Abstract
A survey of potato fields located in the south-eastern region of Himachal Pradesh (India) was carried out in order to find out the natural pathogens infecting the white grub, Brahmina coriacea. About 88 % population of the infected grubs were found to exhibit symptoms of natural bacterial infection during the years 2007–2008. Hence, we attempted to isolate and characterize the most potent bacteria for the management of B. coriacea and tested their insecticidal activity. In this study, ten different bacterial isolates belonging to genera Bacillus, Psychrobacter, Paracoccus, Paenibacillus, Mycobacterium, Staphylococcus and Novosphingobium were isolated from B. coriacea. Bacterial species were identified based on morphology, biochemical tests and homologies of 16S rRNA gene sequences. Pathogenicity tests for all isolated bacteria at 1.0 × 108 cfu/ml of broth were performed on late first instar grubs. Among the bacteria tested, Bacillus cereus induced highest mortality level of 51.85 % within 7 days of treatment followed by Psychrobacter pulmonis (33.33 %), Bacillus psychrodurans (25.93 %), Bacillus pumilus (25.93 %), Paenibacillus tylopili (22.22 %) and Novosphingobium capsulatum (18.52 %). Mortality levels were further increased up to 100 % by B. cereus followed by 88.89 % by P. pulmonis after 30 days of treatment. Our results indicate that B. cereus, P. pulmonis, B. psychrodurans, B. pumilus, P. tylopili and N. capsulatum may be valuable biological control agents for white grubs, B. coriacea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The geographical range of potato (Solanum tuberosum L.) is worldwide and is grown as a major source of food in most countries in temperate climates. Since potato is not a native crop of the sub-tropical region, it is therefore susceptible to many diseases and pest attacks prevalent in abundance in such agro-climates. Potato growing areas located in the north-western hills of India suffer serious damage by soil-dwelling pests belonging to the order Coleoptera including Brahmina coriacea (Hope) (Coleoptera: Scarabaeidae) (Sushil et al. 2008). Initially, first instar grubs of B. coriacea feed on the mother tuber and roots of developing plants. Second instar grubs tend to nibble potato tubers by making small superficial holes. However, the third instar is most active and causes severe damage by making large, shallow and circular holes in potato tubers, thus rendering them unfit for marketing. They remain concealed while feeding on the tubers, and plants continue to grow normally without any reflection of injury on the aerial parts of the plants. Because of this concealed behavior, white grubs remain unnoticed in potatoes during crop development. Populations suddenly increases beyond the economic injury level in places having plentiful tubers. Up to 80 % yield losses in severely infested potato fields have been observed in the higher hills of Shimla in Himachal Pradesh (Chandel et al. 1993; Chandla et al. 2001).
A number of approaches have been employed for controlling the white grubs around the world. As a means of soil pest control, farmers use harmful chemicals without considering pest biology, the residual effect of pesticides in the soil environment or their effect on the health of the consumers. The use of the chemical insecticides is not advisable as the potato tubers are used as food (Ezekiel et al. 1999). Many cultural practices have been used for controlling the white grubs in Himachal Pradesh without an effective outcome (Misra and Chandel 2003). These factors have led to a focus on the development of alternative control measures instead of pesticides. Therefore, integrated pest management has placed great hopes on bio-control agents. The best possible alternatives can be microbial agents like bacteria, fungi, nematodes, viruses, protozoa, and botanical control agents. Microbial control is compatible with biological, toxicological, environmental, and social requirements. Only a few strains of bacteria like Paenibacillus popilliae, Bacillus thuringiensis and Serratia entomophila have been tested against white grubs of different scarab species (Suzuki et al. 1992; Hori et al. 1994; Alm et al. 1997; Hurst et al. 2000; Koppenhofer et al. 2000; Tan et al. 2006). B. cereus has been used successfully as a microbial control agent for the grubs of Amphimallon solstitiale, Melolontha melolontha, Anomala dimidiata and Holotrichia seticollis (Sezen et al. 2005; Selvakumar et al. 2007; Sushil et al. 2008). Other entomopathogenic bacteria isolated from Coleopteran insects include B. circulans, B. sphaericus, P. polymyxa, Streptococcus spp., Micrococcus spp., Yersinia spp. and Enterobacter spp. (Demir et al. 2002; Selvakumar et al. 2003; Sezen et al. 2005).
Given the variety of likely bacterial diseases associated with the white grubs in the south-eastern region of Himachal Pradesh, there is a likelihood of discovering several useful novel strains. Thus, this study was aimed at isolating and identifying potential bacterial pathogens for the management of the important white grub species of the region. Here, we report ten bacterial isolates associated with B. coriacea. Using biochemical tests and sequence analysis of the bacterial 16S rRNA gene, we identified these bacterial isolates by comparing them with those from GenBank. The insecticidal activities of these bacterial isolates were tested against late first instar grubs of B. coriacea. Six out of ten isolates exhibited significant insecticidal activity, suggesting their role as valuable potential bio-control agents.
Materials and methods
Soil sampling and rearing of white grubs
Soil sampling for white grub incidence infesting potato crop was carried out in the potato farms in the south-eastern region of Himachal Pradesh, India, including Shimla (31°N, 77°E, 2,202 m amsl), Shillaroo (31°N, 77°E, 1,820 m amsl) and Kheradhar (30°N, 77°E, 1,048 m amsl) during June to September 2007 and 2008. Sampling was done from the root zone of randomly selected potato plants in 30-cm2 areas up to 20-cm depth and minimum of ten samples from each quadrate of selected location were taken. The samples were placed immediately in containers with soil from the collection site and transported to the laboratory for further studies. Grubs were identified on the basis of (1) body length, (2) head capsule width, and (3) raster pattern (Misra and Chandel 2003). Grubs collected from different locations were reared and kept according to their developmental stage. First instar grubs were reared in trays with maize seedlings as feed (20 grubs/tray). Second and third instar grubs were kept individually in pots, containing a mixture of fine soil and well-rotted farm yard manure (FYM) (1:1) and fed with sliced potato. Collected grubs were checked periodically for any infection until the emergence of adults. Dead larvae were carefully separated and transferred into sterilized vials in order to assess the causes of mortality, whether due to bacteria or any other reasons.
