Abstract
Two-spotted spider mite (TSSM) and onion thrips are serious pests of potatoes in the Ardabil region (Iran). In the present study, anthocorid species were identified in potato fields of this region during 2006 and 2007. The results of the abundance study indicate that Orius niger (Wolff) and O. minutus (Linnaeus) are major predators of these pests in potato fields. The life table parameters of these predators were compared when they were fed 2nd instar larvae of onion thrips or female TSSM on potato leaves. In these experiments, O. niger had a lower nymphal mortality, longer oviposition period, higher net reproductive rate (R 0), and higher intrinsic rate of natural increase (r m ) when fed thrips instead of mites. O. minutus feeding on mites compare to thrips had a lower nymphal mortality, longer oviposition period, higher net reproductive rate, and higher intrinsic rate of natural increase. Based on these results, it can be concluded that these predators could be useful as biological agents in potato fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two-spotted spider mite (TSSM), Tetranychus urticae Koch, and onion thrips (OT), Thrips tabaci Lindeman, are important crop pests throughout the world (Lewis 1997; Venzon et al. 2001). These pests regularly cause economic damage to the potato crop in the Ardabil region of Iran. Potato growers in the region often use insecticides at high doses and short intervals to control pests. Continuous use of insecticides is not a suitable control method of TSSM and OT, since these pests has developed resistance to most of the available insecticides in many parts of the world (Cranham and Helle 1985; Lewis 1997). Therefore, there has been an increasing interest in using biological control agents, especially Orius species, against these pests (Tommasini and Nicoli 1993; Yasunaga 1997; Kohno and Kashio 1998; Lattin 1999; Blaeser et al. 2004; Zhang et al. 2006). Yano et al. (2002) mentioned that the use of indigenous predator species is preferable to the use of imported species. The longevity and fecundity of Orius species vary according to the consumed prey species (Kiman and Yeargan 1985; Venzon et al. 2001, 2002; Deligeorgidis 2002). The purpose of this research was to (a) identify the dominant anthocorid species in potato fields infested with OT and TSSM, (b) determine the relationship between the densities of the dominant species of anthocorid and OT and TSSM, and (c) study the life table parameters of Orius niger (Wolff) and O. minutus (Linnaeus) when fed OT and TSSM on potato leaves.
Materials and methods
Abundance study
The abundance study was conducted at the Agricultural Station of the University of Mohaghegh Ardabili in the Ardabil province of Iran (elevation of 1,332 m; longitude 48°17′E; latitude 38°15′N). Potato tubers (cv. Agria) were planted in four experimental fields during the spring of 2006 and 2007. These experiments were conducted in a randomized complete block design with four replicates. Alfalfa had been planted in these experimental fields in the previous years. Each field consisted of 14 rows of potato plants with 75 cm spacing between rows. These fields were managed according to the local practice with weekly flood irrigation and hand weeding. No insecticide was applied to the plants. Dithane fungicide (Mancozeb, Rohm and Haas Co., Philadelphia, PA) was used to prevent foliar disease before inflorescence emergence. In each experimental field, 40 randomly selected plants were sampled at each of the four growth stages of potato (inflorescence emergence, full flowering, petal fall, and ripening stages) between 10:00 and 11:00. These samples consisted of a mixture of adults and nymphal stages of anthocorid species. Nymphal stages of anthocorid species were reared on potato leaves infected with OT and TSSM inside 2 l rearing units until adult emergence to identify the species. These units were maintained in a growth chamber at 24 ± 1°C, 50 ± 5% RH and a photoperiod of 16:8 h (L:D). Anthocorid species in each sample were identified under the stereomicroscope by their morphological characteristics (Pericart 1996). In each sample, the number of every anthocorid species, OT and TSSM were recorded for 2 years.
Life table study
O. niger and O. minutus were collected by sweep net from unsprayed potato fields (cv. Agria) in the Ardabil plain during July of 2007. These predators were reared on alfalfa (cv. Hamadan) planted in plastic pots, which were placed inside 10 l rearing units. These units were maintained in a growth chamber at 24 ± 1°C, 50 ± 5% RH and a photoperiod of 16:8 h (L:D). They were provided with flour moth eggs (Ephestia kuheniella Zeller) and corn pollen on a piece of napkin every day. Ten green bean pods were placed on the top of the soil inside a unit as oviposition substrate for the predators (Cocuzza et al. 1997; Steiner and Goodwin 1998; Honda et al. 1998; Kakimoto et al. 2005). One-day-old eggs of O. niger and O. minutus on green bean pods were used in the laboratory experiments.
