Abstract
The aim of this study was to investigate the side-effects of fungicides on the physiological activities of plant growth promoting rhizobacteria with intrinsic phosphate-solubilizing potential. The fungicide-tolerant and phosphate-solubilizing bacterial strain PS19 was isolated from the mustard rhizosphere and identified as Klebsiella sp. following 16S rDNA sequencing. The Klebsiella sp. strain PS19 normally, produced plant growth promoting (PGP) substances in substantial amount. In this study, four fungicides of different chemical families (tebuconazole, hexaconazole, metalaxyl, and kitazin) at the recommended, two and three times of the recommended rates decreased the PGP attributes of the strain PS19 in fungicide-concentration dependent manner. Moreover, fungicides at the recommended dose had slight inhibitory effect while the dose higher than the recommended ones reduced the PGP traits (phosphate solubilization, salicylic acid, 2,3-dihydroxy benzoic acid, and indole-3-acetic acid production except exo-polysaccharides, hydrogen cyanate and ammonia production) significantly. Of the four fungicides, tebuconazole generally showed the maximum toxicity to the PGP activities of the strain PS19. The results of this study inferred that fungicides, which are used to control various fungal pests detrimental for the crop productivity, must be examined in vitro for their possible adverse impacts on plant-beneficial rhizobacteria before the field application. This study also revealed an additional aspect of the toxicological mechanisms of the fungicides through which they may suppress the plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various phytopathogens are responsible for the great damage for the agricultural productivity all over the world. One of the major approaches for the control of crop diseases caused by phytopathogens is the chemical control (Jaharamma et al. 2009). The overuse of pesticides including fungicides in agricultural soils has led to the lethal consequences to the useful arthropods and beneficial microbes as well as their inherent plant growth-promoting activities (Moorman 1989; Wani et al. 2005). Besides, a significant proportion of pesticides may accumulate into soils after repeated and injudicious application and has led to the soil pollution (Rodriguez and Fraga 1999). Further, pesticide concentrations higher than the recommended field rates not only adversely affect the phosphate solubilizing potential of rhizobacteria but also decrease the crop yield significantly (Ahemad and Khan 2011). Regular application of chemical pesticides escalated other soil problems such as structural degradation, reduction of organic matter and soil colloidal content, accumulation of phosphorous as an insoluble form and agricultural residues in soils (Rodriguez and Fraga 1999). Phosphate-solubilizing bacteria are known to solubilize phosphate (P) and utilize carbohydrates of crop residues. Therefore, P-solubilizing and plant growth promoting rhizobacteria (PGPR) with multiple functional traits are preferred to enhance mineralization and decomposition process (Jaharamma et al. 2009). Moreover, siderophores and cyanide production ability in various P-solubilizing bacteria are linked to antagonistic and disease suppressing activity against various plant pathogens (Oves et al. 2009). Plant growth promoting rhizobacteria (PGPR) including phosphate solubilizing bacteria improve the growth and health of plants by P solubilization, biological nitrogen fixation, improving nutrient-uptake and synthesizing plant growth regulators (Zaidi et al. 2009). Moreover, biological control of plant pathogens through antibiotics, lytic enzymes, hydrogen cyanide, and siderophores produced by PGPR can also improve plant growth (Khan et al. 2009).
Although Klebsiella has been used as a versatile model to study a range of N2-fixation processes and other mechanisms, plant growth-promoting traits of Klebsiella sp. of rhizosphere-niche have not comprehensively been explored. Further, the most direct approach to analyze the specific agrochemical (including fungicides) induced changes in microbial community is the use of tolerant microorganisms against the same agrochemical (Oves et al. 2009; Ahemad and Khan 2011). In addition, studies on the effect of various fungicides have largely been focused on the changes in populations of soil microflora including PGPR. Furthermore, the reports concerning in vitro PGP activities of PGPR, specifically Klebsiella sp. in the presence of fungicides are rare. Considering these scientific gaps, the present study was, therefore, designed to evaluate the effects of four fungicides, namely, tebuconazole, hexaconazole, metalaxyl and kitazin (Table 1), at the recommended (1×), double (2×) and three times the recommended rates (3×) on the survival and in vitro PGP activities of rhizobacterium fungicide-tolerant Klebsiella sp. as a model PGPR.
