Abstract
The ecology of the coccid Protopulvinaria pyriformis infesting the laurel Laurus nobilis was studied from October 2003 to September 2005 in SW Greece. The coccid is parthenogenetic and oviparous, producing increased amounts of honeydew throughout the year. It settles mainly on the lower leaf surface. The scale overwinters as egg, 1st and 2nd instar nymph and adult and completes several overlapping generations every year. The life cycle was estimated to last in nature ~52 days during winter and 29–33 days during summer. Infestation density ranged between 0.3 and 2.8 live scales per cm2 of leaf surface. Metaphycus helvolus was the only wasp found to parasitize P. pyriformis. Parasitism rate reached 31.2% during the second year of the study. The scale was able to resist parasitization by encapsulating the parasitoid’s eggs. Maximum encapsulation rate was estimated up to 23% of the adult scales. Encapsulated eggs ranged from 1 to 5 eggs per adult scale. Predation rate reached 7% and it was attributed to the coccinellid Chilocorus bipustulatus. The above information could be important for planning a sustainable control strategy for this new pest in Greece.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heart-shaped or pyriform scale Protopulvinaria pyriformis (Cockerell) (Hemiptera: Coccidae), is widely distributed in tropical and subtropical regions of Africa, Middle East, North and South America, and throughout the Palearctic region (Ben-Dov 1993). As far as the Mediterranean basin is concerned the coccid has been recorded in France (Canard 1996), Israel (Blumberg and Blumberg 1991), Italy (Pellizzari 2003), Portugal (Carvalho and Aguiar 1997) and Spain (Spina 2001). Its presence has been recently noted for the first time in Greece, on the laurel Laurus nobilis L. (Lauraceae) (Ben-Dov et al. 2003).
It is a polyphagous insect with more than 100 plant hosts belonging to 34 families (Wysoki 1985) and it is considered as a serious pest of fruit trees and ornamentals in many parts of the world (Ben-Dov 1993). In the countries where the pyriform scale has been recorded it has been found to infest several fruit trees and ornamental plants. The scale produces abundant honeydew, and sooty mould develops subsequently all over the host plant. Affected leaves dry out and fall, reducing the growth of the plant. Sap removal, host debilitation, honeydew production and sooty mould can have major economic effects (Gill 1988; Diaz et al. 2005). As far as its biology is concerned, females are reproducing parthenogenetically (Ben-Dov 1993). Males may occur in the population at a low proportion. Its entire life cycle is spent on the lower leaf surface (Gill 1988). The fecundity of the scale is estimated to reach 200 eggs per female on avocado (Spina 2001). Its phenology has been studied in Israel (Blumberg and Blumberg 1991) and Spain (Spina 2001), where the scale completes two generations per year as well as in Florida, where it completes several overlapping generations (Gill 1988).
Many parasitoids mainly encyrtids of Metaphycus sp. (Blumberg and Swirski 1984; Wysoki 1985; Toit et al. 1991; Blumberg and Goldenburg 1991; Blumberg et al. 1993; Guerierri and Noyes 2000) and a few coccinellid predators (Robertson et al. 1986; Hadar 1993; Swirski et al. 1995; Trjapitzin and Triapitsyn 2003), have been reported to act against P. pyriformis. Variable encapsulation rates of eggs of many parasitoids by P. pyriformis have been demonstrated in other studies (Blumberg 1991; Blumberg and DeBach 1981; Blumberg and Goldenburg 1991; Blumberg et al. 1993). Blumberg and Swirski (1984) report that encapsulation can take place at several developmental stages of the scale. The mortality of a Metaphycus species (Metaphycus swirski) has been attributed to its encapsulation solely at the egg stage by P. pyriformis, while the same parasitoid was found encapsulated also at the larval stage by Coccus capparidis (Blumberg and Swirski 1984). The encapsulation frequency depends on several factors, such as the host plant, the temperature, the age or the species of the scale, and supperparasitism. The encapsulation of eggs of Metaphycus stanleyi by P. pyriformis in several hosts was found significantly higher during the warm period of the year (Blumberg 1991). The encapsulation rate of two parasitoids (M. swirskii and Encyrtus lecanorium) by Coccus hesperidum was lower in young female scales than in mature scales (Blumberg and Goldenburg 1991, Blumberg 1982). Superparasitism reduces the encapsulation frequency, due to the weakness of the parasitized scale. Coccus hesperidum which had been weakened by Coccophagus sp. parasitism was not able to encapsulate eggs of M. swirskii (Blumberg 1982). This resistance to parasitization occurred by encapsulation has been regarded as the main cause of the inability of many parasitoids to prevent outbreaks of the pest (Blumberg 1991).
