Abstract
We describe the results of four laboratory studies designed to measure the effect of temperature and wireworm appetence, weight, and degree of Metarhizium infection on their ability to damage wheat seedlings. Wireworm activity, measured from wireworm speed, increased linearly from 6 to 18 °C and leveled off thereafter. Plant emergence and growth increased exponentially from 6 to 22 °C for wheat cultivars AC Barrie and AC Unity VB. Plant root:shoot ratio at Zadoks 13 was highest at 14 °C and lowest at 22 °C for AC Barrie. Wireworm weight and degree of infection with Metarhizium did not affect their ability to kill wheat seedlings, but wireworms in a feeding state caused significantly more damage than those in a non-feeding state when wheat was grown at 10, 14, 18, and 22 °C. Wireworms (ww) in a feeding state destroyed 1.8 seedlings/ww in 14 days at 22 °C if there were 1 or 2 wireworms in a pot, and 1.5 seedlings/ww if there were 4 wireworms in a pot. If 5 wireworms were placed in a pot, wireworms in a feeding state destroyed 0.3, 1.0, 0.9, 1.3, and 1.4 seedlings/ww in 46, 32, 25, 25, and 25 days at 6, 10, 14, 18, and 22 °C, respectively. Wireworm mortality from Metarhizium during 60 days of containment in pots in the study was higher in non-feeding than in feeding wireworms, and higher if wireworms were selected from a Metarhizium-infected colony than those selected from a non-infected colony. Some of the implications of these results for wireworm management and laboratory trials are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of wireworms to cause economic damage to wheat has been studied extensively since Comstock and Slingerland (1891), with most reports on wireworm management focusing on crop protection rather than wireworm mortality (Vernon et al. 2009; Vernon and van Herk 2012). Wireworm physiology and behavior have also been studied extensively, particularly in the 1940s to 1960s, and many papers detail how wireworms respond to stimuli such as light, moisture, temperature, gravity, plant extracts, and insecticides (e.g., Evans and Gough 1942; Lees 1943a, b; Evans 1944; Falconer 1945a, b; Crombie and Darrah 1947; Davis 1957, 1971; van Herk et al. 2008a, b). Some of these studies originated from the need to understand wireworm phenology and seasonal vertical movement in the soil, a behavior that has long intrigued researchers and complicated wireworm management, as pest wireworm species are not always detectable or cause damage even when present at economic levels (Vernon and van Herk 2012). Periodic “fasting” by wireworms, and their consequent temporal variability in damaging field crops throughout the year, was already reported in the first paper on wireworm physiology and behavior (i.e., Bierkander 1779, in Curtis 1860), and has been pointed out by others since (e.g., Burrage 1963; Doane 1981). These periods of “fasting” may account for 80 % of each larval instar (e.g., in Agriotes ustulatus, Furlan 1998), and are said to precede molting (Evans and Gough 1942), though the authors have observed wireworms actively feeding within 1 week of ecdysis.
That a randomly selected sample of wireworms will contain some larvae in a feeding and some in a non-feeding state, and that this will likely introduce considerable variability in wireworm studies, was first reported by Falconer (1945a), who regretted his oversight of not selecting larvae that were in the same physiological state. Generally, however, wireworm appetence and other wireworm state variables are disregarded by researchers past and present, though in recent years we have attempted to raise awareness for this (e.g., van Herk and Vernon 2007a, b; van Herk et al. 2010). Equally surprising, considering the large body of research on wireworm management in wheat and on wireworm behavior and physiology, is that, with the exception a small lab study done by Falconer (1945a), there seem to be no reports of how temperature affects the ability of wireworms to damage wheat plants.
To address this apparent void in the literature and demonstrate the importance of considering wireworm state when selecting them for studies, we conducted four related laboratory studies. The initial study, conducted at room temperature (RT 22.0 °C), assessed the effect of wireworm number and weight on wheat seedling emergence, growth, and mortality, and determined wireworm weight gain and survival. The second and third studies assessed the effect of temperature on, respectively, wheat seedling emergence and growth in the absence of wireworms, and wireworm activity. The final study assessed the effect of temperature and several wireworm state variables on their ability to damage wheat seedlings. State variables assessed include wireworm appetence, size, and degree of Metarhizium anisopliae infection, and responses measured include plant growth and wireworm survival.
Methods
To permit comparability, an effort was made to keep methodologies consistent between studies, as well as between the initial feeding study and a previous feeding study conducted at 15 °C (van Herk and Vernon 2011). The methods of all four studies described in this article are therefore given together.
Temperature and light
All studies were conducted in controlled environment walk-in coolers (Coldmatic Refrigeration, Concord, ON) and an insect observation room, all set at a constant temperature accurate to ±0.2 °C, at the Pacific Agri-Food Research Centre (PARC) in Agassiz, British Columbia, Canada. The initial wireworm feeding study was conducted at RT (22.0 °C), and the other three studies were conducted at 6.0, 10.0, 14.0, 18.0, and 22.0 °C. Plants in all studies were subjected to a 12:12 light:dark regimen of fluorescent lighting (van Herk and Vernon 2011).
Soil
All soil, a sandy clay loam, used in studies was collected at PARC, sifted through a 2 × 2 mm mesh to remove rocks, roots, and other coarse organic matter, and made up to 20 % moisture by weight. Wheat plants were grown in 2.25 l plastic pots (Richards Packaging, Richmond, BC), filled with 2.0 l soil which was compacted lightly by hand prior to and again immediately after planting.