Materials and methods
Isolation and screening of phosphate solubilizing bacteria
Three rhizosphere soil samples (10 g each) of mustard (Brassica campestris) cultivated in experimental fields (alluvial sandy clay loam, sand 667 g kg−1, silt 190 g kg−1, clay143 g kg−1, organic matter 6.2 g kg−1, Kjeldahl N 0.75 g kg−1, Olsen P 16 mg kg−1, pH 7.2 and water holding capacity 0.44 ml g−1, cation exchange capacity 11.7 cmol kg−1 and 5.1 cmol kg−1 anion exchange capacity) of Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh (27°29′ latitude and 72°29′ longitude), Uttar Pradesh, India were collected in sterile polythene bags (15 × 12 cm2) and thoroughly mixed. In order to identify the P-solubilizing bacteria, a serial dilution assay was carried out in 0.9% NaCl solution and 10 μl of diluted suspension was spread plated on Pikovskaya agar medium (Pikovskaya 1948) (g/l glucose 10, Ca3 (PO4)2 5, (NH4)2 SO4 0.5, NaCl 0.2; MgSO4·7H2O 0.1, KCl 0.1, yeast extract 0.5, MnSO4 and FeSO4 trace, agar 15, pH 7.0). The plates were incubated at 28 ± 2°C for 7 days. The isolates showing clear halo within 7 days around bacterial colonies were considered as P-solubilizers. A total of 50 P-solubilizing isolates with maximum halo sizes and different pigmentations and morphological parameters were selected.
Assessment of bacterial strains for pesticide tolerance
For this study, four fungicides (tebuconazole, hexaconazole, metalaxyl and kitazin) (Table 1) were specially selected owing to their widespread application in agricultural fields. The concentrations of the selected fungicides were calculated by the percent active ingredients in their formulations. The stock solution of each fungicide was prepared by dissolving the fungicides in dimethyl sulfoxide (DMSO) and to prevent degradation of the fungicides, the stock solutions were prepared just prior to each experiment.
The bacterial strains were tested further for their sensitivity/resistance to the four fungicides by agar plate dilution method using minimal salt agar medium (g/l KH2PO4 1, K2HPO4 1, NH4NO3 1, MgSO4·7H2O 0.2, CaCl2·2H2O 0.02, FeSO4·7H2O 0.01, agar 15, pH 6.5) as described by Ahemad and Khan (2009). The freshly prepared agar plates amended separately with increasing concentration (0–3,200 μg ml−1; at a two fold dilution interval) of fungicides were spot inoculated with 10 μl of 108 cells ml−1 of bacterial strains. The plates were incubated at 28 ± 2°C for 3 days and the highest concentration of fungicides supporting bacterial growth was defined as the maximum tolerance level (MTL). Out of 50, a total of 18 bacterial isolates showing higher MTL values (>600 μg/ml) against the fungicides as well as greater halo size (>4 mm) were selected and maintained on solid Pikovskaya agar medium until use.
Bacterial characterization
Among the 18 bacterial strains, the strain PS19 showing higher MTL values and P solubilization (Fig. 1) was further selected. Morphological, physiological and biochemical properties of the strain PS19, that included Gram reaction, citrate utilization test, indole production test, methyl red test, nitrate reduction, Voges Proskaur, catalase test, carbohydrates (dextrose, mannitol and sucrose) utilization test, starch hydrolysis, and gelatin liquefaction test was determined as per the standard methods according to Bergey’s manual of determinative bacteriology (Holt et al. 1994).
16S rDNA based identification
The sequencing of 16S rDNA of the strain PS19 was carried commercially by DNA Sequencing Service, Macrogen Inc., Seoul, South Korea using universal primers, 518F (5′CCAGCAGCCGCGGTAATACG3′) and 800R (5′TACCAGGGTATCTAATCC3′). Later, nucleotide sequence data were deposited in the GenBank sequence database.