Taking into account the lack of experimental data concerning the presence of pyriform scale not only in Greece but also in many Mediterranean countries, this study aims at providing further significant biological and ecological data to enhance the integrated management of this scale pest. Phenological details such as the appearance of 1st instar nymphs, the number of generations and the population peaks give useful information about the time of applying insecticides or releasing a biocontrol agent. In addition, it was considered important to investigate in the present study some aspects of the ecology of the pyriform scale in Greece, concerning the predators and parasidoids of the scale as well as their activity, the possibility of the scale to control the development of parasitoids by encapsulation, the frequency and seasonal fluctuation of encapsulation and the age of the scale and parasitoid in which encapsulation occurs.
Materials and methods
Field experiments were conducted during October 2003–September 2005 on scales infesting the laurel L. nobilis in Kalamata, Messinia Co., SW Greece. The study was made on 40 laurel srubs, 5 years old, about 1.5–2 m high, planted in a public garden of 6,000 m2. The weed control in the garden was mechanically performed. No chemicals were sprayed in the garden during the study period.
For the laboratory studies, samples of about 50–60 leaves of infested laurel were cut, labeled and put into plastic bags and transferred to the laboratory, every 15 days. The lower surface of 12 of these leaves was examined under a binocular stereoscope and the number of all the scales of each instar as well the numbers of predated, parasitized and dead scales were recorded. As predated scales were recorded only the partially destroyed ones (the half-eaten scales) because the totally consumed individuals (such as crawlers) obviously could not be estimated. The numbers of scales at each developmental stage, as well as the numbers of predated, parasitized and dead scales, were expressed as percentage (%) of live scales. Females containing one or more encapsulated (melanized) parasitoid eggs were also noted. Dark encapsulated eggs were easily distinguished inside the transparent yellowish scale body. Encapsulation frequency was assessed as:
-
1.
scales containing encapsulated eggs as percentage (%) of live adult scales and
-
2.
percentage parasitized scales wherein encapsulation completely prevented parasitoid development, which reflects the rate of Efficient encapsulation (Ee):
Infestation density was estimated by the reduction of live scales to 1 cm2 of leaf surface. The leaf surface (E) was estimated using the ellipse surface area equation:
where: π = 3.14, α : minimum radius, b: maximum radius.
Parasitized coccids were placed individually in vials in an incubator under controlled conditions (25°C, 16:8 L:D, 65–75% RH), until adult wasp emergence. The identification of the parasitoid species was made by the third author, and voucher specimens are deposited at the Institute of Zoology, Tbilisi, Georgia, and in Suleiman Demirel University, Isparta, Turkey.
At each sampling the above-mentioned infested shrubs were visually observed for 30 min for the detection of predatory insects. All predators were counted and identified to species.
Outdoor experiments were conducted for the estimation of P. pyriformis life cycle in field conditions. Ten laurel plants 2 years old, were artificially infested by laying infested leaves for 2 days. Artificial infestation took place on 26 October 2003, 7 June 2004 and 6 July 2004. Infested plants were checked daily to observe the development of the coccid until hatching.
A thermograph, housed in a meteorological screen located ~500 m of the infested plants, was used to record daily temperatures.
