Abstract
Bemisia tabaci is a significant pest for many crops, but there are few population studies of this insect on sweet pepper (Capsicum annuum). In this study, stage frequency data were generated with B. tabaci in sweet pepper plants in various situations, and the Bellows and Birley method was used to obtain population parameters from the data. The Akaike Information Criterion (AIC) was used to select the best option of the Bellows and Birley method and, in some cases, to estimate the parameters of the population using model averaging. The ratios estimated/observed for each population parameter were calculated to assess bias and were used to correct the estimations if the ratios were different from 1. The effects of different factors on the estimations of population parameters were analysed. The total duration of development was affected by the experimental conditions (laboratory vs. greenhouse) and temperature, but it had the highest precision. The final survival rate was affected by temperature, and the estimation of individuals entering each stage was affected only by the options included in the Bellows and Birley method. AIC helped to detect differences in the daily survival rate among the different experiments between N1 (first instar) (range 0.842–0.923), and the egg (range 0.989–1.0) and N4 (fourth instar) (0.990). The methodology used can be employed in field population studies. For example, the final survival rate in the greenhouse experiments varied between 0.624 and 0.097, depending on if the parasitoids were present or not, and the total development varied between 420.6 and 440.7 degree days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The whitefly, Bemisia tabaci (Gennadius), is a significant pest in many crops around the world (Oliveira et al. 2001). In regions where there is a great density of vegetable crops cultivated in greenhouses, as is found in the southeast of Spain, the whitefly becomes a serious threat in terms of the increase of its population and the potential transmission of several viral diseases (Navas-Castillo et al. 2000; Segundo et al. 2004; Ruiz et al. 2006). Crops such as sweet peppers, tomatoes, melons, cucumbers, green beans and others may be seriously affected by this pest. Many studies have focused on the biology of this species in different crops and on the analysis of life tables to investigate different parameters of the population or the key factors that regulate its population (Von Arx et al. 1983; Horowitz et al. 1984; Baumgartner et al. 1986; Baumgartner and Yano 1990; Naranjo and Ellsworth 2005; Asiimwe et al. 2007). Several of these studies have compared different models. The biology of B. tabaci has been studied in sweet peppers (Capsicum annuum) under controlled (laboratory) conditions (González-Zamora and Gallardo 1999; Muñiz 2000; Muñiz et al. 2002), but no studies have been presented on the biology of this species with sweet peppers under field conditions.
Stage frequency data are analysed in different ways to obtain information on populations. One way is to use a model or models, which can be as simple or as complicated as needed under the circumstances (for a review see Manly 1990; Southwood and Henderson 2000). If different models are used to analyse the data, the results must be compared to select the most suitable one. Different biological conclusions may be drawn from the data depending upon the final model selected, and therefore, it is important to have a method that selects the best model and measures the strength of the evidence for each one. The Akaike Information Criterion (AIC) is widely used in biological studies to select the best model due to the advantages it has over other criteria, and it is used to estimate parameters by model averaging (Burham and Anderson 2002; Johnson and Omland 2004; Posada and Buckley 2004). In the field of entomology, the application of AIC or other information criteria is generally used to select models that help explain different aspects of the biology and behaviour of insects, and to select models that can be used in the field of crop protection (Luh and Croft 1999; Hansen et al. 2001; Hemerik and van der Hoeven 2003; Umble and Fisher 2003; Sileshi 2006; Takeuchi 2006; Saint-Germain et al. 2007). A study undertaken by Sileshi (2006) is one of few examples of the use of AIC for insect count data or applications for life table analyses. Model averaging is applied when none of the set of models is clearly the best, and several can be used. In such case, the parameters of interest are estimated based on the relative importance (or weight) of the models. To date, no examples have been found on the use of model averaging to estimate population parameters.
This work had different objectives corresponding to the information that can be obtained from stage frequency data of B. tabaci in sweet peppers, both from the laboratory and field. We studied the bias generated after using a model (in this case, the Bellows and Birley method), comparing the observed and estimated parameters, and how different factors can influence this bias. The other objective of this study was to demonstrate the application of model selection and averaging with the AIC to accurately estimate population parameters. The Bellows and Birley method produces different parameters from stage frequency data and, with the help of the AIC, it can be of great interest in population studies due the information generated, such as, for example, survival rates, development time, number of entering stages, and others. Finally, population parameters from field studies are presented to show the potential of this methodology.
Materials and methods
Experimental conditions
The study was carried out in the facilities of the I.F.A.P.A. (Instituto para la Formación Agraria y Pesquera de Andalucia) of “La Mojonera-La Cañada” (36º47′18.57″ N and 2º42′13.87’″ W) in Almería (southeast Spain). The experiments were conducted under laboratory conditions with potted plants and in a plastic greenhouse using sweet pepper plants (Capsicum annum) cv. “Espartaco”. The pots had a diameter of 13.8 cm and a volume of 1.2 l. The substratum was coconut fibre, and the plants were periodically fertilised with Multi Poli-Feesd® (Haifa Chemical).