The online program BLAST was used to find out the related sequences with known taxonomic information in the databank at NCBI website (http://www.ncbi.nlm.nih.gov/BLAST) to accurately identify the strain PS19.
Bioassays of plant growth-promoting activities under fungicide-stress
The PGP activities (P-solubilization, indole acetic acid, siderophore, exo-polysaccharides, hydrogen cyanide and ammonia production) of P-solubilizing bacteria were assayed both in the presence and absence of the selected fungicides under in vitro conditions.
Phosphate solubilization by bacterial strains
The bacterial strains showing P-solubilizing activity during screening process were inoculated into Pikovskaya agar medium supplemented with 0, 1×, 2× and 3× of the recommended rate of each fungicide and incubated at 28 ± 2°C for 7 days and observed for halo formation. The halo formed around the each bacterial colony was measured and the bacterial strains were further used to determine the extent of P-solubilization in Pikovskaya broth by the chlorostannous-reduced molybdophosphoric acid blue method (King 1932; Jackson 1967). In brief, 100 ml of Pikovskaya broth treated with 0, 1×, 2× and 3× of each fungicide, was inoculated with 1 ml of 108 cells/ml of each culture. The flasks were incubated for 7 days with shaking (120 g) at 28 ± 2°C. A 20 ml culture broth from each flask was removed and centrifuged (9,000g) for 30 min. and the amount of soluble P released into the supernatant. To 10 ml of supernatant, 10 ml chloromolybdic acid and five drops of chlorostannous acid was added and volume was adjusted to 50 ml with distilled water. The absorbance of developing blue color was read at 600 nm. The amount of P solubilized was calculated using the calibration curve of KH2PO4. The change in pH following tri-calcium phosphate (TCP) solubilization was also recorded.
Bioassay for indole-3-acetic acid production
The indole-3-acetic acid (IAA) was quantitatively analyzed by the method of Gordon and Weber (1951) later modified by Bric et al. (1991) as described by Wani et al. (2007). In brief, the phosphate solubilizing bacterial strains were grown in Luria–Bertani (LB) broth (g/l tryptone 10, yeast extract 5, NaCl 10 and pH 7.5). A 100 ml LB broth with 100 μg tryptophan/ml supplemented with 0, 1×, 2× and 3× of each fungicide was inoculated with 1 ml culture containing 108 cells/ml bacterial isolate and was incubated for 24 h at 28 ± 2°C with shaking at 125 rpm. After 24 h, 5 ml of each culture was centrifuged at 9,000g for 15 min and 2 ml of Salkowsky reagent (2% 0.5 M FeCl3 in 35% perchloric acid) was added to 2 ml of supernatant and incubated at 28°C in darkness for 1 h. The IAA concentration in the supernatant was determined using a spectrophotometer (λ 540 nm) against a standard curve.
Bioassay for siderophore production
Chrome azurol S (CAS) agar plates supplemented with 0, 1×, 2× and 3× of each fungicide were prepared separately and spot inoculated with 10 μl of 108 cells ml−1. Plates were incubated at 28 ± 2°C for 4 days. Development of yellow to orange halo around the growth indicated siderophore production (Alexander and Zuberer 1991).
The siderophore produced by the test strains was also quantitatively assayed using Modi medium (K2HPO4 0.05%, MgSO4 0.04%, NaCl 0.01%, mannitol 1%, glutamine 0.1%, NH4NO3 0.1%). Modi medium amended with 0, 1×, 2× and 3× of each fungicide was inoculated with 100 μl of 108 cells ml−1 of bacterial strains and incubated at 28 ± 2°C for 5 days. Cultures were centrifuged (4,528g) and the catechol type phenolates (salicylic acid (SA) and 2,3-dihydroxybenzoic acid (DHBA)) in the supernatant were measured using a modification of the ferric chloride-ferricyanide reagent of Hathway (Reeves et al. 1983). In brief, ethyl acetate extracts was prepared by extracting 20 ml of supernatant twice with an equal volume of solvent (ethyl acetate) at pH 2. Hathway’s reagent was prepared by adding 1 ml of 0.1 M ferric chloride in 0.1 N HCl to 100 ml of distilled water and to this, was added 1 ml of 0.1 M potassium ferricyanide. For the assay, one volume of the reagent was added to one volume of sample and absorbance was determined at 560 nm for salicylates with sodium salicylate as standard and at 700 nm for dihydroxy phenols with DHBA as standard.