Isolation and identification of bacteria
Grubs showing symptoms of bacterial infection and cadavers of dead grubs were surface-sterilized using 95 % ethanol and 1 % sodium hypochlorite for 2 min each, and then thoroughly washed with sterile distilled water. For the isolation of different pathogenic bacteria combination of methods were used (Fuxa et al. 1997; Travers et al. 1987). Initially non-spore-forming bacteria were isolated by sampling hemolymphs from each larva as the first step, and then each of the same larva was used for the isolation of endospore-forming bacilli. The hemolymph was collected into a microtube by puncturing the grub’s body with a hypodermic needle, and then serially diluted in sterile distilled water and plated on Standard nutrient agar (Sigma Aldrich) media plates. For the isolation of endospore-forming bacilli, surface-sterilized specimens were homogenized in 0.1 % sterile tryptose (Sigma Aldrich) and serially diluted 1,000 times. The supernatant was heated at 80 °C for 10 min, plated on Standard nutrient agar media plates and incubated at 30 °C for 24 h. Bacterial colonies showing distinctive morphologies were isolated on fresh agar plates and subsequently maintained on nutrient agar slants for further use. These served as pure stock cultures for subsequent Gram staining and biochemical characterization. Single colony was subcultured in a tube of nutrient broth and this subculture was used to inoculate test media. Purification was confirmed by Gram staining. Spore staining and acid fast staining were done using staining kits. The sterility of each test medium was carried out by incubating one uninoculated test medium along with the inoculated test medium. Further, biochemical characterization was carried out according to Bergeys’ manual of systematic bacteriology (Kocur 1984; Wayne and Kubica 1986; Juni 2005; Yabuuchi and Kosako 2005; Logan and De Vos 2009; Priest 2009; Schleifer and Bell 2009).
16S rRNA sequence-based identification of bacteria
Genomic DNA was isolated from ten bacterial isolates by CTAB method with some modifications (Doyle and Doyle 1990). Partial fragments of 16S rDNA from genomic DNA samples of bacteria were amplified by PCR utilizing a pair of universal bacterial primers BCF1 5′-CGGGAGGCAGCAGTAGGGAAT-3′ and BCR2 5′-CTCCCCAGGCGGAGTGCTTAAT-3′ (Cano et al. 1994). PCR was performed using 1 μl of the genomic DNA, 0.5 μmol each primer, 10 mM Tris–HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs and 1–2 U Taq polymerase (Qiagen, Germany) in a 50-μl reaction. PCR was performed in a thermal cycler (Bio-Rad, USA) under conditions: initial denaturation at 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 54 °C for 40 s and 72 °C for 1 min; followed by a final extension of 7 min at 72 °C. PCR products were analyzed on 1.5 % agarose gel with ethidium bromide staining. The bands of desired size were cloned into pGEM®-T easy vector (Promega, USA) and sequenced. Sequencing was performed using Big Dye® Terminator cycle sequencing kit (v.3.1; Applied Biosystems, USA) on an automated DNA Sequencer (ABI Prism 3130xl,; Applied Biosystems, USA). Sequences were analyzed using BLASTN program (Zhang et al. 2000) at NCBI (www.ncbi.nlm.nih.gov). Phylogenetic and molecular evolutionary analyses were performed using MEGA 3.1 software (Kumar et al. 2004). The phylogenetic tree was constructed by the neighbor-joining method (Saitou and Nei 1987) using the distance matrix from the alignment, and distances were calculated by the Kimura 2-parameter (Kimura 1980). The reliability of the tree was measured by bootstrap analysis with 1,000 replicates (Felsenstein 1985).
Pathogenicity test and analysis of data
Pathogenicity tests of bacterial isolates against late first instar grubs of B. coriacea were carried out as described by Jackson and Saville (2000) for soil-dwelling insect pests. Each bacterial isolate was grown on 300 ml of LB broth at 37 °C for 72 h, and subjected to constant shaking (150 rpm). The concentration of cfu in the broth culture was estimated by the spread plate technique after heat shocking of the culture broth, and was adjusted with sterile distilled water to obtain 1.0 × 108 cfu/ml of broth. A mixture of soil and FYM (1:1) autoclaved at 15 psi and 121 °C on three alternative days, was used as the substrate for testing isolates against late first instar grubs. A dose of 300 ml of broth culture (1.0 × 108 cfu/ml) was applied to 3 kg of the soil: FYM medium mixed thoroughly and placed 100 g in each plastic pot (15 cm). Final soil moisture content of 15–20 % was maintained by using sterile distilled water throughout the bioassay period by maintaining the initial gravimetric weight. Ten healthy late first instar grubs were released into each pot. Each treatment was replicated thrice. Maize seedlings were transplanted into the containers after the roots were dipped in the respective bacterial culture. Similarly controls were maintained on sterile soil and FYM mixture with maize seedlings. Disease incidences were determined at 7, 15, 21, and 30 days after the treatment. Grubs that appeared to be sluggish with characteristic symptoms of bacterial infection were categorized and monitored for mortality. Grub mortality was recorded for 4–6 weeks. The pathogenesis of all isolates was confirmed by re-isolation of the bacteria from the hemolymph and their bioassay through methodology detailed above. Re-isolation was done by taking three–four grubs as representative samples. Mortality was corrected using Abbott’s formula (Abbott 1925). Corrected mortality data were statistically analyzed using one-way ANOVA using MSTAT-C software. The difference of two means between treatments exceeding critical difference value was taken as significant.