Seed and planting
For both the initial feeding study and the plant emergence study (grown at different temperatures), ten untreated wheat seeds (cv AC Barrie) were placed 1.5 cm apart and 2 cm deep in a single row across the diameter of the pot. The initial feeding study was set up with 36 pots for each of four wireworm densities (0, 1, 2, and 4 wireworms), though we discarded several “slow” wireworms (mobility score > 0; van Herk and Vernon 2013) at wireworm insertion, changing the number of pots/density slightly (Table 1); and the plant emergence study was set up with 20 pots per temperature (Table 2). For the wireworm feeding at different temperatures study, 42 pots were set up per temperature, with each pot containing 10 wheat seeds (cv AC Unity VB) placed 2 cm deep and 2 cm apart in two parallel rows spaced 5 cm apart. Seeding in studies containing wireworms occurred 1 day after wireworm introduction into pots. Both seed types are modern varieties of Canada Western Red spring wheat used extensively on the Canadian prairies. All pots (except those in control treatments) in the final feeding study contained 5 wireworms.
Wireworms
Wireworms, identified to A. obscurus, were collected from a long-term pasture at PARC in 2011 and held without food in 10 l Rubbermaid containers with clean, sifted soil at 10 °C for 2–6 mos, until needed. All wireworms were weighed and assessed for mobility and health (i.e., the absence of morbidity or Metarhizium infection symptoms) within 1 day of placement in pots or observation arenas to ensure wireworms were healthy (mobility score = 0; van Herk and Vernon 2013). Only feeding wireworms were used in the initial feeding and wireworm movement studies; both feeding and non-feeding wireworms were used in the feeding at different temperatures study. To collect feeding wireworms, the tubs containing wireworms were moved to 14 °C and small bait traps, consisting of 10 ml wheat seed placed in 150 ml cups of moistened, coarse Vermiculite, were placed in the approximate center of the tubs for 4–5 days. We have previously observed this method to remove up to 90 % of the wireworms in a tub in a single baiting session. Wireworms that did not respond to two consecutive baitings within 2 weeks were manually removed from tubs and considered to be non-feeders, similar to methods used in previous studies (van Herk and Vernon 2011).
At the time of wireworm collection at PARC, a number of larvae with Metarhizium infection were found in a localized part of the field. All wireworms collected from this area were kept separate from all the other wireworms, which were collected within 100 m of this “Metarhizium” site, but otherwise were handled and stored identically. Wireworms collected from the “Metarhizium” site had a much higher incidence of mortality during storage (>50 % in ~6 mos) compared to wireworms collected from the nearby sites (<10 %) despite the same handling and storage conditions, indicating that the former population had a higher exposure, or higher susceptibility to the pathogen. Both types of wireworms (hereafter referred to as wireworm “type”) were used in the final feeding study to determine if the level of Metarhizium infection/exposure affected their ability to damage wheat plants. Feeding and non-feeding wireworms were selected in equal numbers from both the “Metarhizium-infected” and “non-infected” colonies.
To determine the effect of wireworm weight on the amount of damage that was done to wheat seedlings in the initial and final feeding studies, all wireworms placed in a pot had a similar weight; within 0.5 and 2.0 mg of each other, respectively. Care was taken that pots used for each wireworm density (initial study) and temperature (final study) study contained wireworms of the same weight range.
Wireworms used in feeding studies were placed in each pot in random positions in divot holes 2 cm deep. Larval health and mobility were again assessed after removal from the pots, 16 and 60 days after insertion in the initial and final feeding studies, respectively. Wireworms used in the initial feeding study were weighed after removal to assess their weight gain or loss.
Wireworm activity
To determine how larval activity is affected by temperature, we recorded the walking speed of wireworms, 25 per temperature, placed in a circular, soil-less bioassay lined with filter paper. This method was chosen as the same bioassay was used to measure walking speed in A. obscurus in a previous study (van Herk et al. 2008a), and as wireworm activity levels have been assessed by measuring walking speed historically (Falconer 1945b). To assess walking speed in this bioassay arena, wireworm positions on a circular 113 cell grid were recorded at precise 3 s intervals for 5 min, and these positions used to calculate linear displacement, as described by van Herk et al. (2008a, b).
Plant emergence, growth, stand assessment, and health
Plant emergence and stand were determined by inspecting all pots several times a day and recording the number of seedling coleoptiles visible. In the initial feeding study, plants were measured to the nearest mm at 5 and 7 days after planting (DAP), during which time nearly all surviving plants were in Zadoks stage 11 (Zadoks et al. 1974). Plant growth was assessed with the Zadoks decimal growth scale for cereal development, as this scale is used widely in cereal research and agriculture today. The scale divides the cereal plant life cycle into ten principal growth stages, labeled 0 (germination) to 9 (ripening), which are further sub-divided into secondary stages to produce a scale from 00 to 99. Plants were measured at the same time of day, and the mean rate of plant growth per hour was calculated by subtracting the 5 DAP length from the 7 DAP and dividing by 48. All plants were considered as independent units for mean calculation and ANOVA (see below). A final stand assessment was performed 13 DAP. Only healthy plants (green, no wilting or browning of tissues) were included in stand counts.
For the plant emergence in the absence of wireworms, emergence, and stand were conducted as above, but plant growth was assessed by measuring plants every 2 days after >80 % of the seeds planted at that temperature had emerged (i.e., at 15, 8, 6, 4, and 3 DAP for 6.0, 10.0, 14.0, 18.0, and 22.0 °C (hereafter 6–22 °C), respectively). Measurements continued for up to 10 days after emergence. Each plant was measured twice in a day, at precise 10 h intervals, to the nearest mm. To compare growth rates (mm/h) between temperatures, rates calculated for plants in the same physiological stage (Zadoks 10) were used (i.e., at 23, 14, 10, 6, and 5 DAP for 6–22 °C, respectively). Zadoks 10 was used, as at this stage >80 % of seeds planted had emerged and plants were on avg. >40 mm above the soil surface at all temperatures. To determine the plant root/shoot ratio, a number (>80) of plants at Zadoks 13 were randomly selected from various pots, carefully removed from the soil, washed, air dried, and weighed.