A 600-m2 plastic greenhouse was used for the greenhouse conditions. The sweet pepper plants were transplanted to the ground in August 1995. The normal agricultural practice in the area for this crop was followed during the period of cultivation, with the spraying of pesticides on the upper part of the plants to control certain diseases and pests, such as powdery mildew (Leveillula taurica (Lev.) Arnaud (Perisporales: Erysiphaceae)) with dinocap and bupirimate; beet armyworm (Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae)) with Bacillus thuringiensis and trichlorfon mixed with wheat bran; and broad mite (Poliphagotarsonemus latus (Banks) (Acari: Tarsonemidae)) with bromopropylate and avermectin. Care was taken to avoid products harmful to whiteflies and their natural enemies.
The adults of B. tabaci that were used to lay the eggs were collected from a different greenhouse planted with peppers (cv. “Espartaco”), where a colony of B. tabaci was constantly reared.
Three trials were carried out in different situations to generate stage frequency data that could be used for the posterior analysis of model selection and model averaging to obtain population parameters. The experiments were carried out under the following two experimental conditions: controlled temperature (laboratory conditions in Trials 1 and 2) versus uncontrolled temperature (field conditions in Trial 3). There were also two scales of observation: individual counts (in Trial 1) versus grouped counts (in Trials 2 and 3). The three trials were as follows:
(1) Trial 1 (individual counts and controlled temperature). Assays were carried out at 20 ± 1, 25 ± 1 and 30 ± 1°C in a growth chamber (KOXKA model MEC-185/F) with 4,000 lux, a 16:8 photoperiod (light:dark) and a relative humidity of 75 ± 10%; and in a breeding chamber at 25 ± 2°C with 6,000 lux, a 16:8 photoperiod (light:dark) and a relative humidity of 65 ± 10%. Each of the studies consisted of one or two potted plants with six to eight leaves each. The plants were infested with high numbers of B. tabaci adults. The adults were confined to one or two leaves per plant by means of a cloth bag for 24 h at the different temperatures defined above. After this time, the adults were eliminated, and the eggs were counted. This time point was considered the initial moment, or zero time point, for the study of development. The eggs were observed and counted daily. When nymphs of the first instar emerged, we waited until they fixed on the leaf, and then their positions were marked with a soft marker (Lumocolor®, Staedtler, Germany). Daily counts of each individual took place until the whitefly adults emerged.
(2) Trial 2 (grouped counts and controlled temperature). Assays were carried out at 20 ± 1 and 30 ± 1°C in a growth chamber (KOXKA model MEC-185/F) with 4,000 lux, a 16:8 photoperiod (light:dark) and a relative humidity of 75 ± 10%; and in a breeding chamber at 25 ± 2°C with 6,000 lux, a 16:8 photoperiod (light:dark) and a relative humidity of 65 ± 10%. Two replicates were done at each temperature. The plant infestation was initiated following the same procedure as described in Trial 1. Once the adults were eliminated, the leaves where the adults had been confined were observed daily. The eggs and individuals that emerged were counted and grouped according to their developmental stage, but they were not marked. The daily counts took place until the adults emerged.
(3) Trial 3 (grouped counts with uncontrolled temperature in a plastic greenhouse). Three experiments were carried out at several time periods throughout the year, as follows: September 19, 1995 to October 23, 1995 (experiment 1); December 1, 1995 to February 26, 1996 (experiment 2); and March 6, 1996 to April 30, 1996 (experiment 3). In each experiment, six to ten plants were selected, and a leaf from each plant was isolated with a cloth bag. Large numbers of adult B. tabaci were introduced into each bag and left for 24 h. The eggs that were laid were counted, and this was considered the starting point for the developmental study. The population was counted daily except in experiment 1, where it was counted every 2 days until all of the adults had emerged. Individuals were counted and grouped according to their developmental stage. The temperature and relative humidity were registered daily during the experiments, with mean temperatures (and ranges) of 24.9°C (12–37°C), 15.9°C (4–30°C) and 18.9°C (8–33°C) for experiments 1, 2, and 3, respectively. Time was measured using degree days (DDs). The DDs were calculated using the maximal and minimal temperatures of two periods in the day (from 00.00–12.00 h and from 12.00 to 24.00 h), and 10°C was the minimum development threshold temperature (Zalom et al. 1985). The following equation was used to calculate DD:
where T is temperature.
In Trials 1 and 2, the different developmental stages, from egg to fourth instar (N4), were observed in the laboratory with a stereobinocular microscope (9× and 45× magnification) by turning the leaf under the microscope. The developmental stages were distinguished as a function of size. The first instar (N1) was the smallest, and the fourth (N4) was the largest. In Trial 3, the different developmental stages from egg to N4 were distinguished using a field lens (8× magnification), and they were separated according to their relative size. In the first greenhouse experiment, the nymphs of the first, second and third instars were counted together.