Results
Field survey for infection incidence of white grubs
A survey in potato fields in the south-eastern region of Himachal Pradesh involving Shimla, Shillaroo and Kheradhar was carried out in order to find natural pathogens infecting the white grub during 2007 and 2008. Grubs were found to exhibit a high occurrence of natural bacterial infection (Table 1). A total of 1,351 grubs were observed of which 148 (10.95 %) grubs were found to be infected with bacteria. Infected grubs were pale/light brown/yellow/milky white in color, with a shrunken body and sluggish movement (Fig. 1). Highest bacterial infection (11.99 %) was observed in the grubs collected from Shilaroo followed by Kheradhar (11.81 %), and least bacterial infection (6.96 %) was found in grubs collected from Shimla. The average body length of second and third instar grubs was 12.68 ± 0.09 × 5.04 ± 0.05 mm and 22.48 ± 0.23 × 9.14 ± 0.04 mm, respectively. Head capsule measured 2.35 ± 0.03 mm and 4.42 ± 0.01 mm. Grubs exhibit raster of two parallel rows of 12 pairs of long, stout, inwardly pointed setae on the last segment of the ventral side of the abdomen. They exhibit a Y-shaped anal slit and randomly placed long, straight and sharply pointed small setae with curved ends up to the anterior end of the palidia with random hairs on the dorsal anal lobe.
Isolation and Identification of bacterial isolates
A total of ten bacteria were isolated and characterized from white grubs, B. coriacea, first according to morphological and biochemical characteristics (Tables 2, 3). These results showed that some of the isolated strains were Gram-positive while others were Gram-negative. On the basis of Gram reaction and spore staining, five groups for identification were prepared, viz., Gram-positive cocci, Gram-positive and Gram-negative sporing bacilli, Gram-positive nonsporing bacilli and Gram-negative coccii. In the case of Gram-positive cocci, the catalase test was positive which differentiates Staphylococci from Streptococci. The alkaline phosphatase test was used to distinguish Staphylococci strains. Paracoccus was differentiated from Staphylococcus on the basis of production of β-galactosidase. One of the isolates was Gram-negative, non-motile, coccus-shaped cells that were catalase and oxidase-positive. It produced growth in 6.5 % NaCl with nonpigmented colonies. These characters were consistent with those of genus Psychrobacter. Gram-positive, spore-forming, thick rod-shaped isolates were identified as Bacilli. The colonies were large, raised irregularly and dull. The egg yolk test was used to differentiate strains of Bacillus. Gram-negative spore-forming rod-shaped Bacillus was identified as Paenibacillus. Gram-negative, non-spore-forming, motile rods were identified as Novosphingobium. Nonmotile, non-spore-forming, acid fast and Gram-positive rods were identified as Mycobacterium massiliense. Since the isolate was acid fast but nonpigmented and fast growing, the NaCl tolerance test was done which was found to be positive. The NaCl tolerance test is often used in the identification of rapidly growing Mycobacteria. Standard reactions as mentioned in Bergey’s manual, like pigmentation, growth rate at 30 °C, nitrate reduction, pyrizinamidase, Tween 80 hydrolysis, arylsulfatase, and urease production were carried out for identification of M. massiliense.
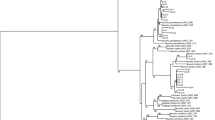
Sequence analysis of the 16S rRNA of isolates indicated that they shared high homologies with other species. All these sequences were submitted to NCBI gene databank, and accession numbers of the isolates were obtained. The partial 547 bp of 16S rRNA sequence of Bacillus psychrodurans strain CPRI3 (GU565559) showed 100 % identity to the strain of B. psychrodurans TSC11. The partial 546 bp of 16S rRNA sequence of Psychrobacter pulmonis strain CPRI4 (GU565560) showed 99 % identity to the strain of P. pulmonis a141. The partial 520 bp of 16S rRNA sequence of Paracoccus marcusii strain CPRI6 (GU565562) showed 98 % identity to the strain of P. marcusii. The partial 546 bp of 16S rRNA sequence of Paenibacillus tylopili strain CPRI7 (GU565563) showed 98 % identity to the strain of P. tylopili MK2. The partial 547 bp of 16S rRNA sequence of Bacillus pumilus strain CPRI18 (GU565570) showed 100 % identity to B. pumilus ZH20. The partial 16S rRNA sequence of 547 bp of Staphylococcus epidermidis strain CPRI9 (GU565564) showed 99 % identity to the strain of S. epidermidis FUA2087. The partial 16S rRNA sequence of 547 bp of Staphylococcus pasteuri strain CPRI11 (GU565566) showed 100 % identity to the strain of S. pasteuri Sp-12. The partial 16S rRNA sequence of 548 bp of B. cereus strain CPRI14 (GU565569) showed 100 % identity to B. cereus WQ9-2. The partial 16S rRNA sequence of 548 bp of Mycobacterium massiliense strain CPRI10 (GU565565) showed 99 % identity to M. massiliense INCQS 594. The partial 16S rRNA sequence of 521 bp of Novosphingobium capsulatum strain CPRI12 (GU565567) showed 99 % identity to N. capsulatum RFNB21. Based on genetic identities, the isolates were selected to construct the phylogenetic tree (Fig. 2).
Phylogenetic tree of bacterial isolates obtained from white grubs. Isolate designations are shown in bold. The optimal tree with the sum of branch length = 0.74379318 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches
Pathogenicity bioassay of bacteria
The results of the insecticidal activities of the ten bacterial isolates at 1.0 × 108 cfu/ml of broth are presented in Table 4 and Fig. 3. In the preliminary pathogenicity bioassay, six out of ten bacterial isolates were found capable of causing a high degree of mortality against late first instar grubs of B. coriacea. Mortality observed in control group was 10 % in 30 days. B. cereus strain CPRI14 was found to be the most pathogenic with the highest mortality rate of 51.85 % after 7 days of treatment. The mortalities recorded for P. pulmonis strain CPRI4, B. psychrodurans strain CPRI3, P. tylopili strain CPRI7, B. pumilus strain CPRI8, and N. capsulatum strain CPRI12 were 25.93, 22.22, 25.93, 18.52, and 33.33 %, respectively, after 7 days of treatment. Significantly low mortalities in the range 3.70–14.81 % were observed for M. massiliense CPRI10, S. pasteuri strain CPRI11, S. epidermidis strain CPRI9, and P. marcusii strain CPRI6. After 15 days of treatments, mortality of grubs was increased by all the isolates, viz., 77.78 % by B. cereus strain CPRI14, 55.56 % by P. pulmonis strain CPRI4, 44.44 % by B. pumilus strain CPRI8, 37.04 % by both, B. psychrodurans strain CPRI3 and P. tylopili strain CPRI7, and 33.33 % by N. capsulatum strain CPRI12. The rest of the isolates were able to cause 3.70–14.81 % mortality after 15 days of treatment. Mortality was further increased up to 96.30 % in the case of B. cereus strain CPRI14, 74.07 % by P. pulmonis strain CPRI4, 66.67 % by B. pumilus strain CPRI8, 62.96 % by B. psychrodurans strain CPRI3, and 59.26 % by each P. tylopili strain CPRI7 and N. capsulatum strain CPRI12. Mortality in the case of other isolates was not increased much and reached up to 14.81–25.93 % only. After 30 days of treatment, B. cereus strain CPRI14 was able to cause 100 % mortality in first instar grubs followed by 88.89 % by P. pulmonis strain CPRI4, 81.48 % by each B. pumilus strain CPRI8 and B. psychrodurans strain CPRI3, 70.37 % by N. capsulatum strain CPRI12, and 66.67 % by P. tylopili strain CPRI7. The rest of the species caused mortalities in the range 7.04–29.63 after 30 days. When re-isolation was carried out, it was possible to get inoculated bacteria, and the inoculated species were found to be predominating (above 98 % in case of all the isolates) as compared to other bacterial species.