Bioassay for exo-polysaccharides
Exo-polysaccharides (EPS) produced by the rhizobacterial strains was estimated by the method of Mody et al. (1989) and later modified by Tank and Saraf (2003). In brief, the bacterial strains were grown in 100 ml basal medium with 5% sucrose supplemented with 0, 1×, 2× and 3× of each fungicide and incubated for 5 days at 28 ± 2°C on shaker (100 rpm). Culture broth was spun (5,433g) for 30 min and EPS was extracted by adding three volumes of chilled acetone to one volume of supernatant. The precipitated EPS was repeatedly washed three times alternately with distilled water and acetone, transferred to a filter paper and weighed after overnight drying.
Bioassays for hydrogen cyanide and ammonia production
Bacterial strains were also tested for the synthesis of hydrogen cyanide (HCN) by adopting the method of Bakker and Schipper (1987). In brief, each bacterial strain was grown in HCN induction medium (g/l tryptic soy broth 30, glycine 4.4, agar 15) supplemented with 0, 1×, 2× and 3× of each fungicide and was incubated at 28 ± 2°C for 4 days. The bacterial strains were then streaked on HCN induction plates. A Whatman filter paper No.1 soaked in 2% sodium carbonate prepared in 0.5% picric acid solution was placed on the top of the plate. The plates were sealed with parafilm and incubated at 28 ± 2°C for 4 days. Development of orange to red color indicated HCN production.
The synthesis of ammonia by the bacterial strains was detected using peptone water supplemented separately with 0, 1×, 2× and 3× of each fungicide. Freshly grown bacterial strains (200 μl of 108 cells ml−1) was inoculated in 20 ml peptone water in tubes and incubated at 28 ± 2°C for 4 days. One ml of Nessler reagent was added to each tube. Development of yellow color indicated a positive test for ammonia production (Dye 1962).
Statistical analysis
The experiments were conducted in three replicates using the same treatments. The difference among the treatment means was compared by honestly significant difference (HSD) using Tukey test at 5% probability level using software, SPSS 10.
Results
Characterization and molecular identification of the strain PS19
The strain PS19 was characterized and identified using standard morphological, physiological and biochemical tests. The characteristics of the strain of PS19 are described in Table 2. On the basis of these features, PS19 was tentatively identified as Klebsiella.
To further consolidate the identity of the strain PS19, 16S rDNA sequence analysis of this strain was performed. 16S rDNA of the strain PS19 was found to be approximately 944 bp in size. Later, the sequences of 16S rDNA of this strain were submitted to GenBank (GenBank accession number FJ705889). A similar search was performed by using the BLAST program that indicated the strain PS19 shared a close relationship with the DNA sequence of Klebsiella sp. WW2 (16S: 98% similarity with the reference strain EF433545) in NCBI database. Such high similar values confirmed the strain PS19 to be Klebsiella sp.
Fungicide-tolerance
In this study, the rhizobacterium Klebsiella sp. PS19 grew well with the varying concentrations of fungicides, and showed a variable tolerance to the tested fungicides amended in C and N sources-deficient minimal salt agar medium. The Klebsiella sp. strain PS19 tolerated the maximum concentration of ketazin (up to 3,000 μg/ml), while in the presence of tebuconazole the strain PS19 demonstrated the least tolerance (up to 1,600 μg/ml). However, the MTL values of the Klebsiella sp. PS19 against each fungicide were unusually high.
Plant growth-promoting activities of the Klebsiella sp. strain PS19
In our study, rhizobacterium Klebsiella sp. strain PS19 displayed plant growth-promoting activities both in normal and fungicide-stressed media. The test strain solubilized inorganic P and produced IAA, siderophores and EPS in substantial amount.