In the individual counts (Trial 1), the fate of each individual was recorded. Thus, it was possible to calculate different parameters, such as the number of individuals entering each instar, the daily survival rate of each instar, the survival rate of each developmental stage, the duration of each instar, the final survival rate of the population and the total development period (González-Zamora and Gallardo 1999). In contrast, in the grouped counts (Trials 2 and 3), only the number of individuals found at each instar was recorded daily. In the grouped counts, the initial number of eggs and the final number of adults that emerged from the empty pupal cases was known. With these data, most of the previous parameters could not be calculated except for the final survival rate of the population and the total development period. Therefore, the other parameters had to be estimated with the help of a model. With individual counts, it was possible to compare the observed values of all parameters with their estimations and to establish the bias and validity of the model used. With Trials 2 and 3, the bias was identified using fewer parameters, such as individuals entering the egg and adult stage, the final survival rate and the total development.
Model fitting
The P1f software package (Manly 1994) was used to analyse the life tables, and it was specifically designed to analyse data from stage-structured populations with different models. In model selection, it is important to have a group of models that are relevant to the data and to the objectives of the analysis, representing a plausible research hypothesis (Burham and Anderson 2002). The Bellows and Birley method (Bellows and Birley 1981) is the most flexible method because it allows estimation of the duration of each stage, the unit time survival rate, the final survival rate, and the numbers entering each stage. This method also allows for different assumptions when different survival parameters for each stage or time of entry in stage 1 are considered (Manly 1990).
All of the experiments were performed using a single cohort, and therefore, the initial number entering stage 1 was known, and no entering distribution was necessary. The Weibull distribution was also used to model the distribution function of each stage. The Weibull distribution function is as follows:
where alpha (α) is the shape parameter, and lambda (λ) determines the spread of the curve along the X-axis. Lambda is also an estimate of the mean duration of each stage.
The P1f program allows for different combinations of the unit time survival rate (needed in the Bellows and Birley method) and the shape parameter (α) of the Weibull distribution, which may be different for each stage in the Bellows and Birley method. The combinations of these two parameters produce the following four options in the program: (1) different survival and shape parameters for each stage; (2) the same survival parameters but different shape parameters for each stage; (3) different survival parameters but the same shape parameters for the stages; and (4) the same survival parameters and shape parameters for all stages. These four options of the Bellows and Birley method produced different estimations of the same population parameters along with fitting of the model to the data as expressed in the log likelihood for each option. For this reason, the four options were considered in this study as models to be selected with a given criterion.
The output estimates produced by the Bellows and Birley method were as follows: (a) individuals entering into each stage (egg, N1, N2, N3, N4 and adult); (b) the stage-specific survival rate (SSSR), which is the survival of a given stage; and (c) duration, which is the developmental time for each stage considered. The P1f program generated the standard deviation for the duration of each stage, but not for the individuals entering the stage or for the SSSR. Two other estimates were calculated using the results of the program as follows: (a) the SRf, which is the final survival rate from egg to adult; and (b) the Durationt, which is the duration of the total developmental period, from egg to adult. However, neither of these two last estimates had an associated standard deviation. The shape parameter (α) of the Weibull distribution and the unit time (daily) survival rate (ϕ) were also estimated in the four options of the Bellows and Birley method. Both of these parameters could be considered equal or different for each stage, and both of them had a standard deviation produced by the P1f program.
Statistical analyses
The output estimates produced by the Bellows and Birley method, such as the individuals entering each stage, the SSSR, the duration of each immature stage, the final survival rate (SRf) and the total duration (Durationt), were compared with the observed values of the same parameters, which were obtained mainly from Trial 1, but also from Trials 2 and 3, to obtain the estimated/observed ratios. The ratios were used to identify the bias of the estimates and to determine if the bias was affected by different factors.
The previous parameters, expressed by relative values in the ratios, were considered as variables that could be affected by different factors. These factors and their levels were as follows: (a) Scale of observation, with two levels, including individual counts (with data coming from the experiments of Trial 1) and grouped counts (with data coming from the experiments of Trial 2); (b) Experimental conditions, with two levels, including controlled temperatures (with data coming from the experiments of Trial 2) and uncontrolled temperatures (with data coming from the experiments of Trial 3); (c) Temperatures, with the three temperatures used in controlled temperatures (with data coming from the experiments of Trials 1 and 2); (d) Options, with the four options of the Bellows and Birley method supported by the P1f program (with the available data from Trials 1, 2 and 3).