Discussion
Recently, there has been an increasing interest in finding more pathogenic and safer bacterial isolates against insect pests. Although there have been several studies for controlling B. coriacea grubs, studies on its microbiota have so far been neglected. Studies on insect microbiota can be the best way to utilize the pathogens of harmful insects for their own biological control. To date, no study has been carried out on the determination of bacterial isolates and the investigation of biological control agents of B. coriacea. For the present study, we employed a range of growth media (data not shown) to examine different samples obtained from diseased grubs, and recovered a total of ten strains of bacteria. Although the majority of biochemical tests were similar to those available in the literature, the increasing availability of gene sequences has contributed greatly to the characterization of bacterial species. For bacterial identification, conventional results are sufficient most of the time for identifying a new bacterial isolate, but occasionally it is very difficult to identify some bacteria based only on conventional tests (Bahar and Demirbag 2007). Hence, we supported identifications of bacterial isolates using 16S rRNA gene sequence analysis, and determined their pathogenicity. Since the 1980s, sequencing of the 16S rRNA gene has been used as an important tool for phylogenetic analysis and classification of bacteria (Lane et al. 1985). The 16S rRNA gene contains regions well conserved in all organisms that are ideal for primer design, PCR, or sequencing and sequence alignment. It also contains species-specific variable regions that allow species identification. Therefore, sequence analysis of the 16S rRNA gene has become a powerful technology for identification of bacterial isolates.
The bacterial genera analyzed in this study encompassed a range of entomopathogenic, phytopathogenic, and soil saprophytic organisms. Insect-associated bacterial flora have been reported earlier on several insects, but bacterial isolates identified in the present study are all new records from grubs of B. coriacea. However, a few of them have already been isolated from other insect and white grub species. Five bacterial isolates, viz. P. pulmonis strain CPRI4, B. psychrodurans strain CPRI3, P. tylopili strain CPRI7, N. capsulatum strain CPRI12, M. massiliense strain CPRI10, and P. marcusii strain CPRI6 had never been reported from any insect species. Earlier, B. cereus has been a well-known insect pathogen and had been isolated from grubs of A. solstitiale, M. melolontha and A. dimidiata (Sezen et al. 2005; Selvakumar et al. 2007; Sushil et al. 2008). P. pulmonis is a non-spore-forming, non-motile bacterium and can grow in 6.5 % Nacl. P. pulmonis had been isolated from Dermanyssus gallinae (Mesostigmata: Dermanyssidae) (Valiente et al. 2009). B. pumilus is known to exhibit antifungal activity and plant growth regulator activity, and its spores are extremely resistant to UV (Bottone and Peluso 2003; Joo et al. 2004; Link et al. 2004). This bacterium is non-toxic to human and animals (USEPA 2004); however, Bentur et al. (2007) has reported central nervous system infection in a child due to B. pumilus. B. pumilus had been reported from several Coleopteran species such as Scolytus multistriatus (French et al. 1984), Limonius canus (Lacey et al. 2007), Dendroctonus micans and Rhizophagus grandis (Yaman et al. 2010), but no report of its identification from white grub has been encountered. B. psychrodurans is spore forming, non motile bacteria and can grow at 30 °C to 35 °C. This bacterium was earlier identified from D. gallinae (Abd El-Rahman et al. 2002; Groudieva et al. 2003; Heuchert et al. 2004). N. capsulatum is a non-spore-formimg, aerobic, non-motile and chemo-organotrophic bacterium which inhabits soil, roots of rose tree, clinical specimens, stocked distilled water, coastal plain sediments, and fluidized bed reactors (Takeuchi et al. 2001). P. tylopili is a spore-forming, chitinolytic bacterium and has been isolated from the mycorhizosphere of Tylopilus felleus (Boletales: Boletaceae) (Kuisiene et al. 2008). P. tylopili is the species which has never been isolated from any arthropod. Four species isolated in the present studies: S. epidermidis, S. pasteuri, P. marcusii, and M. massiliense are well-known human pathogens. S. pasteuri has earlier been isolated from grubs of Costelytra zealandica (Ray et al. 2007). S. epidermidis has previously been recorded from many insects (Davidson et al. 2000; Zouache et al. 2009). P. marcusii has been shown to produce carotenoids and is under intellectual property protection (Harke et al. 1998). M. massiliense was validated as a species separate from the M. chelonae––M. abscessus group in 2006 (Euzeby 2007). It has been isolated from several pulmonary specimens (Adekambi et al. 2004; Simmon et al. 2007; Kim et al. 2007).