For the wireworm feeding at different temperatures study, plant emergence was assessed as above and all emerged plants measured when nearly all were in the Zadoks 11 stage (i.e., at 32, 18, 11, 11, and 11 DAP for 6–22 °C, respectively). Plant stand was assessed weekly after this initial measurement, until the study was terminated 4 weeks after plants at 6 °C reached Zadoks 11 (i.e., at 60 DAP). Only the plant stand at 2 and 4 weeks after measurement is reported here.
Data analysis
The time for 50 and 90 % of plants in a pot to emerge (ET50 and ET90, respectively) was calculated by regressing the number of plants visible above the soil surface to the amount of time (in h) required for those plants to emerge. To obtain an accurate estimate, only those observations when a new plant became visible, and the last recorded observation at which no plants were visible, were included in this calculation. These ET50 and ET90 values were used to calculate average ET50 and ET90 values for each temperature and for regression analysis.
All statistical analysis were conducted using SAS (v9.2). Normality of data was assessed using the UNIVARIATE procedure, and data were transformed with sqrt (x + 0.5) where necessary. Multiple comparisons of mean were conducted with the Ryan–Einot–Gabriel–Welch (REGWQ) procedure (α = 0.05). Specific analysis used for each study are indicated below.
Results
Effect of wireworms on wheat seedlings at 22 °C
Effect on seedling emergence
The mean number of plants that emerged in pots decreased as the number of wireworms present increased (Table 1), with 89 % emerging when no wireworms were present and only 48 % emerging at the highest wireworm density (4 ww/pot). Analyzing the effect of wireworm presence or absence on the number that emerged indicated wireworm presence significantly reduced plant emergence (F = 37.42, df = 1.142, P < 0.0001). The next analysis assessed the effect of the number of wireworms present (1–4/pot) and wireworm weight, which indicated that wireworm weight (range 5.6–55.2 mg) had no significant effect on plant emergence (P > 0.5), which was unexpected, but that the number of wireworms present was highly significant (F = 17.67, df = 3.103, P < 0.0001). Our final analysis eliminated wireworm weight and included the number of wireworms present (0–4/pot) alone (Table 1), which indicated that plant emergence was reduced by 1 plant for each wireworm added to the pot.
Reduction and variability in plant growth as a result of wireworm feeding
To assess plant growth, we measured all plants that had emerged by 5 DAP and did not show damage symptoms, and measured these again at 7 DAP. Nearly all plants had entered Zadoks 11 during this time. The rate of plant growth between 5 and 7 DAP decreased as the number of wireworms increased. In the absence of wireworms, plants grew 1.80 mm/h over a 48 h period (Table 1), which decreased to 1.25 mm/h at the 4 ww/pot density. Analyzing the growth rate using all plants as separate units indicated that both the number of wireworms present and the pots that plants were grown in had a significant effect on the growth rate, suggesting some variability between pots (Table 1; effect of pot F = 2.51, df = 139.823, P < 0.0001). Repeating the analysis with only those pots which contained wireworms indicated that wireworm weight had no significant effect on plant growth rate (P > 0.3).
Inspection of the data indicated considerable variability in plant growth within many pots, likely due to wireworms assembling at and selectively feeding on only one or a few plants in the pot. To quantify this variability we obtained the standard deviation (SD) of the plant growth rate for each pot and compared these among wireworm densities, which indicated plant growth variability was significantly lower in the absence of wireworms (mean = 0.31, SE = 0.03) than if 1, 2, 3, or 4 ww/pot were present (mean and SE of 0.49 (0.04), 0.58 (0.05), 0.49 (0.17), and 0.56 (0.06), respectively) (F = 5.91, df = 4.136, P = 0.0002). The weight of the wireworms present in the pots had no significant effect on plant growth variability (P > 0.5). Regression of plant growth variability (i.e., mean SD of pots with a particular wireworm density) to the number of wireworms present in the pots to determine if wireworms are feeding on one plant in a pot did not show a significant trend. However, further inspection of data revealed that in pots with 0, 1, 2, and 4 ww/pot; 1, 8, 13, and 21 plants (respectively) did not grow at all (0 mm/h), and an additional 4, 16, 13, and 21 plants (respectively) grew very slowly (0.05–0.5 mm/h). If individual wireworms were causing the same amount of per-plant damage in each pot regardless of wireworm density, and were not attacking plants jointly, we would expect 6/34 pots with 2 wireworms to have 2 non-growing plants, and 6/32 pots with 4 wireworms to have 4 non-growing plants, as (corrected for the non-growing plant in the control treatment) 7/38 pots with 1 wireworm had 1 (and never more than 1) non-growing plant. Instead only 2 pots had 2 or 3 non-growing plants in pots with 2 wireworms, and only 5 pots had 2 non-growers (and none had more) in pots with 4 wireworms. Although circumstantial, this suggests that 1 wireworm can cause a plant to stop growing, and further suggests that in those pots with >1 wireworm, wireworms were attacking seeds jointly rather than attacking separate plants. Similarly, since (corrected for slow-growing plants in the control treatment) 12/38 pots with 1 wireworm had 1 plant that grew slowly, we would expect (if wireworms randomly attack plants) 11 pots with 2 wireworms to have 2 plants each that grew poorly, and 10 pots with 4 wireworms to have 4 plants each that grew poorly, but instead there are 4 and 2 pots (respectively) with that number of poorly growing plants.