The ratios were not transformed in any way, and they were first analysed to test the homogeneity of the variances within factors using Cochran’s C contrast, Bartlett’s contrast and Levene’s tests. If the probability associated with any of them was less than 0.05, the Kruskall–Wallis test was used to analyse the data. In contrast, if the P value was greater than 0.05 in all of them, a one-way ANOVA was used to analyse the data. If the P value of the Kruskall–Wallis statistic was less than 0.05, the means of the different levels within the factor were separated using Mann–Whitney’s U test (Steel and Torrie 1988). If the P value of the ANOVA test was less than 0.05, the means of the different levels within the factor were separated using Tukey’s honestly significant difference (HSD) test at P = 0.05. The ratios were then tested to determine whether they differed from 1 using the contrast hypothesis test, with P = 0.05. All analyses were performed using the Statgraphics package (Statistical Graphics 2000).
Model selection
There are different ways of comparing models to select the most appropriate one. This study used the AIC, which is a powerful method for model selection and the inference of ecological data (Burham and Anderson 2002). With AIC, the goal is to select the model with the least number of parameters that represents the data adequately (i.e., the principle of parsimony) (Franklin et al. 2001; Mazerolle 2004). The AIC was used to select the best option of the Bellows and Birley method, and it is defined as follows:
In this equation, K is the number of estimated parameters included in each model (or the options of the Bellows and Birley method in this study). The log-likelihood of the model given the data are readily available in the statistical output, and reflects the overall fit of the model. When there was a comparison of models, the model with the smallest AIC was selected. With count data, as was the case here, it is normal to find overdispersion. Therefore, the AIC was modified to obtain QAIC c (Burham and Anderson 2002).
Two measures, delta AIC (Δ) and Akaike weights (w), associated with the AIC and equally with QAIC c were used to compare models (Burham and Anderson 2002). The delta AIC is a measure of each model relative to the best model. As a rule of thumb, when Δ i is less than two, it suggests substantial evidence in support of the model. When the values are between three and seven, it indicates that the model has considerably less support. When Δ i is greater than ten, it indicates that the model is unlikely. Akaike weights provide another measure of the strength of evidence for each model, and they represent the ratio of delta AIC (Δ i ) values for each model relative to the whole set of candidate models (the four options of the Bellows and Birley model). Akaike weights also indicate the probability that the model is the best among the set of candidate models.
Selecting a model from a set of candidate models may produce a new problem. When no single model is clearly the best, predictions cannot be based on the model ranked in first place. In some cases, the best model may have competitors for the top rank (e.g., when Δ i < 2). A solution to this problem is to base the inference on the entire set of models, an approach called “multimodel inference” or “model averaging” (Burham and Anderson 2002; Johnson and Omland 2004; Posada and Buckley 2004). When this situation happened in this study, a weighted average of the estimates was computed using the Akaike weights.
To conduct model averaging, the estimate of the parameter for each model was weighted by the Akaike weights as follows:
where \( \hat{\theta }_{i} \) denotes the estimate for model i. Similarly, the precision (as standard error, SE) of the model averaged estimate may also be computed and is called the unconditional SE (Burham and Anderson 2002). In many cases, model averaging reduces bias and increases precision, which are desirable properties (Burham and Anderson 2002). Once the model averaged estimates and SE were calculated, confidence intervals were used to assess the magnitude of the effect. After using AIC, or model averaging if needed, the observed parameters and their final estimates were used to calculate the coefficients of determination R 2.
Results
Differences among the observed parameters and their estimates from the Bellows and Birley method in its different options were observed. The discrepancies were measured by calculating the estimated/observed ratios (Table 1), which were used to identify the bias of the method in the different parameters and to assess the effect of the factors on estimations of the same parameters. The ratios were less than one in most cases, but some were close to one. Table 2 shows the statistics obtained for the analyses and their significance. There were no differences in the ratios of each variable studied within the scale of observation (individual counts vs. grouped counts), although the experimental conditions (controlled temperature vs. uncontrolled temperature) showed a significant difference in the ratios only in the total duration of development. The temperature factor displayed differences only in the final survival rate and total duration. Finally, the option factor, which must be considered as appertaining to the Bellows and Birley method, demonstrated significant differences within each factor only in the entering individuals. The estimated/observed ratios were used to correct the estimated parameters in each situation, but only when the ratio was significantly different from one, by dividing the estimated parameter by the ratio value. This correction was used to obtained the final values of each parameter, but it was not used to select the best option with the AIC.