In the preliminary pathogenicity bioassays, six out of ten bacterial isolates, viz. B. cereus strain CPRI14, B. psychrodurans strain CPRI3, B. pumilus strain CPRI8, P. tylopili strain CPRI7, P. pulmonis strain CPRI4, and N. capsulatum strain CPRI12 were found to be highly pathogenic against late first instar grubs of B. coriacea. Other bacterial isolates were also found capable of causing varying degree of mortality. B. cereus strain CPRI14 caused 100 % mortality in first instar grubs after 30 days of treatment. B. cereus had already been reported to cause pathogenicity in other insects and a few white grub species. The mortalities of the grubs of A. solstitiale has been found to be 90 % with B. cereus isolated from A. solstitiale and 75 % with B. cereus from M. melolontha within 10 days (Sezen et al. 2005). B. cereus strain WGPSB-2 had been reported to cause 92 and 67 % mortality in grubs of A. dimidiata and H. seticollis, respectively (Selvakumar et al. 2007; Sushil et al. 2008). Though some strains of B. cereus have been known as opportunistic human pathogens with their ability to produce enteric toxins, some strains of B. cereus have many agronomically useful traits, such as antibiotic production for plant disease suppression (Handelsman et al. 1996). The insecticidal property of B. cereus has been attributed to the production of the lipase toxin phospholipase C (Lysenko 1972a, b) and the paralytic toxin sphingomyelinase C (Nishiwaki et al. 2004). A synergistic action between the antibiotic zwittermicin produced by B. cereus and the crystal toxins of B. thuringeinesis var. kurstaki has been reported in controlling the gypsy moth (Broderick et al. 2000). Recently, Abi Khattar et al. (2009) reported that dlt operon of B. cereus is required for resistance to cationic antimicrobial peptides and virulence in insects. Pathogenicity of B. cereus against other scarab grub species has also been reported previously. A high degree of mortality due to P. pulmonis strain CPRI4 (88.89 %) was recorded for the first time in the present study. There are no previous reports of such studies conducted on this bacterium or its association with any insect. Human infection by Psychrobacter species is rare; however, a few case reports of infection by other Psychrobacter species have been reported (Bowman et al. 1996; Guttigoli and Zaman 2000). Other isolates in the present study, B. pumilus strain CPRI8 and B. psychrodurans strain CPRI3, caused 81.48 % mortality in the larvae of B. coriacea after 30 days of treatment. B. pumilus is a known insect pathogen for possible use in controling insect pests (Thiery and Frachon 1997). An insecticidal activity of B. pumilus has been earlier reported by Erturk et al. (2008) and was found to be highly toxic to the larvae of L. decemlineata causing 95.7 % mortality. B. psychrodurans is recently discovered, psychrotrophic bacterium which is able to grow at lower temperature −2 °C to 0 °C (Abd El-Rahman et al. 2002). Thus, the use of B. psychrodurans may be a plus point at the temperate environments of potato growing areas. Pathogenicity test of B. psychrodurans against any insect had not been done earlier. No report of human infection by B. psychrodurans and P. tylopili has yet been encountered. P. tylopili is a chitinolytic bacterium (Kuisiene et al. 2008) and no insect pathogenicity studies have been previously carried out. N. capsulatum has also caused significant mortality of first instar grubs, but again there is no previous report of its pathogenecity for any insect. N. capsulatum is also a psychrophilic bacterium and no reports of human pathogenecity have been encountered till date. Significantly low mortalities (18.52–29.63 %) were caused by M. massiliense strain CPRI10, P. marcusii strain CPRI6, S. pasteuri strain CPRI11, and S. epidermidis strain CPRI9. M. massiliense and P. marcusii are well-known human pathogens (Chiba et al. 2000; Adekambi et al. 2004; Simmon et al. 2007; Leao et al. 2009), but have never been found associated with any insect, and their pathogenecity studies against any insect had not previously been carried out. However, the remaining two species, S. pasteuri and S. epidermidis, are also known for infections in human and other animals (Roth and James 1988; Kawamura et al. 1998; Olayide and Bamidele 2008; Nelson et al. 2009), but they have also been found associated with insects, and the pathogenicity of S. epidermidis has been reported in Drosophila melanogaster (Diptera: Drosophilidae) (Needham et al. 2004).
The long time taken by the grubs to die is attributed to the fact that grubs feeding in the soil consume spores which germinate in the larval gut and penetrate into the hemocoel. A period of vegetative growth is followed by asynchronous sporulation and death of the larvae. The time taken from ingestion to establishment of bacteria is a few days to several weeks during which grubs generally change in color, stop feeding, show sluggish movement, and die due to bacterial septicemia (Sushil et al. 2008; Mashtoly et al. 2009). As the feeding is stopped, because of the cascade initiated by the bacteria, even if the grub is not dead, no damage is expected from a dying grub, after dying and decomposition of the grub’s body, billions of spores are released into the soil which can initiate further cascades in other grubs if ingested.
In the present study, most of the potential bacteria are spore-forming; however, P. pulmonis is a non-spore-forming bacterium with high pathogenesis. Spore-forming bacteria are generally known to be more effective, whereas non-spore-forming bacteria are considered less pathogenic when they occur in the digestive tract of an insect, but may be very pathogenic if they are able to enter the insect’s hemocoel. Thus, diseases caused by non-spore-forming bacteria generally rely on a conditional factor to gain entrance into the hemocoel. Once in the hemocoel, many non-spore-forming bacteria multiply rapidly and may cause death of the insect from bacterial septicemia. According to Coppel and Martins (1977), non-spore-forming bacterial pathogens include all of the potential pathogens for the insects. Potential pathogens do not normally multiply in the gut, but they can establish themselves in the haemocoel if they have enough time to pass through the wall and enter susceptible cells. Several non-spore-forming bacteria, Serratia entomophila, Serratia marcescens and Melissococcus pluton are the well-known insect pathogens (Jackson et al. 1992; Yilmaz et al. 2006; Muratoglu et al. 2009; Sevim et al. 2010). Hence non-spore-forming bacteria like P. pulmonis and N. capsulatum, in addition to spore-formers, can prove beneficial in the control of B. coriacea grubs. Further testing of all the potential bacteria against second and third instar grubs, eggs, and beetles can provide more information on their pathogencity.