Results
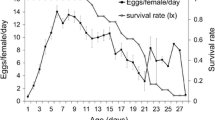
During the present study P. pyriformis was found mainly on the lower leaf surface of L. nobilis, while a few individuals settled on the shoots. The developmental stages of P. pyriformis recorded on leaves, are presented in Fig. 1. The infestation density (mean of live scales ± SE/cm2 of 12 leaves) and the total number of scales found per sampling (on 12 leaves) are shown in Fig. 1a. The infestation density ranged between 0.3 (20 January 2005) and 2.8 (20 May 2005) live scales per cm2 and the total number of scales found per sampling ranged between 139 (20 January 2005) and 993 (20 May 2004) scales. The composition of all developmental stages of the scale is presented in Figs. 1b–f. The scale overwintered as egg, 1st and 2nd instar nymph and adult. Pest population consisted mainly by 2nd instar nymphs (40–50%) from October until April, during both years of the study. On the other hand, no crawlers were observed from January until March. The scale completes several overlapping generations annually, in Greece.
The life cycle was estimated to last ~52 days in nature during winter. This is based on the fact that crawlers of the next generation were observed on 17 December 2003 on laurel plants which had been artificially infested with crawlers on 26 October 2003. Similarly, the scale completes development at about 33 and 29 days during summer (7 June–10 July 2004 and 6 July–4 August 2004, respectively).
Dead scales are presented in Fig. 2a whereas dead individuals from unknown causes comprised 2.0–81.1% of the whole pest population. Predated scales reached 7.0% in June 2004 (Fig. 2b). During visual observations minor numbers of larvae and adults of Chilocorus bipustulatus (Linnaeus) (Coleoptera: Coccinellidae) were found. Their population peak was recorded during June 2005 (4 adults and 7 larvae).
Composition of Protopulvinaria pyriformis population found on laurel from October 2003 to September 2005 in Greece: a dead from unknown causes, b predated, c parasitized, (% of live scales) d containing encapsulated eggs of Metaphycus helvolus (% of live adults) and e % efficient encapsulation (Ee) (% of parasitized scales), f monthly average temperatures
Parasitized scales are presented in Fig. 2c. Parasitism was recorded only in adult scales and it was attributed to Metaphycus helvolus (Compere) (Hymenoptera: Encyrtidae), since this was the only parasitoid that emerged from parasitized scales. Parasitism rate was maximized during July 2004 (26.8%), and June 2005 (31.2%). Parasitized scales contained 1–3 wasp individuals.
Protopulvinaria pyriformis reacted to M. helvolus parasitism by encapsulating the parasitoid eggs. Preovipositing and ovipositing female adults of the scale were recorded to contain 1–5 encapsulated eggs (menanized) of the parasitoid. The highest levels of encapsulation were recorded during summer (August 2004 and July 2005) reaching 17% and 23% of adult scales, respectively (Fig. 2d) when the Efficient encapsulation (Ee) at the same periods was 43–100% (Fig. 2e). The 29.1% of the total number of females containing encapsulated eggs were preovipositing females and the 70.9% ovipositing females (Fig. 2d). Encapsulation was observed only at the egg stage of the parasitoid, which means that parasitoid development was entirely prevented.
Discussion
Protopulvinaria pyriformis completes two generations per year in Israel, feeding on avocado (oviposition during early May and late October) and three generations feeding on common ivy (oviposition during late March, mid August and late October) (Blumberg and Blumberg 1991). It is evident from the current study that pyriform scale completes several overlapping generations annually, in Greece. However, estimation of the exact number of generations based on the seasonal occurrence of each developmental stage is inapplicable, due to the simultaneous presence of many developmental stages (crawlers, nymphs, adults) during most time of the year (Fig. 1b–f). The same phenomenon has been observed when the scale infested avocados and ornamental plants in Florida (Gill 1988).
The increase in infestation density (live scale individuals per cm2) during the warm period (Fig. 1a), can be attributed to the high numbers of newly-hatched crawlers present at the same time (Fig. 1b). The following peak on dead scales (Fig. 2a) is ascribed to the increased mortality of crawlers which presumably is recorded soon after their hatching.