The different parameters of the AIC analysis for each Trial (Table 3) indicated that the data were overdispersed (c between 1 and 5; Burham and Anderson 2002). Each trial comprised its own set of data, and therefore, the results are shown separately. The four options supported by the P1f program with the Bellows and Birley method were compared with the delta AIC (Δ) and the Akaike weights (w). The first option (i.e., different daily survival rates for the stages) was selected as the best with the experiments carried out at the lowest temperature under controlled conditions (20°C in both individual and grouped counts, Trials 1 and 2, respectively). In contrast, the second option (i.e., the same daily survival rate for all stages) was selected as the best at the intermediate temperature (25°C in both individual and grouped counts, Trials 1 and 2, respectively). At the higher temperature (30°C in both individual and grouped counts, Trials 1 and 2, respectively), the selection was not as clear, but the options with the same survival rate for the stages (options 2 and 4) generally had higher weights. Similar selections of options occurred in the experiments carried out in the greenhouse (Trial 3). In the experiment carried out in winter (experiment 2 with a mean temperature of 15.9°C), the first option (i.e., different daily survival rates for the stages) was clearly selected. In experiment 3 (mean temperature of 18.9°C and at the beginning of spring), the third option (i.e., different daily survival rate for each stage) was the most important. In experiment 1, under greenhouse conditions (warm temperatures with a mean of 24.9°C and at the beginning of autumn), the second and fourth options (both with the same daily survival rate for each stage) were more important.
Finally, the final population parameters for each experiment (with their own standard errors), were obtained using multimodel inference when necessary, according to the w factor of Table 3. The coefficients of determination R 2 between the observed and estimated parameters (corrected with the ratio if necessary) were calculated as follows: 0.909 (41), 0.925 (20), 0.988 (20), 0.985 (12) and 0.999 (13) for the individuals entering the different stages, the stage-specific survival rate (SSSR), the duration of development in the different stages, the final survival rate, and the duration of total development period, respectively (the numbers between brackets are the number of points used to calculate the coefficient of determination in each case).
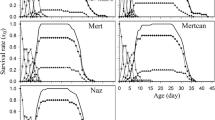
In 6 out of 13 experiments, the first and/or third option of the Bellows and Birley method (i.e., in which the daily survival rates were considered differently for each instar) produced higher Akaike weights (Table 4). Three of the experiments demonstrated a clear difference with non-overlapping confidence intervals between the daily survival rates of the egg and N1 stages, and in one experiment between the N1 and N4 stages (Table 4). In several cases, there was only a light overlap in the confidence intervals of the daily survival rate of the N4 with the egg stage. Also, the estimates of the daily survival rate with the different options in the individual counts (Trial 1) were similar to the observed daily survival rates, which were included in the 95% confidence intervals of the estimates (Table 4). The observed values of daily survival rate showed statistical differences in the stage and temperature factors and their interaction (F 4,5 = 68.5, P < 0.001; F 2,5 = 113.8, P < 0.001; and F 8,5 = 16.6, P = 0.003, respectively). The differences between stages within the temperature were consistent with the results from the estimations and their confidence intervals at 20°C (N1 was different from egg and N4; Table 4). At 30 and 25°C, however, differences were found between the egg stage and the remaining instar stages (Table 4 and data not shown).
The final estimates obtained for the greenhouse experiments are presented in Table 5, and they show the type of output obtained using the AIC and the multimodel inference. From the results, the similarity of the total duration among the three experiments (ranging from 420.6 to 440.7 DD) was significant, whereas the final survival rates (ranging from 0.097 to 0.624) indicate that the environmental conditions in the three experiments were different.
Discussion
The study presented in this article had two objectives. The first objective was to show the potential of the Bellows and Birley method to estimate parameters from stage frequency data. As in any estimation, the parameters estimated may differ from the observed values of the same parameters. The observed parameters are not always known. In this study, however, the observed parameters were known in most of the cases, and they were used to identify the bias of the Bellows and Birley method as presented by the software P1f program. The second objective was to use a procedure to select a model that provides the best trade-off between bias and accuracy (i.e., AIC selected the most parsimonious model from those used to adjust the data). If different models support the data similarly, a multimodel inference (e.g., model averaging) can be considered to obtain a precision estimator for the different parameters.
The Bellows and Birley method produced several estimates that were different from the observed values, as shown by the ratios in Table 1. The ratios of the estimated/observed parameters are useful for identifying the bias of the estimates and for determining if this bias is affected by the different factors considered. It is of particular interest that no difference of the ratios within the scale of observation (individual vs. grouped counts) was observed in the variables estimated. However, there were statistical differences in the ratios within the experimental conditions and temperatures, which affected variables representing general values (i.e., final survival rate and total duration of development). There were differences among the experiments carried out in the greenhouses and among the different temperatures in the laboratory. However, there was a remarkably high precision in several of the estimated variables, such as the total duration of development, across all factors.
The option factor was associated with the Bellows and Birley method of the P1f program, and it had a significant effect on one of the studied variables. The second option of the Bellows and Birley method (i.e., same survival parameter but different shape parameter for each stage) had a remarkable effect on the estimations of entering individuals, with a ratio close to 0.5 (Table 1).
With the analysis presented herein, we have a tool to help answer the question of whether there are different daily survival rates at each stage. The options of the Bellows and Birley method with different daily survival rates in each stage (options 1 and 3) obtained better support at lower temperatures (both in controlled conditions and the plastic greenhouse), whereas the options with equal survival rates (options 2 and 4) were selected at medium and higher temperatures. The factors that affect mortality in each situation may be different and may change in other experiments, but their identification and quantification may only be answered with an adequate sampling methodology that identifies them and relates them to the results of the analysis.