OT and TSSM were collected from the same potato fields and reared on bean pods (Phaseolus vulgaris Linnaeus) that were planted in pots placed inside 10 l rearing units. These units were maintained in a growth chamber at 24 ± 1°C, 50 ± 5% RH and a photoperiod of 16:8 h (L:D). Newly emerged 2nd instar larvae of OT and female TSSM were collected from the colony and used in the laboratory experiments as preys because 2nd instar larvae of OT and female TSSM did not fly and were observed more easily on potato leaves.
The life table parameters of O. niger and O. minutus, fed either 2nd instar larvae of OT or female TSSM, were studied in the laboratory. In these experiments, 1-day-old eggs of O. niger and O. minutus on green bean pods were transferred separately into transparent cylindrical plastic cages (10 cm diameter and 25 cm high) with a mesh lid. These units were maintained in a growth chamber at 24 ± 1°C, 50 ± 5% RH and a photoperiod of 16:8 h (L:D). After the predator’s egg hatched, 30 2nd instar larvae of OT or 30 female TSSM on the lower surface of a potato leaf (with 5 leaflets) were offered separately to each newly hatched nymph of O. niger and O. minutus. Every 24 h, the status of the predators’ nymphs, the death or completion of their development to the adult stage, and their gender (the apical segment of abdomen in female is straight and in male is curved and swollen) was recorded and the potato leaves were renewed in each unit. Twenty-four hours after the adult emergence, 30 2nd instar larvae of OT and 30 female TSSM on the lower surface of the potato leaf were offered separately to each of the adult pairs of O. niger and O. minutus inside a unit using protocol similar to the one described above. The potato leaves were renewed every 24 h for all treatments. Survival of Orius females and the number of eggs laid on the potato leaves were counted under the stereomicroscope every 24 h until the death of the predators’ females. If the male of O. niger and O. minutus died before the mated females began to oviposit, another newly emerged male from the same treatment was introduced. Non-ovipositing females were also included in calculations of longevity and fecundity of each predator species. Each treatment was replicated 40 times.
Data analyses
Prior to analysis, in order to correct for the heterogeneity of variance, all the data were log-transformed (ln x + 2). In the field experiments, the abundance of every anthocorid species, OT and TSSM at four growth stages of potato in 2 years were analyzed by a combined analysis, split plot design in 2 years, and the differences were compared by Tukey’s HSD test (PROC GLM, SAS Institute 1999). A simple regression analysis was also performed between the densities of OT and TSSM with the densities of O. niger and O. minutes, respectively, in potato fields during 2006 and 2007 (PROC CORR, SAS Institute 1999). In the laboratory experiments, the data of the life cycle parameters of the two predator species when fed two prey species were analyzed by two-way ANOVA, and the differences were compared by the SNK test (PROC ANOVA, SAS Institute 1999). The intrinsic rate of natural increase (r m = ln(R 0)/T), the net reproductive rate (R 0 = ∑ x l x m x ), generation time (T = ∑ x l x m x x/R 0), the finite rate of increase (day−1) (λ = e2), the intrinsic birth rate (day−1) (b = ∑e−r(x + 0.5) l x ), the intrinsic death rate (day−1) (d = b − r), gross reproductive rate (eggs/female) (GRR = Σ M x ), and doubling time (days) (DT = (ln 2)/r) were calculated for two Orius species when fed 2nd instar larvae of OT and female TSSM (Birch 1948; Laughlin 1965). In these formulas, x was the age of the female in days, l x is survival of the female until x, m x is the number of female offspring produced at age x, and M x is the total number of offspring (= number of eggs) produced at age x (Birch 1948; Laughlin 1965).
Results
Abundance study
In this study, four anthocorid species, O. niger, O. minutus, O. horvathi (Reuter), and Anthocoris pilosus (Jackovlev), were collected and identified in potato fields of the Ardabil region. The abundance of anthocorid species were significantly different at four phenological stages of potato in 2 years (F = 25.47; df = 9, 936; P = 0.0001). The abundance of O. niger was higher than O. minutus at inflorescence emergence, full flowering and petal fall stages (P ≤ 0.05), whereas the abundance of O. niger was similar to O. minutus at the ripening stage (P > 0.05) (Fig. 1). For the four phenological stages of potato, the populations of O. horvathi and A. pilosus were significantly lower when compared with O. niger and O. minutus (P ≤ 0.05) (Fig. 1).