Among the parasitoids which are referred as natural enemies of the pyriform scale in bibliography, two species only are reported in Greece. Metaphycus helvolus, in several areas of the country (Kapatos et al. 1977) and Metaphycus sp. near stanleyi Compere, in Corfu island (NW Greece) (Viggiani and Mazzone 1977), have been collected from infestations of the black scale Saissetia oleae (Oliver) (Homoptera : Coccidae). The former species was the sole wasp recorded during the present study causing notable parasitism to the coccid demonstrating two peaks on July 2004 (26.8%) and on June 2005 (31.2%). This is regarded as quite an increased parasitism rate when compared with the respective rates by M. stanleyi (10–12%) (Blumberg and Blumberg 1991) and the parasitoid complex of Metaphycus sp., Coccophagus sp. (Hymenoptera: Aphelinidae) and Tetrastichus sp. (Hymenoptera: Eulophidae) (25.2%) (Toit et al. 1991).
The pyriform scale is capable of resisting to some extent to parasitization by encapsulating the parasitoid eggs. Encapsulation is a common phenomenon in P. pyriformis and it has been thoroughly studied (Blumberg 1991; Blumberg and Blumberg 1991; Blumberg et al. 1993, and others). The highest encapsulation rates were recorded during June and August. This phenomenon is due to the positive correlation between the encapsulation ability of a soft scale and the ambient temperature (Blumberg and DeBach 1981; Blumberg 1982; Blumberg et al. 1993). Similar results have been reported in other field studies where encapsulation by P. pyriformis was maximized during the summer period (Blumberg 1991; Blumberg and Blumberg 1991; Blumberg et al. 1993).
Encapsulation rate reached 17–23% of adult scales. It is evident that encapsulation by scales infesting laurel in Greece is significantly less intense compared to that by scales infesting ornamental plants (78–100%) (Blumberg 1991; Blumberg et al. 1993), and avocado trees (49–62%) (Blumberg 1991; Blumberg and Blumberg 1991; Blumberg et al. 1993), in Israel. Taking into account that encapsulation is significantly influenced not only by ambient temperature (Blumberg and DeBach 1981; Blumberg 1982; Blumberg et al. 1993), but also by the parasitoid species (Salt 1963) the differences among those studies and the current one may be explained. The abovementioned studies were conducted in Israel, an area with warmer climate than SW Greece, and dealt with various parasitoids species other than M. helvolus, such as its conspecifics M. swirskii Annecke & Mynhardt, Μ. galbus Annecke and M. stanleyi. Although, it is very probable that most encapsulated eggs observed during the present study belong to M. helvolus, we cannot exclude the possibility that other parasitoids failed to complete development inside P. pyriformis due to the encapsulation of their eggs.
Although M. helvolus is referred as a natural enemy of P. pyriformis (Robertson et al. 1986; Toit et al. 1991) it is not included in other studies among the parasitoids being encapsulated by the pyriform scale (Blumberg 1991; Blumberg and Swirski 1984; Blumberg and Blumberg 1991; Blumberg and Goldenburg 1991; Blumberg et al. 1993). Therefore, in the present study, data on encapsulation of M. helvolus by P. pyroformis are given for the first time. Encapsulation of M. helvolus by P. pyriformis ranged between 1 and 5 eggs in the present study, while 20 or more encapsulated eggs of M. helvolus were found per scale in the soft brown scale Coccus hesperidum (Blumberg and DeBach 1981) and up to 8 or more eggs of the parasitoid E. infelix oviposited in a single scale of P. pyriformis, became encapsulated (Blumberg and Goldenburg 1991). This difference in the numbers of encapsulated eggs could be attributed to differences in host scales, parasitoid species and host plants.
In our study, encapsulation was observed more frequently in ovipositing than preovipositing females. Similar results have been reported in other studies, where egg encapsulation of M. swirkii was higher by ovipositing than preovipositing female adults of P. pyrifomris (Blumberg and Swirski 1984) whereas encapsulation of Encyrtus lecanorium eggs by Coccus hesperidum was significantly lower in young female scales than in mature scales (18.4 vs. 83.5%) (Blumberg and Goldenburg 1991).