The daily survival rates of the stages was of particular interest (Table 4). The observed values of the daily survival rates in the experiments of Trial 1 fell within the confidence interval of their estimations, reflecting their accuracy. It may be assumed that the estimations in the other experiments shown in Table 4, where no observed values were generated, were equally accurate. Although the daily survival rates were different among some stages within the temperatures, as confirmed by the ANOVA of the observed values (even though these results must be considered with caution due the low number of replications of the observed data), this was not confirmed with the Bellows and Birley method in some cases (e.g., the experiment performed at 25°C, not shown in Table 4, the experiment performed at 30°C in Trial 1, shown in Table 4, or one of the experiments performed at 20°C in Trial 2, shown in Table 4).
For differences among stages, studies that relate this variation to environmental variables, such as climate, number of predators, number of parasitoids, food availability or any other variable, may help to explain these differences (Manly 1990). In experiments conducted at a controlled temperature at 20°C, the temperature and possible manipulations under the stereomicroscope may be considered as factors affecting the survival of N1. Other factors may have produced these differences in the experiments carried out with uncontrolled temperatures (Trial 3 in the greenhouse).
The egg and N1 and N4 instar stages had the greatest effect on the B. tabaci population in the life table analysis in different locations and crops (Horowitz et al. 1984; Naranjo and Ellsworth 2005; Asiimwe et al. 2007). Predation (Naranjo and Ellsworth 2005) and parasitisation (Asiimwe et al. 2007) were the principal factors responsible for decreasing populations, affecting mainly the N4 instar, and dislodgement was second in most of the previous studies. In general, these conclusions agree with the results obtained in this study for the greenhouse experiments (although with only three experiments), where N1 and N4 had the highest mortalities, with estimated survival rates of 0.758 and 0.474, respectively, in experiment 2, and 0.372 and 0.515, respectively, in experiment 3 (Table 5). In greenhouses, the N1 instar is the most exposed to environmental factors, such as peaks of high temperature and low humidity typical of greenhouses in southeast Spain and low temperatures in some periods of the year. The N1 instar is also subject to other factors related to the plant itself, such as nutritional factors and cuticle thickness (Byrne and Bellows 1991). Predation by lacewings or beetles on whiteflies was not observed in Trial 3 (greenhouses). However, Eretmocerus mundus Mercet adults, which are the main parasitoid of B. tabaci in greenhouses in Almería (Rodríguez-Rodríguez et al. 1994), and subsequently, parasitised N4, were detected. The high mortality found in N4 (mainly from parasitism but also from direct feeding) and the low final survival rates recorded in experiments 2 and 3 (SRf = 0.143 and SRf = 0.097, respectively) (Table 5) were due to E. mundus in contrast to the values obtained in the first experiment in which no E. mundus was detected.
The level of parasitism due to E. mundus in the N4 instar may be high (González-Zamora et al. 1996; Téllez et al. 2003; Stansly et al. 2005). Predation by E. mundus adults on different instars of the whitefly B. tabaci is well known and has been evaluated (Gerling and Fried 2000; Urbaneja et al. 2007), and is considered an important factor in population regulation (Téllez et al. 2003; Zang and Liu 2008).
The methodology used in this work allowed the bias of the Bellows and Birley method to be identified in different experimental situations (laboratory vs. greenhouse and individual vs. grouped counts) and with the use of the P1f program. The final estimates obtained from the best option of the Bellows and Birley method selected with the AIC or with model averaging, when it was needed, include the correction with the estimated/observed ratio when the ratios were different from one. The estimations may be used to analyse life tables to, for example, study the key factors that affect the survival of a population with a high enough number of generations (Southwood and Henderson 2000). However, this was not the objective of this work. The final estimates for all of the experiments were compared with the observed values, obtaining, in general, a good agreement between them, as reflected in the coefficient of determination R 2. It is especially remarkable that the total duration of development had an R 2 = 0.999, which reflects the robustness of its estimation in both experimental conditions (Table 1). The duration of development of each stage and the final survival rates also had high values of R 2 (0.988 and 0.985, respectively). The high similarity between the observed and final estimated parameters with the field experiments in greenhouses is shown in Table 5.
The final estimates for the experiments carried out in the greenhouses (Table 5) are of particular interest for their implications in population studies in field conditions. The final estimation of the total duration of development may be compared with other studies, such as those carried out in cotton, to study the developmental time of B. tabaci (Zalom et al. 1985; Zalom and Natwick 1987). The mean generation time of B. tabaci in cotton was found to be between 316.0 and 369.5 DD, which differs from the values obtained for sweet peppers in the present study. The duration of each instar also indicates the difference among instars. The development times of the egg and N4 stages were longer than the other instars, in agreement with results from laboratory studies on sweet peppers and other crops (González-Zamora and Gallardo 1999; Muñiz 2000).