Mean abundance of four anthocorid species at four different phenological stages of potato in 2006 and 2007 (different letters indicate significant differences at P ≤ 0.05). The columns 1–8 represent O. niger 2006, O. niger 2007, O. minutus 2006, O. minutus 2007, O. horvathi 2006, O. horvathi 2007, A. pilosus 2006 and A. pilosus 2007
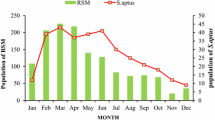
The abundance of OT and TSSM were significantly different for the four growth stages (F = 348.86; df = 3, 312; P = 0.0001). The abundance of OT and TSSM increased at inflorescence emergence, full flowering, and petal fall stages, but their abundance was significantly decreased during the ripening stage (P ≤ 0.05) (Fig. 2).
A significant and positive correlation was observed between the population densities of O. niger and OT (F = 14.05; df = 1, 6; P = 0.01; R 2 = 0.701) (Fig. 3) and O. niger and TSSM (F = 8.48; df = 1, 6; P = 0.027; R 2 = 0.58) (Fig. 4). A significant and positive correlation was observed between the population densities of O. minutus and TSSM (F = 51.6; df = 1, 6; P = 0.0001; R 2 = 0.896) (Fig. 5), whereas the correlation between the population densities of O. minutus and OT was not significant (F = 4.04; df = 1, 6; P = 0.09; R 2 = 0.402) (Fig. 6).
Life table study
The nymphal development time and pre-oviposition period of O. niger were not significantly different when fed 2nd instar larvae of OT instead of female TSSM, and the nymphal development time and pre-oviposition period of O. minutus were not significantly different when feeding on mites compare to thrips (F = 3.47; df = 1, 156; P = 0.064 and F = 3.76; df = 1, 156; P = 0.054) (Table 1).
O. niger had significantly lower nymphal mortality percentages, higher sex ratio, and a longer oviposition period when fed 2nd instar larvae of OT instead of female TSSM, however, O. minutus had significantly lower nymphal mortality percentages, higher sex ratio, and a longer oviposition period with feeding on mites compare to thrips (F = 30.79; df = 1, 156; P = 0.0001 and F = 14.11; df = 1, 156; P = 0.0002 and F = 39.74; df = 1, 156; P = 0.0001) (Table 1).
O. niger fed 2nd instar larvae of OT when compared with female TSSM showed a higher intrinsic rate of natural increase (r m ), higher net reproductive rate (R0), higher gross reproductive rate (GRR), higher intrinsic birth rate (b), and higher finite rate of increase (λ) (Table 2). However, the intrinsic rate of natural increase, the net reproductive rate, the gross reproductive rate, the intrinsic birth rate, and the finite rate of increase of O. minutus were higher when fed mites (Table 2).
O. niger fed 2nd instar larvae of OT had a lower intrinsic death rate (d), whereas the intrinsic death rate of O. minutus was lower when fed mites (Table 2). The generation time (T) of O. niger was longer when feeding on thrips, however, O. minutus feeding on mites compare to thrips had longer generation time (Table 2). The doubling time (DT) of O. niger and O. minutus was shorter when feeding on thrips and mites, respectively (Table 2).
Discussion
Our data on the abundance and life table studies suggest that O. niger is a primary predator of OT and O. minutus is a primary predator of TSSM. There are also other important biotic and abiotic factors that can affect the abundance of these predators. Atakan and Gencer (2008) concluded that the populations of O. niger were more abundant in normal-planted cotton fields and its population density significantly correlated with the Frankliniella occidentalis (Pergande) population. With regard to predator–thrip interactions, they suggested that O. niger might be an efficient biological control agent to regulate western flower thrips, especially in normal-planted cotton.
