Abstract
The transgenic rice lines Kemingdao 1 (KMD1) and Kemingdao 2 (KMD2) contain a synthetic cry1Ab gene derived from Bacillus thuringiensis Berliner and are highly resistant to rice stem borers and foliage-feeding lepidopterans. Propylea japonica (Coleoptera: Coccinellidae) is an important predator of rice insect pests; it also uses rice pollen as a food source under natural conditions. In the present study, the effects of KMD1 and KMD2 pollen expressing Cry1Ab protein on the fitness of P. japonica were assessed in the laboratory. P. japonica larvae and adults were provided with the following four diets: KMD1 pollen with the aphid Myzus persicae, KMD2 pollen with M. persicae, nontransgenic Xiushui 11 (parent variety of KMD1 and KMD2) pollen with M. persicae, and M. persicae only (KMD1–pollen, KMD2–pollen, XS11–pollen, and aphid treatments, respectively). The results showed that the longevity of female adults in the KMD1–pollen treatment was significantly lower than that in the XS11–pollen treatment, but was not significantly different from that in the KMD2–pollen and aphid treatments. Newly emerged males in the KMD2–pollen treatment were evidently less vital than those in the XS11–pollen treatment, but not significantly different from those in the KMD1–pollen and aphid treatments. The development, survival and reproduction indices for the three pollen treatments did not differ significantly from one another. In short, Bt toxin expressed in Bt rice pollen had no evident negative impacts on P. japonica fitness when the pollen was used as a food by this beetle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The striped stem borer (SSB), Chilo suppressalis (Walker) (Lepidoptera: Pyralidae), and the yellow stem borer (YSB), Scirpophaga incertulas (Walker), are two of the most serious pests of rice in temperate and subtropical Asia (Dale 1994). Over the past decade, several synthetic genes including cry1Ab, cry1Ac and cry1B derived from Bacillus thuringiensis Berliner (Bt) have been transferred into indica or japonica rice to confer resistance to SSB and YSB (Fujimoto et al. 1993; Wünn et al. 1996; Ghareyazie et al. 1997; Nayak et al. 1997; Wu et al. 1997; Cheng et al. 1998; Datta et al. 1998; Alam et al. 1999; Breitler et al. 2000; Khanna and Raina 2002). In China, the obtained transgenic Bt rice lines have been proven to be highly resistant to rice stem borers (including SSB and YSB) and foliage-feeding lepidopterans under field conditions (Shu et al. 2000; Ye et al. 2001).
Adverse effects of Cry1Ab-expressing Bt plants on arthropod predators may arise as a result of several factors, including high concentration of Bt protein in pollen (a food source for predators), modifications of the introduced cry1Ab gene, and expression of Bt protein throughout the plant (Jepson et al. 1994; Pilcher et al. 1997). Thus, potential risk exists for Bt plants to adversely affect the fitness of predators. In the case of Bt rice, Bernal et al. (2002) reported that both the mirid predator Cyrtorhinus lividipennis Reuter and its prey, the brown planthopper Nilaparvata lugens Stål, could be exposed to Bt toxins from Bt rice, but this exposure might not affect the fitness of C. lividipennis. Laboratory observation has indicated that Bt rice has no marked effect on the predation capacity of the wolf spider Pirata subpiraticus (Liu et al. 2003a). In rice fields, Bt rice also has no significant effect on the population dynamics of dominant spider species, or the community of arthropod predators (Liu et al. 2002, 2003b). According to Schoenly et al. (2003), however, planting Bt rice appeared to alter the species richness of predators.
Predaceous coccinellids play important roles in natural control of pest insects (Obrycki and Kring 1998). Previous studies demonstrated that Bt crop pollen expressing Bt proteins, such as Cry1Ab, Cry3A and Cry3Bb1, poses little risk to this group of predators (Pilcher et al. 1997; Riddick and Barbosa 1998; Duan et al. 2002). However, to clarify conclusively the impact of Bt plant pollen on predaceous coccinellids, more extensive experiments are needed (Schoenly et al. 2003).
Propylea japonica (Thunberg) (Coleoptera: Coccinellidae) is a very common and abundant predator in many crop systems throughout China including rice (Yang 1983; Ma and Liu 1985; Zou et al. 1986; Zhou and Xiang 1987; Cheng 1996). Both larval and adult P. japonica are predaceous, feeding predominantly on aphids (Song et al. 1988). When insect prey is scarce, they also use plant pollen as a supplemental food source (Li et al. 1992). In rice fields, P. japonica preys on the aphid Macrosiphum avenae (F.), young larvae of the rice leaffolder Cnaphalocrocis medinalis Guenée, young nymphs of N. lugens, and others (Song et al. 1988); it can also feed on rice pollen during both larval and adult stages (personal observation). In this study, development, survival, and reproduction of P. japonica feeding on Bt rice pollen were observed. The objectives were to determine effects of Bt rice pollen on the fitness of this beetle, and subsequently to broaden our knowledge about the ecological risk of transgenic Bt plants pending commercial release.
Materials and methods
Propylea japonica
Propylea japonica adults were collected from rice plants during the flowering stage on the experimental farm of Zhejiang University during August 2003. Pairs were placed in glass tubes (1.4 cm diameter, 10 cm long) containing the green peach aphid, Myzus persicae (Sulzer), and maintained in a climatically controlled chamber with photoperiod 16:8 h (L:D), 28±1°C, and 60–80% RH. M. persicae was supplied daily to the tubes. As egg deposition occurred, adults were transferred to other clear glass tubes, and the tubes bearing eggs were maintained until egg hatch. The newly hatched larvae were subjected to experiments. A water-saturated cotton ball was placed in each glass tube to maintain moisture. The M. persicae had been raised on Brassica oleracea var. capitata L. in the climatically controlled chamber.