Phosphate solubilization under fungicide-stress
Phosphate-solubilizing capability of the Klebsiella sp. strain PS19 in the presence of three concentrations of fungicides was assayed both qualitatively and quantitatively using solid and liquid Pikovskaya medium (Table 3). In general, when the concentration of each fungicide was increased, the size of the halo decreased substantially. The effect of 1× and 2× of all fungicides on zone diameter was less noxious. In contrast, the highest concentration (3×) of each fungicide showed the most toxic effect on the halo formation. The order of toxicity of fungicides at 3× on halo size (solubilization index) was found as: tebuconazole > hexaconazole > metalaxyl > kitazin (Table 3).
Moreover, the amount of P solubilized in liquid medium decreased with increasing concentrations of fungicides from 1× to 3×. The greatest decline in P-solubilizing potency of the strain PS19 in broth was found 83, 90 and 94% at 1×, 2× and 3× μg/l of tebuconazole, respectively over control. The fungicide toxicity (percent decrease over control) at 3× was observed as: tebuconazole (94) > hexaconazole (91) > metalaxyl (89) > kitazin (83). However, no correlation was observed between the halo size and the amount of P solubilized in broth (Table 3).
Siderophore production under fungicide-stress
The Klebsiella sp. strain PS19 exhibited substantial siderophore producing potential as evidenced by the formation of an orange colored zone of 13 mm size on CAS agar medium in the absence of fungicides. The size of siderophore zone also decreased with increasing concentration of each fungicide. The order of percent decline in siderophore synthesis expressed as zone size relative to untreated control for all fungicides at 3× was observed as: tebuconazole (31) > hexaconazole (23) = metalaxyl (23) > kitazin (15).
Additionally, the ethyl acetate extraction from culture supernatant of the Klebsiella sp. strain PS19 grown in the Modi medium devoid of fungicides yielded 29 and 21 μg/ml SA and DHBA type siderophores. Fungicide-concentration dependent progressive decline for both types of iron-binding molecules was observed. Nevertheless, degree of fungicide-mediated decline in SA and DHBA production varied with the chemical nature of fungicides (Table 3). Overall, hexaconazole displayed the highest inhibitory effect on biosynthesis of both SA and DHBA. At 3×, the order of percent decline in SA synthesis for each fungicide was observed as: hexaconazole > tebuconazole > metalaxyl = kitazin. Moreover, trend of toxicity of fungicides at 3× on bacterial DHBA synthesis was: hexaconazole > tebuconazole = metalaxyl = kitazin (Table 3).
Indole-3-acetic acid production under fungicide-stress
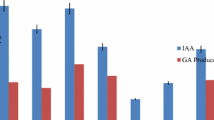
The effect of three concentrations of each fungicide on IAA synthesized by the Klebsiella sp. strain PS19 varied considerably. In the untreated medium, the Klebsiella sp. strain PS19 produced the maximum (42 μg/ml) amount of IAA. In contrast, the quantity of IAA released by the Klebsiella sp. strain PS19, however, decreased progressively with the graded-increment of each fungicide in LB broth. The Klebsiella sp. strain PS19 produced a substantial amount of IAA both in the absence and presence of fungicides. Among the tested fungicides, tebuconazole at 3× reduced the IAA production maximally by 93%, while metalaxyl exhibiting least toxicity decreased IAA by 74%, compared to the untreated control (Table 3).
Exo-polysaccharides, hydrogen cyanide and ammonia production under fungicide-stress
Unlike the trend of fungicide-concentration dependent inhibition of other PGP activities of the Klebsiella sp. strain PS19, EPS synthesis by the strain PS19 increased gradually with regular enhancement of each fungicide. Among all the tested fungicides, the maximum stimulation of EPS secretion was observed in the presence of the recommended dose of tebuconazole. At 3×, the maximum induction in EPS secretion (percent increase over control) was found as: hexaconazole (61) > tebuconazole (56) > metalaxyl (39) > kitazin (17). Moreover, the traits of HCN and ammonia production in the presence of all fungicides were not affected.