The life table parameters of predator species are important for their capability of biological control. This study showed that the type of prey can strongly influence the life table parameters of Orius species. In the laboratory experiments, feeding with 2nd instar larvae of thrips resulted in better survival and higher fecundity of O. niger while feeding with female mites resulted in better survival and higher fecundity of O. minutus. Also, the analysis of the nymphal development time, nymphal mortality percentage, pre-oviposition, and oviposition period indicated that the population growth of O. niger was restricted mostly by a high nymphal mortality, low sex ratio, and low r m when fed female mites, and the population growth of O. minutus was restricted mostly by a high nymphal mortality, low sex ratio, and low r m when fed 2nd instar larvae of thrips.
Few studies address the intrinsic rate of natural increase for O. niger and O. minutus when fed OT and TSSM. Deligeorgidis (2002) demonstrated that O. niger strongly preferred 2nd instar larvae of OT when compared with the 2nd instar larvae of F. occidentalis, and concluded that O. niger was a suitable predator to control OT. Baniameri et al. (2005) estimated a high r m (0.113) for O. niger at 26°C on a diet of E. kuehniella eggs and suggested that the population growth rate of O. niger was restricted mostly by juvenile mortality. Toyoshima (2006) reported that O. minutus had the potential to control TSSM populations on apple trees in Japan.
The intrinsic rate of natural increase of O. niger varies by both the different species of prey offered and the greater difficulty for Orius species to catch adult thrips than nymph thrips (Salas-Aguilar and Ehler 1977; Teerling et al. 1993). For example, Teerling et al. (1993) showed that O. tristicolor (White) responded to specific semiochemical cues from western flower thrips and preferred to feed on it in comparison with other thrip species. Lichtenauer and Sell (1993) concluded that in a no-choice test, O. insidiosus (Say) and O. minutus consumed more thrip larvae than adults. The fecundity of O. insidiosus varied when it consumed thrips, mites, and the eggs of moths (Kiman and Yeargan 1985). Venzon et al. (2001, 2002) reported that O. laevigatus (Fieber) was attracted more to the TSSM-infected cucumber than western flower thrip-infected plants. Fritsche and Tamo (2000) found that the control of Megalurothrips sjostedti Trybom by O. albidipennis (Reuter) was less efficient than the control of Ceratothripoides cameroni (Priesner) and F. schultzei Trybom.
Our results indicate that O. niger and O. minutus are relatively abundant in Ardabil potato fields and these predators can be useful to biologically control onion thrips and TSSM.
References
Atakan E, Gencer O (2008) Influence of planting date on the relationship between populations of Frankliniella flower thrips and predatory bug Orius niger in cotton. J Pest Sci 81:123–133
Baniameri V, Soleiman-Nejadian E, Mohaghegh J (2005) Life table and age-dependent reproduction of the predatory bug Orius niger Wolff (Heteroptera: Anthocoridae) at three constant temperatures: a demographic analysis. Appl Entomol Zool (Jpn) 40:545–550. doi:10.1303/aez.2005.545
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26. doi:10.2307/1605
Blaeser P, Sengonca C, Zegula T (2004) The potential use of different predatory bug species in the biological control of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). J Pest Sci 77:211–219. doi:10.1007/s10340-004-0057-2
Cocuzza GE, De Clercq P, Van De Veire M, De Cock A, Degheele D, Vacante V (1997) Reproduction of Orius laevigatus and Orius albidipennis on pollen and Ephestia kuehniella eggs. Entomol Exp Appl 82:101–104. doi:10.1023/A:1002931622011
Cranham JE, Helle W (1985) Pesticide resistance in Tetranychidae. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier, Amsterdam, pp 405–421
Deligeorgidis PN (2002) Predatory effect of Orius niger (Wolff) (Hem., Anthocoridae) on Frankliniella occidentalis (Pergande) and Thrips tabaci Lindeman (Thysan., Thripidae). J Appl Entomol 126:82–85. doi:10.1046/j.1439-0418.2002.00603.x