Conclusion
This is the first detailed investigation of isolation and identification of ten bacterial species belonging to seven genera associated with B. coriacea white grubs based on homologies of 16S rRNA gene sequences. All bacterial species encountered in the present study are new records from white grubs except B. cereus and S. pasteuri, and some of the species viz., B. psychrodurans, P. tylopili, P. pulmonis, M. massiliense, and P. marcusii, have not previously been reported from any insect. In preliminary pathogenecity tests, some of them appear to be promising for use as bicontrol agents. The present studies have provided us a framework for further investigation and for generating hypothesis to conduct pathogenicity tests in different developmental stages of white grubs. Further studies are needed for strain optimization and development of better substrates for mass production and practical uses. Application of isolates on different stages of grubs including second and third instar grubs, eggs, and beetles are also needed to understand their mortality effects. Side effects and safety tests with beneficial insects, mammals, and human cells need to be conducted.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Abd El-Rahman HA, Fritze D, Sproer C, Claus D (2002) Two novel psychrotolerant species, Bacillus psychrotolerans sp. nov. and Bacillus psychrodurans sp. nov., which contain ornithine in their cell walls. Int J Syst Evol Microbiol 52:2127–2133
Abi Khattar Z, Rejasse A, Destoumieux GD, Scoubas JM, Sanchis V, Lereclus DA, Givaudan M, Kallassy C, Nielsen L, Gaudriault S (2009) The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects. J Bacteriol 191(22):7063–7073
Adekambi T, Reynaud-Gaubert M, Greub G, Gevaudan MJ, La Scola B, Raoult D, Drancourt M (2004) Amoebal coculture of Mycobacterium massiliense sp. nov. from the sputum of a patient with hemoptoic pneumonia. J Clin Microbiol 42:5493–5501
Alm S, Villani M, Yeh T, Shutter R (1997) Bacillus thuringiensis serovar japonensis strain Buibui for control of Japanese and Oriental beetle larvae (Coleoptera: Scarabaeidae). Appl Entomol Zool 32:477–484
Bahar AA, Demirbag Z (2007) Isolation of pathogenic bacteria from Oberea linearis (Coleptera: Cerambycidae). Biologia 62:13–18
Bentur HN, Dalzell AM, Riordan FAI (2007) Central venous catheter infection with Bacillus pumilus in an immunocompetent child: a case report. Ann Clin Microbiol Antimicrob 6:12
Bottone EJ, Peluso RW (2003) Production by Bacillus pumilus (MSH) of an antifungal compound that is active against Mucoraceae and Aspergillus species: preliminary report. J Med Microbiol 52:69–74
Bowman JP, Cavanagh J, Austin JJ, Sanderson K (1996) Novel Psychrobacter species from Antarctic ornithogenic soils. Int J Syst Bacteriol 46:841–848
Broderick NA, Goodman RM, Raffa KF, Handelsman JO (2000) Synergy between zwittermicin A and Bacillus thuringiensis subsp. kurstaki against gypsy moth (Lepidoptera: Lymantridae). Environ Entomol 29:101–107
Cano RJ, Borucki MK, Higby-Schweitzer M, Poinar HN, George O, Poinar JR, Kerri JP (1994) Bacillus DNA in fossil bees: an ancient symbiosis? Appl Environ Microbiol 60:2164–2167
Chandel RS, Chander R, Gupta PR (1993) Toxicity of some soil insecticides to immature stages of Brahmina coriacea (Hope). J Soil Biol Ecol 13(2):103–107
Chandla VK, Raj D, Sharma A, Garg ID, Verma KD, Raman R (2001) Serious attack of white grub in potato fields at Shimla. J Indian Potato Assoc 28(1):125–126
Chiba M, Kono M, Hoshina S, Komatsu M, Kitagawa Y, Iizuka M, Watanabe S (2000) Presence of bacterial 16S ribosomal RNA gene segments in human intestinal lymph follicles. Scand J Gastroenterol 35:824–831
Coppel CH, Martins JW (1977) Biological insect pest suppression. Springer, Berlin
Davidson EW, Rosell RC, Hendrix DL (2000) Culturable bacteria associated with the whitefly, Bemisia argentifolii (Homoptera: Aleyrodidae). Florida Entomol 83:159–171
Demir I, Sezen K, Demirbag Z (2002) The first study on bacterial flora and biological control agent of Anoplus roboris (Sufr., Coleoptera). J Microbiol 40:104–108
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Erturk O, Yaman M, Irfan A (2008) Effects of four Bacillus spp. of soil origin on the Colorado potato beetle Leptinotarsa decemlineata (Say). Entomol Res 38:135–138
Euzeby JP (2007) List of bacterial names with standing in nomenclature. Society for systematic and veterinary bacteriology, Toulouse, France. http://www.bacterio.cict.fr/m/mycobacterium.html. Accessed 4 Oct 2011
Ezekiel R, Sukumaran NP, Shekhawat GS (1999) Potato: a wholesome food. In: Technical Bulletin, vol 49. Central Potato Research Institute, Shimla, p 132
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
French JRJ, Robinson PJ, Minko G, Pahl P (1984) Response of the European elm bark beetle, Scolytus multistriatus, to host bacterial isolates. J Chem Ecol 10:1133–1149
Fuxa JR, Kunimi Y, Nakai M (1997) Research methods for microorganisms interacting with arthropods in soil. In: Hurst CJ, Kundsen GR, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology, vol 2. ASM Press, Washington, DC, p 894
Groudieva T, Grote R, Antranikian G (2003) Psychromonas arctica sp. nov., a novel psychrotolerant, biofilm-forming bacterium isolated from Spitzbergen. Int J Syst Evol Microbiol 53:539–545
Guttigoli A, Zaman MM (2000) Bacteremia and possible endocarditis caused by Moraxella phenylpyruvica. South Med J 93:708–709