Plant mortality
The mean number of plants in pots that survived to the end of the study decreased as the number of wireworms present increased (Table 1), with 84 % surviving when no wireworms were present and only 25 % surviving at the highest wireworm density. Analyzing the effect of wireworm presence or absence on the number that survived indicated wireworm presence significantly reduced plant survival (F = 59.53, df = 1.142, P < 0.0001). The next analysis assessed the effect of the number of wireworms present (1–4/pot) and wireworm weight, which indicated that wireworm weight had no significant effect on plant survival (P > 0.6), but that the number of wireworms present was highly significant (F = 18.99, df = 3.103, P < 0.0001). Our final analysis eliminated wireworm weight and included the number of wireworms present (0–4/pot) alone (Table 1), and indicated that plant survival at 14 DAP was reduced by 1.8 plants for each wireworm added to the pot for 1–3 wireworms, and by 1.5 plants if 4 wireworms were added to the pot. Regression of the difference in the number of plants surviving in pots with a particular number of wireworms and the mean number surviving in pots without wireworms, to the number of wireworms gave the following model:
The lower number of plants killed per wireworm at the 4 wireworm/pot density may be due from the lower number of remaining plants (i.e. the wireworms were competing for plants to feed on), as evident from the plants in these pots being smaller (on average) than plants grown in pots with fewer wireworms (data not shown).
Wireworm weight gain
The amount of weight gained by wireworms during the study fluctuated considerably (Table 1), and formal analysis indicated that weight gain was not affected by the number of wireworms present in the pot (Table 1), but depended mostly on their original weight (F = 73.31, df = 1.91, P < 0.0001). Subsequent regression of absolute weight increase to their original weight indicated that heavier wireworms gained less weight during the study than lighter larvae
This finding suggests that larger wireworms may need to ingest more food than smaller larvae just to maintain their body mass, and as we did not observe an increase in plant damage with wireworm size, it suggests that small and large larvae fed similarly and that the rate of wireworm growth slows as they increase in size.
Wireworm mortality
Most wireworms were recovered from the pots at the end of the study, with 17.5 % dead or missing. Of these, 86 % were confirmed to have died from Metarhizium, 4.7 % were dead but showed no symptoms of Metarhizium (which generally takes 1–2 days to emerge after death at RT), and 9.3 % were missing. As wireworms that die from the fungus eventually disintegrate completely, it is likely that all missing and dead wireworms died from Metarhizium. The number of wireworms confirmed to have died from Metarhizium increased with the number of wireworms placed in the pot (Table 1), but there was no difference in the proportion of wireworms dead (range 0.17–0.21) among wireworm densities (P > 0.9). Wireworm weight had no significant (P > 0.05) effect on wireworm mortality due to Metarhizium during the study.
Effect of temperature on wheat seedling emergence and early growth
The mean number of plants that emerged was similar (88–92 %) for all temperatures except 6 °C (83 %), causing slightly significant (P = 0.042) differences in the mean emergence among temperatures (Table 2). As expected, plant emergence was first detected at 22 °C and last at 6 °C (Table 2), and the time for 50 % (ET50) and 90 % (ET90) of plants emerging differed significantly between all temperatures (Table 2). Modeling the emergence time indicated an exponential relationship between emergence time and temperature for both ET50 and ET90
As expected, wheat seedling growth at Zadoks 10 was also highly influenced by temperature, with the growth rate lowest at 6 °C (0.34 mm/h) and highest at 22 °C (1.65 mm/h) (Table 2). The pots that plants were grown in had no significant effect on growth rate (P > 0.3). Modeling the seedling growth rate again indicated an exponential relationship between growth and temperature.
Analysis of the plant root:shoot ratio at Zadoks 13 indicated that this ratio was highest at 14 °C, and lowest at 22 °C, indicating that the optimum temperature for root growth was lower than that of the shoot growth. This analysis indicated that the pots in which plants were grown in also had a significant effect on the root:shoot ratios obtained (F = 3.92, df = 36.306, P < 0.0001).
Response of wireworms to temperature
Wireworm movement was strongly affected by temperature, with average walking speeds ranging from 3.4 to 20.7 cm/min. Analysis of wireworm movement with ANCOVA indicated both temperature and wireworm weight (range 15.0–57.1 mg) influenced walking speed significantly (Table 3), with speed increasing with increases in both variables. As this increase appeared to be linear from 6 to 18 °C, after which wireworm speed began to level off (Table 3), wireworm speed was regressed to both temperature and wireworm weight for 6–18 °C data to obtain the following linear relationship:
This suggests that wireworm walking speed would effectively stop at 4.8, 3.9, and 3.0 °C for wireworms that are 20, 30, and 40 mg (respectively) in this particular bioassay.