Discussion
Fungicide-tolerance
Tolerance/resistance in microorganisms against pesticides including fungicides is a complex process which is regulated at both physiological and genetic level of microorganism. Generally, the microorganisms that developed resistance against pesticides are frequently capable of biodegrading them (Ortiz-Hernández and Sánchez-Salinas 2010). The tolerance against pesticides in general, is attributed to microbial genetics or the physiological changes that induce the microbial metabolism for the formation of a new metabolic pathway to bypass a biochemical reaction inhibited by a specific pesticide (Bellinaso et al. 2003; Herman et al. 2005). Our study, however, documented abnormally higher tolerance levels of the Klebsiella sp. strain PS19 against the selected fungicides amended in minimal salt agar medium. Since the medium used to assess the MTL values of the Klebsiella sp. strain PS19 did not contain any C and N sources except the tested fungicides, the Klebsiella sp. strain PS19 might have utilized these fungicides as the only energy source.
In vitro production of plant growth promoting substances
In the present study, the Klebsiella sp. strain PS19 exhibited the PGP traits like P solubilization, production of siderophores, phytohormone and exo-polysaccharides in substantial amount. In general, rhizospheric bacteria promote the plant growth by different mechanisms. One of the important mechanisms is the solubilization of mineral phosphate in the rhizosphere through which the growing plants receive the soluble forms P (Oves et al. 2009). In fact, phosphorus is one of the most important nutrients for plant growth and development and can only be assimilated as soluble phosphate species (Illmer and Schinner 1992). However, the amount of soluble P in soil is generally very low, normally at levels of 1 mg kg−1 or less (Goldstein 1994). Phosphate solubilizing bacteria play a crucial role in soils by making available solubilized fraction of various phosphate minerals to growing plants. The P-solubilizing property is due to a drop in pH, which has been associated with their ability to secrete low molecular weight organic acids such as gluconic, 2-ketogluconic, oxalic, citric, acetic, malic, succinic, etc. (Zaidi et al. 2009). In our study, the Klebsiella sp. strain PS19 solubilized the inorganic phosphate considerably even in the presence of the recommended and the higher doses of fungicides.
In the aerobic environment, iron occurs principally as Fe3+ and is likely to form insoluble hydroxides and oxyhydroxides, thus making it generally inaccessible to microorganisms. The most commonly found strategy in bacteria to acquire sufficient iron is the secretion of siderophores which are low-molecular mass iron chelators with high association constants for complexing iron. Thus, siderophores act as solubilizing agents for iron from minerals or organic compounds under conditions of iron limitation (Indiragandhi et al. 2008). Accordingly, the secretion of siderophores including SA and DHBA by the rhizobacteria under iron stress confers an added advantage upon these organisms, resulting in exclusion of phytopathogens on account of iron starvation (Khan et al. 2009). The production of siderophores like SA and DHBA by the Klebsiella sp. strain PS19 indicated that this bacterial strain may be used as a bio-control against soil borne phytopathogens.
Plant growth enhancing hormone, IAA synthesized by plant growth-promoting rhizobacteria affect many physiological activities of plants, such as, cell enlargement, cell division, root initiation, growth rate, phototropism, geotropisms, apical dominance, etc. (Khan et al. 2009). Moreover, this phytohormone also acts as a signaling molecule during the onset of symbiosis in legumes (Barker and Tagu 2000). In our study, substantial IAA was produced by the Klebsiella sp. strain PS19 even exposed to three times the recommended dose of each fungicide.
The EPS production is an important trait of rhizobacteria because it protects the bacterial cell against desiccation, phagocytosis and phage attack and also helps in nitrogen fixation by preventing high oxygen tension (Tank and Saraf 2003). Interestingly, the amount of EPS secreted by bacterial strain PS19 in this study increased progressively with gradual increase in fungicide-concentrations. The increase in EPS following fungicide-treatments suggested that the fungicides might have acted as the inducers of EPS biosynthesis. The EPS synthesized excessively by the Klebsiella sp. strain PS19 is likely to provide it protection against the stressed environment.