Handelsman J, Raffel J, Mester EH, Wunderlich L, Grau CR (1996) Biological control of damping off of alfalfa seedlings with Bacillus cereus UW 85. Appl Environ Microbiol 56:713–718
Harke M, Hirschberg J, Oren A (1998) Paracoccus marcusii sp. nov., an orange Gram-negative coccus. Int J Syst Evol Microbiol 48:543–548
Heuchert A, Glockner FO, Amann R, Fischer U (2004) Psychrobacter nivimaris sp. nov., a heterotrophic bacterium attached to organic particles isolated from the South Atlantic (Antarctica). Syst Appl Microbiol 27:399–406
Hori H, Suzuki N, Ogiwara K, Himejima M, Indrasith LS, Minami S, Sato R, Ohba M, Iwahana H (1994) Characterization of larvicidal toxin protein from Bacillus thuringiensis serovar Japonensis strain Buibui specific for scarabaeidae beetles. J Appl Bacteriol 76:307–313
Hurst MR, Glare TR, Jackson TA, Ronson C (2000) Plasmid located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J Bacteriol 182:5127–5138
Jackson TA, Saville DJ (2000) Bioassays of replicating bacteria against soil-dwelling pests. In: Navon A, Ascher KRS (eds) Bioassays of entomopathogenic microbes and nematodes. CABI, Wallingford, pp 73–94
Jackson TA, Pearson JF, O’Callaghan MO, Mahanty HK, Willocks MJ (1992) Pathogen to product-development of Serratia entomophila (Enterobacteriaceae) as a commercial biological control agent for the New Zealand grass grub (Costelytra zealandica). In: Jackson TA, Glare TR (eds) Use of pathogens in scarab pest management. Intercept, Andover, pp 191–198
Joo GJ, Kim YM, Lee IJ, Song KS, Rhee IK (2004) Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macroides and Bacillus pumilus. Biotechnol Lett 26:487–491
Juni E (2005) Genus Psychrobacter, Juni and Heym 1986, 389VP. In: Boone DR, Brenner DJ, Castenholz RW, Garrity GM, Krieg NR, Staley JT (eds) Bergey’s manual of systematic bacteriology, Part A, vol 2, 2nd edn. Springer, New York, pp 437–441
Kawamura Y, Hou XG, Ferdousi S, Hirose K, Miyake M, Shu SE, Ezaki T (1998) Distribution of Staphylococcus species among human clinical specimens and emended description of Staphylococcus caprae. J Clin Microbiol 36(7):2038–2042
Kim HY, Yun YJ, Park CG, Lee DH, Cho YK, Park BJ, Joo SI, Kim EC, Hur YJ, Kim BJ, Kook YH (2007) Outbreak of Mycobacterium massiliense infection associated with intramuscular injections. J Clin Microbiol 45:3127–3130
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kocur M (1984) Genus Paracoccus, Davis 1969, 384AL. In: Krig NR, Hold JG (eds) Bergey’s manual of systematic bacteriology, Section-4, vol 1. Williams & Wilkins, Baltimore, pp 399–402
Koppenhofer AM, Wilson M, Brown I, Kaya HK, Gaugler R (2000) Biological control agents for white grubs (Coleoptera: Scarabaeidae) in anticipation of the establishment of the Japanese beetle in California. J Econ Entomol 93:71–80
Kuisiene N, Raugalas J, Sproer C, Kroppenstedt RM, Stuknyte M, Chitavichius D (2008) Paenibacillus tylopili sp. nov., a chitinolytic bacterium isolated from the mycorhizosphere of Tylopilus felleus. Folia Microbiol 53(3):433–437
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Lacey LA, Unruh TR, Simkins H, Thomsen-Archer K (2007) Gut bacteria associated with the pacific coast wireworm, Limonius canus, inferred from 16S rDNA sequences and their Implications for Control. Phytoparasitica 35(5):479–489
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci USA 82:6955–6959
Leao SC, Tortoli E, Viana-Niero C, Ueki SYM, Lima KVB, Lopes ML, Yubero JM, Maria C, Garcia MJ (2009) Characterization of mycobacteria from a major Brazilian outbreak suggests that revision of the taxonomic status of members of the Mycobacterium chelonae-M. abscessus group is needed. J Clin Microbiol 47(9):2691–2698
Link L, Sawyer J, Venkateswaran K, Nicholson W (2004) Extreme spore UV resistance of Bacillus pumilus isolates obtained from an ultraclean spacecraft assembly facility. Microb Ecol 47:159–163
Logan AN, De Vos P (2009) Genus Bacillus Cohn De Vos 1872, 174AL. In: De Vos P, Garrity GM, Krieg NR, Ludwid W, Rainey FA, Schleifer KH, Whileman WB (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, pp 21–128
Lysenko O (1972a) Pathogenicity of Bacillus cereus for insects. I. Production of phospholipase C. Folia Microbiol 17:221–227
Lysenko O (1972b) Pathogenicity of Bacillus cereus for insects. II. Toxicity of phospholipase C for Galleria mellonella. Folia Microbiol 17:228–231
Mashtoly TA, EL-Zemaity MS, Hussien MI, Alm SR (2009) LC and LD50 values of Bacillus thuringiensis Serovar japonensis strain buibui toxin to oriental beetle and northern masked chafer larvae (Coleoptera: Scarabaeidae). J Econ Entomol 102(5):1891–1895
Misra SS, Chandel RS (2003) Potato white grubs in India. In: Technical Bulletin, vol 60. Central Potato Research Institute, Shimla, p 8
Muratoglu H, Kati H, Demirbag Z, Sezen K (2009) High insecticidal activity of Leclercia adecarboxylata isolated from Leptinotarsa decemlineata (Col.: Chrysomelidae). Afr J Biotechnol 8(24):7115
Needham AJ, Kibart M, Crossley H, Ingham PW, Foster SJ (2004) Drosophila melanogaster as a model host for Staphylococcus aureus infection. Microbiology 150:2347–2355
Nelson A, Hultenby K, Hell E, Riedel HM, Brismar H, Flock JI, Lundahl J, Giske CG, Marchini G (2009) Staphylococcus epidermidis isolated from newborn infants express pilus-like structures and are inhibited by the cathelicidin-derived antimicrobial peptide LL37. Pediatr Res 66(2):174–178