Effect of temperature and wireworm state on damage to wheat seedlings
Effect of wireworms on seedling emergence
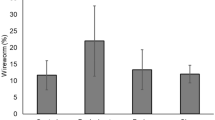
The mean number of plants that emerged in pots without wireworms did not differ significantly among temperatures (range 91–96 %; Table 4). In contrast, it was apparent that the presence and appetence of wireworms in the pots had a considerable effect on plant emergence, and this effect differed among temperatures (Table 4). Analyzing plant emergence numbers per pot was done as follows. For the first analysis, we tested the effects of temperature and wireworm presence/absence, which indicated temperature was nearly significant (F = 2.36, df = 4.200, P = 0.055), while both wireworm presence/absence and the interaction between these variables were significant (F = 87.67, df = 1.200, P < 0.0001; F = 3.44, df = 4.200, P = 0.0096, respectively). Repeating this analysis per temperature indicated that at each temperature, significantly more plants emerged in the absence of wireworms than in their presence (P < 0.05). We subsequently analyzed for the effects of wireworm type (wireworms from Metarhizium vs. non-Metarhizium fields), appetence, and weight on seedling emergence, per temperature. This indicated that for all temperatures except 22 °C, both wireworm type and weight (range 9.8–53.6 mg) had no significant effect on emergence (P > 0.14); at 22 °C, wireworm weight had a slightly significant effect (F = 5.22, df = 1.24, P = 0.0314), with emergence decreasing as wireworm weight increased. For this reason, we repeated the analysis including temperature, and wireworm presence and appetence as the only variables (i.e., wireworms were said to be feeding, non-feeding, or not present), and constructed Table 4 accordingly. This analysis indicated that for all temperatures, plant emergence was greater where wireworms were in a non-feeding state than in a feeding state (Table 4), and that at all temperatures even non-feeding wireworms appeared to reduce the number that emerged, though this reduction was only significant at 14 and 18 °C (Table 4).
As expected, plant emergence was again first detected at 22 °C and last at 6 °C (Table 4). The time taken for 50 % of plants that did emerge (i.e., seedlings that had effectively germinated and were not killed by wireworms) to emerge (ET50), was modeled similar to the plant emergence at different temperatures study. The initial analysis for the effects of temperature and wireworm presence/absence revealed that temperature had a highly significant effect on ET50 (F = 5604.02, df = 4.200, P < 0.0001) but that wireworm presence/absence and the interaction were not significant (P > 0.95). Hence, mean ET50 and ET90 values were calculated using all pots grown at a particular temperature, and the analysis repeated with the temperature variable only (Table 4). Modeling the emergence time again indicated an exponential relationship between emergence time and temperature
Effect of wireworms on seedling growth
To assess plant growth at different temperatures in the presence of wireworms, we measured the plants when they had entered Zadoks 11, which was 1 week after 90 % emergence (of surviving plants) was observed. As before, we first analyzed the effect of temperature and wireworm presence/absence on plant length, which indicated both temperature and wireworm presence/absence had a significant effect (F = 58.20, df = 4.200, P < 0.0001; F = 18.49, df = 1.200, P < 0.0001, respectively), but not the interaction between the two (P > 0.9). Analyzing for the effect of wireworm type, appetence, and weight for each temperature indicated that for each temperature, either wireworm type, or weight had a significant effect on plant length (P > 0.15). We therefore repeated the analysis including only temperature and wireworm appetence/presence as factors (as above), which indicated that at all temperatures except 6 °C the average plant length per pot was significantly shorter when wireworms were in a feeding state than when they were in a non-feeding state (Table 4), and that there was no significant difference in plant lengths if wireworms were absent or present in the non-feeding state (Table 4).
Effect of wireworms on seedling mortality
A considerable amount of seedling mortality was observed over the course of the study, with mortality differing among temperatures and between pots with feeding and non-feeding wireworms. To quantify these differences, we first analyzed the effect of temperature and wireworm presence/absence on the number of plants surviving at 2 weeks (46, 32, 25, 25, 25 DAP for 6–22 °C, respectively), and 4 weeks, which indicated that both factors and their interaction were significant [(temperature F = 12.84, df = 4.200, P < 0.0001; F = 25.12, df = 4.200, P < 0.0001, respectively); (wireworm presence/absence F = 129.22, df = 1.200, P < 0.0001; F = 166.72, df = 1.200, P < 0.0001, respectively); (interaction F = 4.05, df = 4.200, P = 0.0035; F = 5.45, df = 4.200, P = 0.0003, respectively)]. Analyzing for the effect of wireworm type, appetence, and weight for each temperature indicated that for each temperature either wireworm type or weight had a significant effect on plant length (P > 0.15). We therefore repeated the analysis including only temperature and wireworm appetence/presence as factors (as above), which indicated that at all temperatures the average number of surviving plants per pot at 2 and 4 weeks after plants were measured was fewer if wireworms (feeding or non-feeding) were present. At 2 weeks after measurement, non-feeding wireworms had caused a significant reduction in plant stand at 14 and 22 °C, and by 4 weeks this was true for all temperatures except 6 °C (Table 4). On both dates, and at all temperatures, feeding wireworms reduced plant stand more than non-feeding wireworms, and at all temperatures except 6 °C this difference was significant. The reduction in stand caused by feeding wireworms was significant at all temperatures, including 6 °C, on both dates (Table 4). Inspection of the data indicates that the reduction of the number of plants surviving at 2 weeks after measurement from the mean number that emerged generally increased with temperature for feeding and non-feeding wireworms (non-feeding 0.6, 0.5, 0.8, 1.4, and 2.5 plants per pot, respectively) (feeding 1.1, 1.3, 2.2, 3.6, and 3.1 plants per pot, respectively) (Table 4). This reduction appears to be exponential, with the decrease at 22 °C being lower than expected. The same pattern holds true for the number of seedlings reduced by 4 weeks after measurement (Table 4).