In our study, HCN and ammonia production by the Klebsiella sp. strain PS19 remained unaffected under fungicide stress. The release of HCN by rhizospheric bacteria into the soil can be toxic to subterranean animals and phytopathogenic organisms (Guo et al. 2007). Similarly, ammonia production by rhizobacterial strains is reported elsewhere (Wani et al. 2008). However, there is no such report wherein the effect of these fungicides was assessed on these PGP activities of rhizobacteria.
Each PGP trait of bacteria is the result of sequential metabolic reactions mediated by enzymes along the defined metabolic pathway. The metabolic pathways for any specific PGP trait may be more than one depending upon the type of the PGP substances and bacterial genera/species. It is well reported that pesticides including fungicides adversely affect protein synthesis and the metabolic enzymes (Kapoor and Arora 1996; Boldt and Jacobsen 1998). Additionally, these chemical agents not only damage the structural proteins essential for growth of organisms but also responsible for genotoxicity (Pham et al. 2004) and eventually lead to the decreased functioning and the survival of organisms (Kumar et al. 2010). Therefore, it seems probable that fungicides employed in this study might have inhibited the functioning of the enzymes involved in synthesis of PGP substances in the Klebsiella sp. strain PS19.
In conclusion, fungicides at all tested rates showed the varying degree of toxicity towards the PGP traits (except EPS) of Klebsiella sp. strain PS19. However, toxicity to these traits was less pronounced at the recommended rate of fungicides and increased beyond the recommended rate. This study revealed that fungicides must be tested in laboratory for their possible side-effects on plant-beneficial soil micro flora prior to field application. These findings would possibly add some new insights in pest management in agricultural practices.
References
Ahemad M, Khan MS (2009) Effect of insecticide-tolerant and plant growth promoting Mesorhizobium on the performance of chickpea grown in insecticide stressed alluvial soils. J Crop Sci Biotechnol 12:213–222
Ahemad M, Khan MS (2011) Pseudomonas aeruginosa strain PS1 enhances growth parameters of greengram [Vigna radiata (L.) Wilczek] in insecticide-stressed soils. J Pest Sci 84:123–131
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Bakker AW, Schipper B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Barker SJ, Tagu D (2000) The roles of auxins and cytokinins in mycorrhizal symbioses. J Plant Growth Regul 19:144–154
Bellinaso ML, Greer CW, Peralba MC, Henriques JA, Gaylarde CC (2003) Biodegradation of the herbicide trifluralin by bacteria isolated from soil. FEMS Microb Ecol 43:191–194
Boldt TS, Jacobsen CS (1998) Different toxic effects of the sulphonylurea herbicides metsulfuron methyl, chlorsulfuron and thifensulfuron methyl on fluorescent pseudomonads isolated from an agricultural soil. FEMS Microbiol Lett 161:29–35
Bric JM, Bostock RM, Silversone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Dye DW (1962) The inadequacy of the usual determinative tests for the identification of Xanthomonas spp. Nzt Sci 5:393–416
Goldstein AH (1994) Involvement of the quinoprotein glucose dehydrogenase in the solubilization of exogenous phosphates by gram-negative bacteria. In: Torriani-Gorini A, Yagil E, Silver S (eds) Phosphate in microorganisms: cellular and molecular biology. ASM Press, Washington DC, pp 197–203
Gordon S, Weber RP (1951) The colorimetric estimation of IAA. Plant Physiol 26:192–195
Guo Y, Zheng H, Yang Y, Wang H (2007) Characterization of Pseudomonas corrugata strain P94 isolated from soil in Beijing as a potential biocontrol agent. Curr Microbiol 55:247–253
Herman PL, Behrens M, Chakraborty S, Crastil BM, Barycki J, Weeks DP (2005) A three component dicamba O-demethylase from Pseudomonas maltiphilia strain DI-6: gene isolation, characterization and heterologous expression. J Biol Chem 280:24759–24767