Nishiwaki H, Ito K, Otsuki K, Yamamoto H, Komai K (2004) Purification and functional characterization of insecticidal sphingomyelinase C produced by Bacillus cereus. Eur J Biochem 271:601–606
Olayide AJ, Bamidele AA (2008) Transmission of bacterial isolates through all the developmental stages of dog ticks (bacteriological evidence). J Anim Vet Adv 7(8):959–962
Priest FG (2009) Genus Paenibacillus Ash Priest & Collins, 1994, 852VL. In: De Vos P, Garrity GM, Krieg NR, Ludwid W, Rainey FA, Schleifer KH, Whileman WB (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, pp 269–295
Ray JL, Helga KA, Sandra Y, Nielsen KM, O’Callaghan M (2007) An assessment of the potential of herbivorous insect gut bacteria to develop competence for natural transformation. Environ Biosafety Res 6:135–147
Roth RR, James WD (1988) Microbial ecology of the skin. Annu Rev Microbiol 42:441–464
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schleifer KH, Bell JH (2009) Genus Staphylococcus, Rosenberg 1884, 18L. In: De Vos P, Garrity GM, Krieg NR, Ludwid W, Rainey FA, Schleifer KH, Whileman WB (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, pp 392–421
Selvakumar G, Sushil SN, Bhatt JC, Singh RD (2003) Isolation of Yersinia sp. from diseased white grub larvae. Indian. J Microbiol 43(3):211–212
Selvakumar G, Mohan M, Sushil SN, Kundu S, Bhatt JC, Gupta HS (2007) Characterization and phylogenetic analysis of an entomopathogenic Bacillus cereus strain WGPSB-2 (MTCC 7182) isolated from white grub Anomala dimidiata (Coleoptera: Scarabaeidae). Biocontrol Sci Technol 17:525–534
Sevim A, Demirbag Z, Demir I (2010) New study on the bacteria of Agrotis segetum Schiff. (Lepidoptera: Noctuidae) and their insecticidal activities. Turk J Agric For 34. doi:10.3906/tar-0902-9
Sezen K, Demir I, Kati H, Demirbag Z (2005) Investigations on bacteria as a potential biological control agent of summer chafer, Amphimallon solstitiale L. (Coleoptera: Scarabaeidae). J Microbiol 43:463–468
Simmon KE, Pounder JI, Greene JN, Walsh F, Anderson CM, Cohen S, Petti CA (2007) Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J Clin Microbiol 45:1978–1980
Sushil SN, Mohan M, Selvakumar G, Bhatt JC, Gupta HS (2008) Isolation and toxicity evaluation of bacterial entomopathogens against phytophagous white grubs (Coleoptera: Scarabaeidae) in Indian Himalayan hills. Int J Pest Manag 54(4):301–307
Suzuki N, Hori H, Ogiwara K, Asano S, Sato R, Ohba M, Iwahana H (1992) Insecticidal spectrum of a novel isolate of Bacillus thuringiensis serovar Japonensis. Biol Control 2:136–142
Takeuchi M, Hamana K, Hiraishi A (2001) Proposal of the genus Sphingomonas sensu stricto and three new genera, Sphingobium, Novosphingobium and Sphingopyxis, on the basis of phylogenetic and chemotaxonomic analyses. Int J Syst Evol Microbiol 51:1405–1417
Tan B, Jackson TA, Hurst MR (2006) Virulen1ce of Serratia strains against Costelytra zealandica. Appl Environ Microbiol 72:6417–6418
Thiery I, Frachon E (1997) Identification, isolation, culture and preservation of entomopathogenic bacteria. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, London, pp 55–77
Travers RS, Martin AWP, Reichelderfer CF (1987) Selective process for efficient isolation of soil Bacillus spp. Appl Environ Microbiol 53:1263–1266
US Environmental Protection Agency Office of Pesticide Programs (2004) Biopesticides registration action document, Bacillus pumilus strain QST 2808 (PC Code 006485)
Valiente MC, Thioulouse J, Chauve C, Normand P, Zenner L (2009) Bacterial taxa associated with the hematophagous mite Dermanyssus gallinae detected by 16S rRNA PCR amplification and TTGE fingerprinting. Res Microbiol 160:63–70
Wayne LG, Kubica GP (1986) Genus Mycobacterium Lehman & Neuman 1896, 363AL. In: Peter HA, Mair NS, Sharpe ME, Hold JG (eds) Bergey’s manual of systematic bacteriology, vol 2, Section-16. Williams & Wilkins, Baltimore, pp 1436–1457
Yabuuchi E, Kosako Y (2005) Genus Novosphingobium Yabuuchi, Yanno, Oaizu, Hashimoto, Ezaki, Yamamoto 1990, 321VP. In: Boone DR, Castenholz RW, Garrity GM, Brenner DJ, Krieg NR, Staley JT (eds) Bergey’s Manual of systematic Bacteriology, Part C, vol 2, 2nd edn. Springer, New York, pp 234–258
Yaman M, Erturk O, Aslan I (2010) Isolation of some pathogenic bacteria from the great spruce bark beetle, Dendroctonus micansand its specific predator Rhizophagus grandis. Folia Microbiol 55(1):35–38
Yilmaz H, Sezen K, Katil H, Demirbag Z (2006) The first study on the bacterial flora of the European spruce bark beetle, Dendroctonus micans (Coleoptera: Scolytidae). Biologia (Bratisl) 61(6):679–686
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7(1–2):203–214
Zouache K, Tran-Van V, Voronin D, Mavingui P (2009) Composition of bacterial communities associated with natural and laboratory populations of Asobara tabida infected with Wolbachia. Appl Environ Microbiol 75(11):3755–3764
Acknowledgments
The authors are grateful to the Indian Council of Agricultural Research, New Delhi, for funding this project. A.S. gratefully acknowledges the Senior Research Fellowship awarded by the Indian Council of Agricultural Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by "Communicated by M. Traugott" .
Rights and permissions
About this article
Cite this article
Sharma, A., Thakur, D.R., Kanwar, S. et al. Diversity of entomopathogenic bacteria associated with the white grub, Brahmina coriacea . J Pest Sci 86, 261–273 (2013). https://doi.org/10.1007/s10340-012-0459-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-012-0459-5