Wireworm mortality from Metarhizium
Considerable mortality of wireworms was observed at the end of the study (range 0–84 %, Table 5, of which 72 % were confirmed to have died from Metarhizium, 7 % died showed no signs of Metarhizium, and 21 % were missing). Analysis of the number of wireworms dead per pot tested for the effect of temperature, wireworm type, appetence, and weight, and all interactions between these variables. This analysis indicated that temperature, wireworm type, and wireworm appetence all had significant effects on wireworm mortality (F = 40.29, df = 4.160, P < 0.0001; F = 31.58, df = 1.160, P < 0.0001; F = 5.71, df = 1.160, P = 0.018; respectively), and that there were significant interaction effects between temperature and wireworm appetence (F = 2.99, df = 4.160, P = 0.021) and between wireworm type and appetence (F = 3.43, df = 1.160, P = 0.066); other variables and interactions were not significant (P > 0.4). Repeating the analysis with wireworms confirmed to have died from Metarhizium infection gave similar results, except that the interaction between temperature and wireworm appetence was no longer statistically significant (P > 0.15). The results of these analysis were kept in mind to present the overall mortality data in Table 5. As shown, for both Metarhizium-infected and non-infected wireworms, wireworm mortality increased with temperature. For both types of wireworms, mortality was generally higher in non-feeding wireworms than in wireworms in the feeding state, though this was more pronounced in the non-infected wireworms (Table 5).
Discussion
Effect of temperature on wireworm movement
Both the numerical values obtained, and the linear relationship between wireworm walking speed between 6 and 18 °C (after which speed leveled off), are similar to that found by Falconer (1945a). Falconer, whose data was first consulted after our own was collected and analyzed, measured the walking speeds of 10 A. lineatus–obscurus through a tube at 8, 14, 19, and 25 °C and concluded that their speed increased linearly from 8 to 19 °C and leveled off thereafter. As Falconer’s paper reports individual walking speeds, we regressed his (correlated) data for speeds obtained at 8–19 °C to derive the following relationship:
This crude analysis of Falconer’s data suggests his “large” wireworms would stop moving at ~2 °C, which is similar to our finding that large wireworms should stop moving at 3 °C. The significant effect of wireworm weight on their movement simply reflects that heavier wireworms are larger and can therefore move faster. Although these findings do not reflect how fast wireworms move through the soil, they do indicate how temperature affects wireworm movement.
Effect of temperature on plant emergence and growth
ET50, ET90, and shoot growth rate values all indicate an exponential relation between temperature and plant growth over the 6–22 °C temperature range. This relationship holds true for both cultivars, and considering the similarity in ET50 and ET90 values obtained for the two cultivars for each temperature, this relationship may hold true for spring wheat in general. This response to temperature would suggest plants become less susceptible to wireworm damage as temperature increases, particularly as wireworm activity increases linearly over this temperature range and then levels off. However, both emergence and shoot growth data do not well reflect root growth. The optimal temperature for root and shoot growth differ for many plants, including wheat. Porter and Gawith (1999) in their review of the effect of temperature on the growth and development of wheat, report the mean optimal temperature for leaf initiation and root growth to be 22.0 and <16.3 °C, respectively, and state that the range between the lowest and highest temperature at which growth occurs is much smaller for roots than for shoots and leaves. This is particularly true and important for the first few weeks after planting (Huang et al. 1991), during which, incidentally, plants are also most susceptible to being destroyed by wireworms. The difference in optimal temperatures for root and shoot growth leads to increased root:shoot ratios in many plants (Equiza et al. 2001), including wheat (Huang et al. 1991), which we also observed in this study.
Variables affecting the ability of wireworms to damage wheat seedlings
One environmental (temperature) and three state variables (appetence, weight, and Metarhizium “type”) were evaluated for their effect on the ability of wireworms to damage wheat seedlings. Of these, both temperature and wireworm appetence had a considerable effect and the other two factors were of little importance.
Very little damage was observed at 6 °C, although the significant reduction in stand 2 and 4 weeks after measurement (46 and 60 DAP) indicated wireworms were feeding even at this temperature. Considerable damage was evident at 10–22 °C, but the number of seedlings that failed to emerge or were dead 2 and 4 weeks after measurement did not increase with temperature as expected, with the emergence at 14 °C in the presence of feeding wireworms significantly higher than at 10 and 18 °C. This may indicate that more rapid root growth occurred at 14 °C, as evident from the higher root:shoot ratio observed in the second study and as predicted by the literature. By the 2 and 4 weeks plant stand assessments, there was more mortality at 18 than 14 °C, and the difference in damage between 10 and 14 °C was no longer significant. As wireworm attack on wheat seedlings typically begins with the roots and moves to the seed and stem, it is possible that plants at 14 °C were initially able to cope with the damage to wireworm due to the favorable temperature for root growth, but lost this ability as wireworms attacked the plants elsewhere. In comparison, Falconer (1945a) studied wireworm feeding on soaked wheat seeds and thought the optimum feeding temperature of A. lineatus–obscurus was 18 °C, and that little feeding occurred at 7 °C or lower temperatures.
It was evident that wireworms in the feeding state caused considerably more damage than those in the non-feeding state. As wireworms spend much of each instar in a non-feeding state, selecting larvae at random for use in laboratory bioassays will likely introduce variability in the results, as feeding and non-feeding larvae will not respond similarly. For example, non-feeding wireworms will not orient to germinating wheat in the soil. Wireworm movement in the soil is non-random. Doane et al. (1975) demonstrated that wireworms follow CO2 gradients in the soil to find food, a behavior we have used extensively in the lab to determine if insecticides are effective, elicit repellency, etc. (van Herk and Vernon 2007a; van Herk et al. 2008b), and which makes it easy to pre-select feeding wireworms (e.g., by placing a bait trap with germinating wheat in a wireworm storage tub). Using non-feeding wireworms, or a mixture of feeding and non-feeding wireworms in laboratory bioassays, will give spurious results if evaluating wireworm attraction or repulsion by insecticides (e.g., will lead to over-estimations of an insecticide’s efficacy due to less reduction in plant stand, or appear to increase an insecticide’s repulsiveness due to a lower incidence of wireworm contact).