Holt JG, Krieg NR, Sneath PHA, Staley JT, Willams ST (1994) Bergey’s manual of determinative bacteriology. Williams and Wilkins, USA
Illmer P, Schinner F (1992) Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol Biochem 24:389–395
Indiragandhi P, Anandham R, Madhaiyan M, Sa TM (2008) Characterization of plant growth-promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Curr Microbiol 56:327–333
Jackson ML (1967) Soil chemical analysis. Prentice-Hall of India, New Delhi
Jaharamma M, Badri Narayanan K, Sakthivel N (2009) Genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads and their simultaneous role in promotion of plant growth and soil health. In: Khan MS, Zaidi A (eds) Phosphate solubilizing microbes for crop improvement. Nova Science, Inc, pp 111–127
Kapoor K, Arora L (1996) Observations on growth responses of cyanobacteria under the influence of herbicides. Pollut Res 15:343–351
Khan, Zaidi A, Wani PA, Ahemad M, Oves M (2009) Functional diversity among plant growth-promoting rhizobacteria. In: Khan MS, Zaidi A, Musarrat J (eds) Microbial strategies for crop improvement. Springer, Berlin, pp 105–132
King JE (1932) The colorimetric determination of phosphorus. Biochem J 26:292–297
Kumar N, Anubhuti Bora JI, Amb MK (2010) Chronic toxicity of the triazole fungicide tebuconazole on a heterocystous, nitrogen-fixing rice paddy field cyanobacterium, Westiellopsis prolific Janet. J Microbiol Biotechnol 20:1134–1139
Mody BR, Bindra MO, Modi VV (1989) Extracellular polysaccharides of cowpea rhizobia: compositional and functional studies. Arch Microbiol 1:2–5
Moorman TB (1989) A review of pesticide effects on the microorganisms and microbial processes related to soil fertility. J Prod Argic 2:14–23
Ortiz-Hernández ML, Sánchez-Salinas E (2010) Biodegradation of the organophosphate pesticide tetrachlorvinphos by bacteria isolated from agricultural soils in Mexico. Rev Int Contam Ambient 26:27–38
Oves M, Zaidi A, Khan MS, Ahemad M (2009) Variation in plant growth promoting activities of phosphate-solubilizing microbes and factors affecting their colonization and solubilizing efficiency in different agro-ecosystems. In: Khan MS, Zaidi A (eds) Phosphate solubilizing microbes for crop improvement. Nova Science, New York, pp 247–263
Pham CH, Min J, Gu MB (2004) Pesticide induced toxicity and stress response in bacterial cells. Bull Environ Contam Toxicol 72:380–386
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiol 17:362–370
Reeves MW, Pine L, Neilands JB, Balows A (1983) Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol 154:324–329
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Tank N, Saraf M (2003) Phosphate solubilization, exopolysaccharide production and indole acetic acid secretion by rhizobacteria isolated from Trigonella foenum-graecum. Ind J Microbiol 43:37–40
Wani PA, Zaidi A, Khan AA, Khan MS (2005) Effect of phorate on phosphate solubilization and indole acetic acid releasing potentials of rhizospheric microorganisms. Ann Pl Protec Sci 13:139–144
Wani PA, Khan MS, Zaidi A (2007) Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere 70:36–45
Wani PA, Khan MS, Zaidi A (2008) Chromium-reducing and plant growth-promoting Mesorhizobium improves chickpea growth in chromium-amended soil. Biotechnol Lett 30:159–163
Zaidi A, Khan MS, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung 56:263–284
Acknowledgments
Authors are grateful to Dr. Naqvi, Parijat Agrochemicals, New Delhi, India, for providing technical grade fungicides and University Grants Commission (UGC), New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. J. Gorman
Rights and permissions
About this article
Cite this article
Ahemad, M., Khan, M.S. Biotoxic impact of fungicides on plant growth promoting activities of phosphate-solubilizing Klebsiella sp. isolated from mustard (Brassica campestris) rhizosphere. J Pest Sci 85, 29–36 (2012). https://doi.org/10.1007/s10340-011-0402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-011-0402-1