The higher encapsulating rate by ovipositing females could be attributed to the positive correlation between the encapsulation rate and the age of the host scale (Blumberg 1982; Blumberg and DeBach 1981) as well as to the fact that the ovipositing females could have encapsulated the eggs of the parasitoid in the nature during their preoviposition period and they continued their development until oviposition (Blumberg and Swirski 1984). The increase of encapsulation rate recorded between July–September 2004 and June–August 2005 (Fig. 2d) could explain the reduction of parasitism rate of the scale during this period (Fig. 2c). The fact that the encapsulation rate was higher during summer is in agreement with the results of other studies where encapsulation was more frequent in summer than in other seasons (Blumberg et al. 1993).
The presence of predated individuals of P. pyriformis is attributed to the action of Chilocorus bipustulatus which proved to be the sole predatory insect observed during the present study. However, these data refer only to partially destroyed scales and do not include totally consumed individuals such as crawlers that (obviously) cannot be estimated. The presence of C. bipustulatus has also been noted on infested by P. pyriformis avocado trees in Israel (Hadar 1993; Swirski et al. 1995). Other coccinellids such as Azya obrigera obrigera Mulsant (Trjapitzin and Triapitsyn 2003), Hyperaspis sp., Chilocorus angolensis Crotch (Robertson et al. 1986), Oenopia conglobata (L.), Scymnus spp. (Coleoptera: Coccinellidae), as well as the common green lacewing Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) (Hadar 1993; Swirski et al. 1995, 1997) have also been reported to feed upon P. pyriformis.
The data of the present study give some information concerning the phenology and ecology of P. pyriformis, which is a new pest in Greece. Although the scale is found at present only on laurel shrubs, it could be considered as a potential serious pest, as it is referred as important pest of fruit trees and ornamental plants in many parts of the world (Ben-Dov 1993). The data of phenology show that the scale is active throughout the year completing several overlapping generations. The predator C. bipustulatus could not result in any significant reduction of the pest. In addition, the action of the parasitoid M. helvolus although higher than that of the predator, could not be able to control the pest, which was found in both years at similar infestation levels. The study on encapsulation is giving important information on the ecology of this new pest, as it is known that the high rates of encapsulation of Metaphycus spp eggs by P. pyriformis during the summer may interfere with the efficient biological control of the pest (Blumberg et al. 1993).
References
Ben-Dov Y (1993) A systematic catalogue of the soft scale insects of the world (Homoptera: Coccoidea: Coccidae) with data on geographical distribution, host plants, biology and economic importance. Flora and Fauna Handbook, No. 9. Sandhill Crane Press, Gainesville
Ben-Dov Y, Stathas GJ, Malliarou JS (2003) The pyriform scale, Protopulvinaria pyriformis (Cockerell) (Hemiptera: Coccidae) in Greece. Agric Res 26:89–91
Blumberg D (1982) Further studies on the encapsulation of Metaphycus swirskii by soft scales. Entomol Exp Appl 31:245–248
Blumberg D (1991) Seasonal variation in the encapsulation of eggs of the encyrtid parasitoid Metaphycus stanleyi by the pyriform scale, Protopulvinaria pyriformis. Entomol Exp Appl 58:231–237
Blumberg D, DeBach P (1981) Effects of temperature and host age upon the encapsulation of Metaphycus staneyi and Metaphycus helvolus eggs by brown soft scale coccus hesperidum. J Invertebr Pathol 37:73–79
Blumberg D, Swirski E (1984) Response of three soft scales (Homoptera: Coccidae) to parasitization by Metaphycus swirskii. Phytoparasitica 12:29–35
