski S, Aluja M, Sivinski J, Wharton R (2000) Hymenopteran parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the southern United States: diversity, distribution, taxonomic status and their use in fruit fly biological control. Integr Pest Manag Rev 5:81–107
Quintero EM, López IC, Kondo T (2012) Manejo integrado de plagas como estrategia para el control de la mosca del botón floral del maracuyá Dasiops inedulis Steyskal (Diptera: Lonchaeidae). Rev Corpoica Cienc Tecnol Agropecu 13:31–40
Santos SAP, Pereira JA, Torres LM, Nogueira AJA (2007) Evaluation of the effects, on canopy arthropods, of two agricultural management systems to control pests in olive groves from north-east of Portugal. Chemosphere 67:131–139
Santos O, Varon EH, Salamanca J (2009) Prueba de extractos vegetales para el control de Dasiops spp., en granadilla (Passiflora ligularis Juss.) en el Huila, Colombia. Rev Corpoica 10:141–151
Shelton AM, Badenes-Perez FR (2006) Concepts and applications of trap cropping in pest management. Annu Rev Entomol 51:285–308
Simon S, Bouvier JC, Debras JF, Saupanor B (2010) Biodiversity and pest management in orchard systems: a review. Agron Sustain Dev 30:139–152
Theiling KM, Croft BA (1988) Pesticide side-effects on arthropod natural enemies: a database summary. Agric Ecosyst Environ 21:191–218
Thomas DB (1993) Survivorship of the pupal stage of the Mexican fruit fly Anastrepha ludens (Loew) (Diptera: Tephritidae) in an agricultural and a nonagricultural situation. J Entomol Sci 28:150–362
Thomas DB (1995) Predation on the soil inhabiting stages of the Mexican fruit fly. Southwest Entomol 20:61–71
Uchôa-Fernandes MA, Zucchi RA (1999) Metodología de colecta de Tephritidae y Lonchaeidae frugívoros (Diptera: Tephritoidea) y sus parasitoides (Hymenoptera). An Soc Entomol Bras 28:601–610
Uchôa-Fernandes MA, Oliveira I, Molina RMS, Zucchi RA (2002) Species diversity of frugivorous flies (Diptera: Tephritidae) from hosts in the cerrado of the State of Mato Grosso do Sul, Brazil. Neotrop Entomol 31:515–524
Uchôa-Fernandes MA, Molina RM, Oliveira I, Zucchi RA, Canal NA, Díaz NB (2003) Larval endoparasitoids (Hymenoptera) of frugivorous flies (Diptera, Tephritoidea) reared from fruits of the cerrado of the State of Mato Grosso do Sul, Brazil. Rev Bras Entomol 47:181–186
Urbaneja A, Garcia Mari F, Tortosa D, Navarro C, Vanaclocha P, Bargues L, Castañera P (2006) Influence of ground predators on the survival of the Mediterranean fruit fly pupae, Ceratitis capitata, in Spanish citrus orchards. Biocontrol 51:611–626
Van Driesche RG, Bellows TS (1996) Biological control. Chapman and Hall, New York
Vayssieres JF, Korie S, Ayegnon D (2009) Correlation of fruit fly (Diptera Tephritidae) infestation of major mango cultivars in Borgou (Benin) with abiotic and biotic factors and assessment of damage. Crop Prot 28:477–488
Wee SL, Tan KH (2005) Male endogenous pheromonal component of Bactrocera carambolae (Diptera: Tephritidae) deterred gecko predation. Chemoecology 15:199–203
Wong MA, Wong TTY (1988) Predation of the Mediterranean fruit fly and the Oriental fruit fly (Diptera: Tephritidae) by the fire ant (Hymenoptera: Formicidae) in Hawaii. Proc Hawaii Entomol Soc 28:169–177
Wyckhuys KAG, O’Neil RJ (2006) Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot 25:1180–1190
Wyckhuys KAG, Lopez F, Rojas M, Ocampo J (2011) The relationship of farm surroundings and local infestation pressure to pest management in cultivated Passiflora species in Colombia. Int J Pest Manag 57:1–10
Wyckhuys KAG, Korytkowski CJ, Martinez B, Rojas M, Ocampo J (2012) Species composition and seasonal occurrence of Diptera associated with passionfruit crops in Colombia. Crop Prot 32:90–98
Acknowledgments
We are grateful to Catalina Camargo, Lorena Garcia, Raul Gomez, Francisco Lopez, Hilary Ramirez, and all farmers that participated in the experiment. Cheslavo Korytkowski, Javier Martinez, and Juan Manuel Perilla provided valuable assistance in identifying Dasiops spp. Jhon Albeiro Quiroz facilitated access to the Museum Francisco Luis Gallego of Universidad Nacional de Colombia sede Medellín, and Luz Stella Fuentes kindly provided access to laboratory space. This work was funded by the Colombian Ministry of Agriculture and Rural Development, with Grant MADR 2008L6772-3445 to KW.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Carrero, D.A., Melo, D., Uribe, S. et al. Population dynamics of Dasiops inedulis (Diptera: Lonchaeidae) and its biotic and abiotic mortality factors in Colombian sweet passionfruit orchards. J Pest Sci 86, 437–447 (2013). https://doi.org/10.1007/s10340-013-0487-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-013-0487-9