Considering the range of wireworm weights used in this study, the failure of weight to have a significant effect on plant emergence, growth, and final stand (other than the marginally significant effect on emergence at 22 °C) in either feeding study is surprising. As the majority of the wireworms used in the feeding studies were >15 mg, this suggests that the amount of damage done by a wireworm differs little if it is 2, 3, or 4 years old. This is an unexpected and counter-intuitive result, although it has been reported for other wireworm species. King et al. (1933) report that early instar Selatosomus aeripennis destructor larvae were as destructive to wheat seeds as late instars due to their mode of attack (i.e., due to damage done to the stem of the seedling immediately above the seed). Additional work should be done to determine if small and large larvae of A. obscurus damage wheat seedlings similarly and if the potential to destroy or retard the growth of seedlings changes with wireworm size.
Wireworms infected with Metarhizium often show no symptoms or reduction in their ability to damage wheat seeds until a few days before they die from the fungus (van Herk, personal observation). It is therefore not surprising that no difference was observed between wireworm types in the amount of damage done to wheat seedlings in the final study. We have previously shown that Metarhizium-infected wireworms can cause considerable damage to wheat in a laboratory study (van Herk and Vernon 2011).
Mortality of wireworms from Metarhizium
Mortality of wireworms during a laboratory study from Metarhizium infection is very common (Comstock and Slingerland 1891), and in a previous paper we reported 52 % of wireworms dying during a laboratory feeding study from the pathogen in 25 days (van Herk and Vernon 2011). It is thought that all wireworms collected at PARC contain Metarhizium spores and that an environmental trigger (e.g., temporary exposure to a high temperature) is necessary to induce infection (van Herk and Vernon 2011; Kabaluk and Ericsson 2007). For this reason, and as wireworms were handled carefully and not exposed to insecticides or other stressors either before or during the study, we do not think that the Metarhizium we observed was a secondary infection on weakened or already dying wireworms but was due primarily to the temperature they were kept at inside the pots. The temperature dependence of Metarhizium development within the insect explains the increase in mortality due to Metarhizium with temperature in feeding and non-feeding wireworms of both types in the final study. The significantly higher incidence of mortality in the Metarhizium-infected wireworms confirms that these larvae had a higher field exposure to the fungus or higher level of infection, and justifies our separation of the wireworms used in the final study into the infected and non-infected classes, despite observing no differences in their appearance or behavior when they were selected. The relatively low rate of mortality from Metarhizium in the initial study compared to the final study and previous work (van Herk and Vernon 2011) underscores the variability of Metarhizium infection/exposure in locally collected wireworms. It is noteworthy that in neither feeding study reported here, wireworm mortality from the fungus appeared to be affected by weight, as was suggested in previous work (van Herk and Vernon 2011). The higher incidence of mortality in the feeding wireworms may be due to changes in their metabolism or ability to resist infection just before or just after molting, assuming that their lack of appetence reflects proximity to molting.
Number of wheat seedlings a wireworm can kill
Previously, we have shown that the number of wheat seedlings killed at 15 °C was 1.0 per wireworm in 25 days (van Herk and Vernon 2011). Here we report that at 22 °C wireworms in the feeding state can destroy ~1.5–1.8 seedlings in 14 days, leading to an increase in wireworm weight of ~1 mg. The increase in the number of seedlings a wireworm can kill as the temperature is increased is evident in the final feeding study. Subtracting the number of plants surviving 2 weeks after measurement from the stand in the pots without wireworms at that temperature, suggests a wireworm in a non-feeding state can destroy 0.2, 0.3, 0.4, 0.6, and 0.7 seedlings in 46, 32, 25, 25, and 25 days, respectively, as the temperature is increased from 6 to 22 °C, respectively. The fact that non-feeding wireworms destroyed any seeds at all suggests some had re-entered a feeding state during the study. Wireworms are in a non-feeding state for 1–2 weeks after molting, and for a longer period immediately before the next molt (Vernon and van Herk 2012), suggesting that the low amount of feeding of these “non-feeding” wireworms was from larvae that had recently molted.
A wireworm in the feeding state destroyed 0.3, 1.0, 0.9, 1.3, and 1.4 seedlings, respectively. Together these numbers give a reasonable estimate of the number of wheat seedlings a single medium–large (i.e., >15 mg) larvae of A. obscurus can destroy, provided it is in the feeding state when the seeds are planted. The caveats are important. This study focused on the damage done to wheat seedlings by A. obscurus larvae, an important pest in Europe, parts of Asia, and Canada. The most damaging wireworm species to wheat and other cereal crops in Canada are Hypnoides bicolor (Esch.) and Selatosomus aeripennis destructor (Brown), both of which differ considerably in size and ecology from A. obscurus, and may be able to cause differing amounts of damage.
In conclusion, it is imperative that both temperature and wireworm state be taken into account when conducting either laboratory or field studies. Those conducting insecticide efficacy studies for managing wireworms on wheat need to be aware of wireworm feeding and molting cycles. Conducting such studies in a laboratory or greenhouse environment at temperatures above 14 °C may—depending on species and location—result in considerable mortality of wireworms due to Metarhizium, and in seedlings being more susceptible to wireworm damage than they would be in the field at lower temperatures.