Blumberg D, Blumberg O (1991) The pyriform scale, Protopulvinaria pyriformis, and its common parasitoid, Metaphycus stanleyi, on avocado and Hedera helix. Alon Hanotea 45:265–269
Blumberg D, Goldenburg S (1991) Encapsulation of eggs of two species of Encyrtus (Hymenoptera: Encyrtidae) by soft scales (Homoptera: Coccidae) in six parasitoid-host interactions. Israel J Ent 25–26:57–65
Blumberg D, Wysoki M, Hadar D (1993) Further studies of the encapsulation of eggs of Metaphycus spp. (Hym.: Encyrtidae) by the pyriform scale, Protopulvinaria pyriformis (Hom.: Coccidae). Entomophaga 38:7–13
Canard M (1996) The pyriform scale Protopulvinaria pyriformis Cockerell, 1894, a cottony scale new for the French fauna (Homoptera, Coccidae). B Soc Entomol Fr 101:131–134
Carvalho JP, Aguiar AMF (1997) Citrus pests in the Island of Madeira. Pragas dos citrinos na Ilha da Madeira Madeira: Secretaria Regional de Agricultura, Florestas e Pescs
Diaz WB, Fabián FV, Zamora JJG (2005) Insectos Plagas del Plato en la Costa Central. http://www.minag.gob.pe/dgpa1/ARCHIVOS/palta_doc0002.pdf, Accessed 7 January 2007
Gill RJ (1988) The scale insects of California—Part 1: The Soft Scales (Homoptera: Coccoidea: Coccidae). California Department of Food and Agriculture, California
Guerierri E, Noyes JS (2000) Revision of European species of genus Metaphycus Mercet (Hymenoptera: Chalcidoidea: Encyrtidae), parasitoids of scale insects (Homoptera: Coccoidea). Syst Entomol 25:147–222
Hadar D (1993) Population dynamics of the pyriform scale, Protopulvinaria pyriformis Cockerell, and its natural enemies in avocado groves. Ph.D. Thesis, The Hebrew University of Jerusalem, Israel (Hebrew, with English summary)
Kapatos ET, Stratopoulou ET, Sahinoglou A (1977) Spatial pattern of Saissetia oleae (Homoptera: Coccidae) in Greece. Environ Entomol 26:1202–1207
Pellizzari G (2003) Hemiptera Coccoidea new or little known for the Italian fauna. Boll Zool Agric Bachic 35:99–106
Robertson CM, Villers EA, De-Villiers EA (1986) Parasites of avocado pest bite the dust. Citrus Subtrop Fruit J 632:637
Salt G (1963) The defence reactions of insects to metazoan parasites. Parasitology 53:527–642
Spina ML (2001) Pyriform cochineal (Protopulvinaria pyriformis Cockerell) infestations in avocado plantules grown from seeds. Inf Fitopatol 51:64–66
Swirski E, Wysoki M, Izhar Y (1995) Avocado pests in Israel. Proceedings of The World Avocado Congress III, Israel, pp 419–428
Swirski E, Ben-Dov Y, Wysoki M (1997) Guava. In: Ben-Dov Y, Hodgson CJ (eds) Soft scale insects—their biology, natural enemies and control, world crop pests, vol 7B. Elsevier, Amsterdam, pp 255–263
Toit WJ, Schutte MS, Steyne WP (1991) Parasitoids of the heart-shaped scale, Protopulvinaria pyriformis (Cockerell) (Hemiptera: Coccidae) on avocados in South Africa. Yearb South Afr Avocado Grow Assoc 14:74–77
Trjapitzin VA, Triapitsyn SV (2003) A new species of Homalotylus (Hymenoptera: Encyrtidae) from Mexico, parasitoid of Azya orbigera orbigera (Coleoptera: Coccinellidae). Entomol News 114:192–196
Viggiani G, Mazzone P (1977) Preliminary notes on the introduction into Italy of Metaphycus near stanleyi Comp. and Diversinervus elegans Silv. (Hym. Encyrtidae), parasites of Saissetia oleae (Oliv.). Boll Lab Entomol Agr Filippo-Silvestri 34:217–227
Wysoki M (1985) Search for the pyriform scale, Protopulvinaria pyriformis (Cockerell) (Homoptera: Coccidae) and its natural enemies in South Africa. Alon Hanotea 39:785–789
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Haye.
Rights and permissions
About this article
Cite this article
Stathas, G.J., Eliopoulos, P.A., Japoshvili, G. et al. Phenological and ecological aspects of Protopulvinaria pyriformis (Cockerell) (Hemiptera: Coccidae) in Greece. J Pest Sci 82, 33–39 (2009). https://doi.org/10.1007/s10340-008-0216-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-008-0216-y