Fritsche ME, Tamo M (2000) Influence of thrips prey species on the life-history and behavior of Orius albidipennis. Entomol Exp Appl 96:111–118. doi:10.1023/A:1004015216361
Honda JY, Nakashima Y, Hirose Y (1998) Development, reproduction and longevity of Orius minutus and Orius sauteri (Heteroptera: Anthocoridae) when reared on Ephestia kuehniella eggs. Appl Entomol Zool (Jpn) 33:449–453
Kakimoto K, Urano S, Noda T, Matuo K, Sakamaki Y, Tsuda K, Kusigemati K (2005) Comparison of the reproductive potential of three Orius species, O. strigicollis, O. sauteri, and O. minutus (Heteroptera: Anthocoridae), using eggs of the Mediterranean flour moth as a food source. Appl Entomol Zool (Jpn) 40:247–255. doi:10.1303/aez.2005.247
Kiman ZB, Yeargan KV (1985) Development and reproduction of the predator Orius insidiosus (Hemiptera: Anthocoridae) reared on diets of selected plant material and arthropod prey. Ann Entomol Soc Am 78:464–467
Kohno K, Kashio T (1998) Development and prey consumption of Orius sauteri (Poppius) and O. minutus (L.) (Heteroptera: Anthocoridae) fed on Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Appl Entomol Zool (Jpn) 33:227–230
Lattin JD (1999) Bionomics of the Anthocoridae. Annu Rev Entomol 44:207–231. doi:10.1146/annurev.ento.44.1.207
Laughlin R (1965) Capacity for increase: a useful population statistic. J Anim Ecol 34:77–91. doi:10.2307/2370
Lewis T (1997) Thrips as crop pests (first edition). CAB International, Oxon, p 740
Lichtenauer A, Sell P (1993) Biological control of insects; predation of different developmental stages of the western flower thrips by adults of Orius minutus (Heteroptera: Anthocoridae). Meded Fac Landbouw Univ Gent 58:397–407
Pericart J (1996) Family Anthocoridae (Fiber). In: Aukema B, Rieger C (eds) Catalogue of the Heteroptera of the Palearctic Region. The Netherlands Entomological Society, Wageningen, pp 108–140
Salas-Aguilar J, Ehler LE (1977) Feeding habits of Orius triticolor. Ann Entomol Soc Am 70:60–62
SAS Institute (1999) SAS/Stat users guide. SAS Institute, Cary
Steiner MY, Goodwin S (1998) Method for collecting and rearing thrips (Thysanoptera) and their natural enemies. Aust J Entomol 37:101–106. doi:10.1111/j.1440-6055.1998.tb01554.x
Teerling CR, Gillespie DR, Borden JH (1993) Utilization of western flower thrips alarm pheromone as a prey-finding kairomone by predators. Can Entomol 125:431–437
Tommasini MG, Nicoli G (1993) Adult activity of four Orius species reared on two preys, integrated control in glasshouses. IOBC WPRS Bull 16:181–184
Toyoshima S (2006) Development, prey consumption, and fecundity of Orius minutus (Heteroptera: Anthocoridae) when fed on Tetranychus urticae (Acari: Tetranychidae). J Acarol Soc Jpn 15:151–156. doi:10.2300/acari.15.151
Venzon M, Janssen A, Sabelis MV (2001) Prey preference, intraguild predation and population dynamics of an arthropod food web on plant. Exp Appl Acarol 25:785–808. doi:10.1023/A:1020443401985
Venzon M, Janssen A, Sabelis MV (2002) Prey preference and reproductive success of the generalist predator Orius laevigatus. Oikos 97:116–124. doi:10.1034/j.1600-0706.2002.970112.x
Yano E, Watanabe K, Yara K (2002) Life history parameters of Orius sauteri (Poppius) (Het., Anthocoridae) reared on Ephestia kuehniella eggs and the minimum amount of the diet for rearing individuals. J Appl Entomol 126:389–394. doi:10.1046/j.1439-0418.2002.00598.x
Yasunaga T (1997) The flower bug genus Orius Wolff (Heteroptera: Anthocoridae) from Japan and Taiwan, part II. Appl Entomol Zool (Jpn) 32:379–386
Zhang S, Wu L, Xu X, Hua B (2006) Predation of Orius minutus on Odontothrips loti. Ying Yong Sheng Tai Xue Bao 17:1259–1263
Acknowledgments
The Research Council of Mohaghegh Ardabili University (Iran) is gratefully acknowledged for their financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Juen.
Rights and permissions
About this article
Cite this article
Fathi, S.A.A. The abundance of Orius niger (Wolf.) and O. minutus (L.) in potato fields and their life table parameters when fed on two prey species. J Pest Sci 82, 267–272 (2009). https://doi.org/10.1007/s10340-009-0250-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-009-0250-4