References
Bierkander C (1779) Transactions of the Academy of Sciences in Sweden, p 285. (Cited in: Curtis, J (1860) Farm Insects. Blackie and Son, London, UK)
Burrage RH (1963) Seasonal feeding of larvae of Ctenicera destructor and Hypolithus bicolor (Coleoptera: Elateridae) on potatoes placed in the field at weekly intervals. Ann Entomol Soc Am 56:306–313
Comstock JH, Slingerland MV (1891) Wireworms. Bulletin 33 of the Agricultural Experiment Station. Cornell University, Ithaca
Crombie AC, Darrah JH (1947) The chemoreceptors of the wireworm (Agriotes spp.) and the relation of activity to chemical constitution. J Exp Biol 24:95–109
Davis GRF (1957) Growth and feeding behavior of larvae of Ctenicera aeripennis detructror (Brown) (Coleoptera: Elateridae) I. Effects of carrot slices and seeds of wheat, flax, barley, rye, and alfalfa. Ann Entomol Soc Am 50:578–584
Davis GRF (1971) Phagostimulatory effects of lipids and related substances on the prairie grain wireworm, Ctenicera destructor Brown. Can J Zool 49:1–4
Doane JF (1981) Evaluation of a larval trap and baits for monitoring the seasonal activity of wireworms in Saskatchewan. Environ Entomol 10:335–342
Doane JF, Lee YW, Klinger J, Westcott ND (1975) The orientation response of Ctenicera destructor and other wireworms (Coleoptera: Elateridae) to germinating grain and to carbon dioxide. Can Entomol 107:1233–1252
Equiza M, Miravé JP, Tognetti JA (2001) Morphological, anatomical, and physiological responses related to differential shoot vs root growth inhibition at low temperature in spring and winter wheat. Ann Bot 87:67–76
Evans AC (1944) Observations on the biology and physiology of wireworms of the genus Agriotes Esch. Ann Appl Biol 31:235–250
Evans AC, Gough HC (1942) Observations on some factors influencing growth in wireworms of the genus Agriotes Esch. Ann Appl Biol 29:168–175
Falconer DS (1945a) On the behaviour of wireworms of the genus Agriotes Esch. (Coleoptera: Elateridae) in relation to temperature. J Exp Biol 21:17–32
Falconer DS (1945b) On the movement of wireworms of the genus Agriotes Esch. (Coleoptera: Elateridae) on the surface of the soil and their sensitivity to light. J Exp Biol 21:33–38
Furlan L (1998) The biology of Agriotes ustulatus Schäller (Col., Elateridae) II. Larval development, pupation, whole cycle description and practical implications. J Appl Entomol 122:71–78
Huang BR, Taylor HM, McMichael BL (1991) Growth and development of seminal and crown roots of wheat seedlings as affected by temperature. Environ Exp Bot 31:471–477
Kabaluk JT, Ericsson JD (2007) Environmental and behavioral constraints on the infection of wireworms by Metarhizium anisopliae. Environ Entomol 36:1415–1420
King KM, Arnason P, Glen R (1933) The wireworm problem in field crops of western Canada. Leaflet 35 of the Canadian Department of Agriculture, Entomology Branch, Saskatoon, SK
Lees AD (1943a) On the behaviour of wireworm of the genus Agriotes Esch. (Coleoptera: Elateridae) I. Reactions to humidity. J Exp Biol 20:43–53
Lees AD (1943b) On the behaviour of wireworm of the genus Agriotes Esch. (Coleoptera: Elateridae) II. Reactions to moisture. J Exp Biol 20:54–60
Porter JR, Gawith M (1999) Temperatures and the growth and development of wheat: a review. Eur J Agronomy 10:23–36
van Herk WG, Vernon RS (2007a) Soil bioassay for observing the orientation, feeding, repellency, and post-contact toxicity behaviours of wireworms (Coleoptera: Elateridae) exposed to insecticide treated wheat seed. Environ Entomol 36:1441–1449
van Herk WG, Vernon RS (2007b) Morbidity and recovery of the Pacific Coast wireworm, Limonius canus, following contact with tefluthrin-treated wheat seeds. Entomol Exp Appl 125:111–117
van Herk WG, Vernon RS (2011) Mortality of Metarhizium anisopliae infected wireworms (Coleoptera: Elateridae) and feeding on wheat seedlings is affected by wireworm weight. J Entomol Soc BC 108:38–40
van Herk WG, Vernon RS (2013) Categorization and numerical assessment of wireworm mobility over time following exposure to bifenthrin. J Pest Sci. doi:10.1007/s10340-011-0381-2
van Herk WG, Vernon RS, Roitberg BD (2008a) Repellency of a wireworm, Agriotes obscurus (Coleoptera: Elateridae) on exposure to synthetic insecticides in a soil-less bioassay. Environ Entomol 37:534–545
van Herk WG, Vernon RS, Moffat C, Harding C (2008b) Response of the Pacific Coast wireworm, Limonius canus, and the dusky wireworm, Agriotes obscurus (Coleoptera: Elateridae), to insecticide-treated wheat seeds in a soil bioassay. Phytoprotection 89:7–19
van Herk WG, Vernon RS, Harding C, Roitberg BD, Gries G (2010) Possible aversion learning in the Pacific Coast wireworm. Phys Entomol 35:19–28
Vernon RS, van Herk WG (2012) Wireworms as pests of potato. In: Giordanengo P, Vincent C, Alyokhin A (eds) Insect pests of potato: Global perspectives on biology and management. Academic Press, Elsevier, Amsterdam, pp 103–164
Vernon RS, van Herk WG, Clodius M, Harding C (2009) Wireworm management I. Stand protection versus wireworm mortality with wheat seed treatments. J Econ Entomol 102:2126–2136
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Acknowledgments
We are grateful to six excellent summer students—Amanda Stepto, James Elwood, Bryanna Lepine, Pierig le Pottier, Emmanuelle Bietz, and Shelby Snow—for their cheerful and reliable assistance, and to two anonymous reviewers for their constructive comments on the MS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
van Herk, W.G., Vernon, R.S. Wireworm damage to wheat seedlings: effect of temperature and wireworm state. J Pest Sci 86, 63–75 (2013). https://doi.org/10.1007/s10340-012-0461-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-012-0461-y