In conclusion, the methodology used in this article allowed the bias of the estimations obtained from the model (the Bellows and Birley method) to be identified. This methodology also permitted the selection of the best model from a set of models (applying model averaging when necessary) to analyse stage frequency data in life tables to shed light on important aspects of a population, which was presented in this study for the whitefly B. tabaci in sweet pepper plants.
References
Asiimwe P, Ecaat JS, Otim M, Gerling D, Kyamanywa S, Legg JP (2007) Life-table analysis of mortality factors affecting populations of Bemisia tabaci on cassava in Uganda. Entomol Exp Appl 122:37–44
Baumgartner J, Yano E (1990) Whitefly population dynamics and modelling. In: Gerling D (ed) Whiteflies: their bionomics, pest status, and management. Intercept Ltd., Newcastle, pp 123–146
Baumgartner J, Delucchi V, Von Arx R, Rubli D (1986) Whitefly (Bemisia tabaci Genn., Stern.: Aleyrodidae) infestation patterns as influenced by cotton weather and Heliothis: hypothesis testing by using simulation models. Agric Ecosyst Environ 17:49–59
Bellows TS, Birley MH (1981) Estimating development and mortality rates and stage recruitment from insect stage-frequency data. Res Popul Ecol 23:232–244
Burham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag, New York
Byrne DN, Bellows TS (1991) Whitefly biology. Annu Rev Entomol 36:431–457
Franklin AB, Shenk TM, Anderson DR, Burham KP (2001) Statistical model selection: an alternative to null hypothesis testing. In: Shenk TM, Franklin AB (eds) Modelling in natural resource management: development, interpretation, and application. Island Press, Washington, pp 75–90
Gerling D, Fried PM (2000) Biological studies with Eretmocerus mundus Mercet (Hymenoptera: Aphelinidae) in Israel. OILB/SROP Bull 23:117–123
González-Zamora JE, Gallardo JM (1999) Desarrollo y capacidad reproductiva de Bemisia tabaci (Gennadius) (Homoptera; Aleyrodidae) en pimiento a tres temperaturas. Bol San Veg-Plagas 25:3–11
González-Zamora JE, Moreno Vázquez R, Rodríguez Rodríguez MD, Rodríguez Rodríguez MP, Mirasol Carmona E, Lastres Garcia Testón J, Manzanares Ruiz C (1996) Evolución del parasitismo en Bemisia tabaci (Genn.) y Trialeurodes vaporariorum (West.) (Homoptera; Aleyrodidae) en invernaderos de Almería. Bol San Veg-Plagas 22:373–389
Hansen EM, Bentz BJ, Turner DL (2001) Temperature-based model for predicting univoltine brood proportions in spruce beetle (Coleoptera: Scolytidae). Can Entomol 133:827–841
Hemerik L, van der Hoeven N (2003) Egg distributions of solitary parasitoids revisited. Entomol Exp Appl 107:81–86
Horowitz AR, Podoler H, Gerling D (1984) Life table analysis of the tobacco whitefly Bemisia tabaci (Gennadius) in cotton fields in Israel. Oecol Appl 5:221–233
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Luh HK, Croft BA (1999) Classification of generalist or specialist life styles of predaceous phytoseiid mites using a computer genetic algorithm, information theory, and life history traits. Environ Entomol 28:915–923
Manly BFJ (1990) Staged-structured populations. Sampling, analysis and simulation. Chapman and Hall Ltd., London
Manly BFJ (1994) Population analysis system: P1f, single species stage frequency analysis. Ver. 3.0. Ecological Systems Analysis, Pullman
Mazerolle MJ (2004) Appendix 1. Making sense out of Akaike’s Information Criterion (AIC): its use and interpretation in model selection and inference from ecological data. University of Laval, Canada. http://www.theses.ulaval.ca/2004/21842/apa.html. Accessed 29 Nov 2005
Muñiz M (2000) Development of the B-biotype of Bemisia tabaci (Gennadius, 1889) (Homoptera: Aleyrodidae) on three varieties of pepper at constant temperatures. Bol San Veg-Plagas 26:605–617
Muñiz M, Nombela G, Barrios L (2002) Within-plant distribution and infestation pattern of the B- and Q-biotypes of the whitefly, Bemisia tabaci, on tomato and pepper. Entomol Exp Appl 104:369–373
Naranjo SE, Ellsworth PC (2005) Mortality dynamics and population regulation in Bemisia tabaci. Entomol Exp Appl 116:93–108
Navas-Castillo J, Camero R, Bueno M, Moriones E (2000) Severe yellowing outbreaks in tomato in Spain associated with infections of Tomato chlorosis virus. Plant Dis 84:835–837
Oliveira MRV, Henneberry TJ, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 2005:709–723
Posada D, Buckley T (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likehood ratio tests. Syst Biol 53:793–808
Rodríguez-Rodríguez MD, Moreno R, Téllez MM, Rodríguez-Rodríguez MP, Fernández-Fernández R (1994) Eretmocerus mundus (Mercet), Encarsia lutea (Masi) y Encarsia transvena (Timberlake) (Hym., Aphelinidae) parasitoides de Bemisia tabaci (Homoptera, Aleyrodidae) en los cultivos horticolas protegidos almerienses. Bol San Veg-Plagas 20:695–702
Ruiz L, Janssen D, Martín G, Velasco L, Segundo E, Cuadrado IM (2006) Analysis of the temporal and spatial disease progress of Bemisia tabaci-transmitted Cucurbit yellow stunting disorder virus and Cucumber vein yellowing virus in cucumber. Plant Pathol 55:264–275
Saint-Germain M, Drapeau P, Buddle CM (2007) Occurrence patterns of aspen-feeding wood-borers (Coleoptera: Cerambycidae) along the wood decay gradient: active selection for specific host types or neutral mechanisms? Ecol Entomol 32:712–721
Segundo E, Martín G, Cuadrado IM, Janssen D (2004) A new yellowing disease in Phaseolus vulgaris associated with a whitefly-transmitted virus. Plant Pathol 53:517
Sileshi G (2006) Selecting the right statistical model for analysis of insect count data by using information theoretic measures. Bull Entomol Res 96:479–488
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell Science Ltd., Oxford
Stansly PA, Calvo J, Urbaneja A (2005) Release rates for control of Bemisia tabaci (Homoptera: Aleyrodidae) biotype “Q” with Eretmocerus mundus (Hymenoptera: Aphelinidae) in greenhouse tomato and pepper. Biol Contr 35:124–133
Statistical Graphics (2000) Statgraphics plus for windows. 5.1.5. Rockville, MD
Steel RGD, Torrie JH (1988) Bioestadística: principios y procedimientos [translation from the 2nd ed. of Principles and procedures of statistics. A biometrial approach]. McGraw-Hill Latinoamericana, Bogotá
Takeuchi H (2006) Estimation of the occurrence of pecky grain damage by a sweeping census of Leptocorisa chinensis Dallas (Hemiptera: Alydidae) in rice fields. Japn J Appl Entomol Zool 50:137–143
Téllez MM, Lara L, Stansly P, Urbaneja A (2003) Eretmocerus mundus (Hyt.: Aphelinidae), parasitoide autóctono de Bemisia tabaci (Hom.: Aleyroridae): primeros resultados de eficacia en judía. Bol San Veg-Plagas 29:511–521
Umble JR, Fisher JR (2003) Influence of below-ground feeding by garden symphylans (Cephalostigmata: Scutigerellidae) on plant health. Environ Entomol 32:1251–1261
Urbaneja A, Sanchez E, Stansly PA (2007) Life history of Eretmocerus mundus, a parasitoid of Bemisia tabaci, on tomato and sweet pepper. Biocontrol 52:25–39
Von Arx R, Baumgartner J, Delucchi V (1983) A model to simulate the population dynamics of Bemisia tabaci Genn. (Stern. Aleyrodidae) on cotton in the Sudan Gezira. Z Angew Entomol 96:341–363
Zalom FG, Natwick ET (1987) Developmental time of sweetpotato whitefly (Homoptera: Aleyrodidae) in small field cages on cotton plants. Fla Entomol 70:427–431
Zalom FG, Natwick ET, Toscano NC (1985) Temperature regulation of Bemisia tabaci (Homoptera: Aleyrodidae) populations in Imperial Valley cotton. J Econ Entomol 78:61–64
Zang LS, Liu TX (2008) Host-feeding of three parasitoid species on Bemisia tabaci biotype B and implications for whitefly biological control. Entomol Exp Appl 127:55–63
Acknowledgments
The first author was granted a postdoctoral scholarship from the Junta de Andalucia (Spain) at the I.F.A.P.A. (Instituto para la Formación Agraria y Pesquera de Andalucia) centre “La Mojonera-La Cañada” (Almeria, Spain), within the research project “Lucha integrada en cucurbitáceas”. The O.E.C.D. (Organisation for Economic Co-operation and Development) supported the first author with a grant to visit Prof. B.F.J. Manly at Otago University (Dunedin, New Zealand). The authors would like to thank Jose Miguel Gallardo for his help in collecting data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Horowitz
Rights and permissions
About this article
Cite this article
González-Zamora, J.E., Moreno, R. Model selection and averaging in the estimation of population parameters of Bemisia tabaci (Gennadius) from stage frequency data in sweet pepper plants. J Pest Sci 84, 165–177 (2011). https://doi.org/10.1007/s10340-010-0337-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-010-0337-y