Rice pollen
Pollen was obtained from field-grown plants of the Bt rice lines Kemingdao 1 (KMD1) and Kemingdao 2 (KMD2), and from plants of the nontransgenic line, japonica rice cv. Xiushui 11 (XS11, parent variety of KMD1 and KMD2). Both KMD1 and KMD2 were homozygous containing a synthetic cry1Ab gene derived from B. thuringiensis. They were transformed by Agrobacterium infection (Cheng et al. 1998; Shu et al. 1998). Expression of the cry1Ab gene is driven by maize ubiquitin promoter (Cheng et al. 1998).
One day before anthesis, inflorescences were cut from plants. In the laboratory, anthers enclosed within glume were collected, lyophilized, ground, and sifted through a 1-mm sieve. The obtained anther powder (containing pollens) was placed in sealed plastic vials and kept at −20°C until use. The concentration of Cry1Ab toxin in anther powder was determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Envirologix).
Effects of Bt rice pollen on preimaginal fitness of P. japonica
Propylea japonica could not complete larval development solely on rice pollen (personal observation). Thus M. persicae, an optimal food source of P. japonica (Guo and Wan 2001), was supplemented in the three pollen treatments. The experimental treatments were KMD1 pollen with aphid, KMD2 pollen with aphid, and XS11 pollen with aphid (described hereafter as KMD1–pollen, KMD2–pollen, and XS11–pollen treatments, respectively). A control diet of only M. persicae (described hereafter as aphid treatment) was used to verify that experimental conditions used in this study were suitable for P. japonica. A total of 200 newly hatched P. japonica larvae from 20 female sources were used, with 50 larvae being subjected to each treatment. In addition, to determine average percentage of larvae that pupated and percentage of pupae that eclosed, the larvae in each treatment were divided into three subgroups.
Larvae were reared individually in glass tubes (1.4 cm diameter, 10 cm long). In the aphid treatment, 10, 20, 40 and 60 M. persicae adults per day were provided to each larva during the first, second, third and fourth instars, respectively. In the pollen treatments, each larva was given 20 anthers together with 5, 10, 20 and 30 M. persicae adults per day during the first, second, third and fourth instars, respectively. To increase the probability that P. japonica would feed on pollen, aphids were not supplied during the first 12 h of each instar.
The experiment was conducted in the climatically controlled chamber described earlier. Water was provided to larvae by placing a water-saturated cotton ball in each tube. Anthers in tubes were refreshed twice daily. Larvae were checked every 3 h for ecdysis and mortality; development time and survival rate of each preimaginal stage were determined. Weight of pupae and adults was measured approximately 12 h after pupation and within 6 h after emergence, respectively.
Effects of Bt rice pollen on adult fitness of P. japonica
For each treatment, adults (only those obtained around emergence peak) were transferred to a Petri dish (10.0 cm diameter, 2.6 cm tall) after being weighed, and were given the same diet as during the fourth-instar stage. After 3 days, adults were paired and placed in glass tubes (1.4 cm diameter, 10 cm long) containing sufficient M. persicae but no rice pollens. Thereafter, oviposition and adult survival were monitored every 3 h. When egg clutches were observed, the P. japonica adults as well as the residual aphids were transferred to other clear glass tubes, and the number of egg clutches and number of eggs in each clutch were recorded. To determine hatch rates, 28–40 egg clutches in each treatment that were deposited around oviposition peak were gathered, divided randomly into three groups, and placed individually in glass tubes (1.4 cm diameter, 10 cm long) containing a wet cotton ball. The number of total and hatched eggs in each group of egg clutches was recorded. Adult longevity was also measured.
Flip time has been used to indicate adult fitness of coccinellids in earlier studies (Smith and Krischik 1999; Lundgren and Wiedenmann 2002). Such an indicator was also used in the present study. To obtain sufficient male adults for the test, another group of P. japonica was raised under each diet treatment described above. At 6 h after emergence, males were placed on their dorsa on a piece of filter paper (90 mm diameter), and the time until the beetle flipped over was measured with a stopwatch to the nearest 1 s. Timing was stopped at 60 s for beetles that did not flip. Flip time was divided into six grades, 1–5, 6–10, 11–15, 16–20, 21–25 and 26–60 s. Percentage of adults in each flip-time grade and average flip time of the adults flipping within 15 s (which accounted for 79.4–86.2% of the individuals observed) were determined.
Verification of pollen ingestion by P. japonica larvae and adults
For each pollen treatment, feces of P. japonica larvae during each instar stage and of adults within 3 days of emergence were treated with I2–KI solution and then observed under a binocular microscope. If there were stained pollens present, it would prove that the pollen used in our experiments had been ingested by the insect.
Data analysis
Development, survival and reproduction data were analyzed using analysis of variance (ANOVA) (SPSS 2002). The χ2 test was conducted to evaluate the relationship between adult flip time and diet treatment at the level P=0.05. Before being subjected to ANOVA, data in percentage were transformed using arcsine square root, and the flip-time data of adults flipping within 15 s was transformed using square root. Means were compared among treatments using the Tukey HSD test at the level P=0.05.
Results
Using ELISA, the concentration of B. thuringiensis protein (Cry1Ab) in the anthers (containing pollens) of transgenic rice lines KMD1 and KMD2 was determined as 13.1±2.6 and 31.4±4.3 μg g−1 lyophilized anther powder, respectively. In the pollen treatments, pollens were found in the feces of P. japonica at each feeding stage (i.e., during each larval instar and within 3 days after emergence), suggesting that pollen had been ingested by the insects.
ANOVA indicated that the type of diet (KMD1–pollen with aphid, KMD2–pollen with aphid, XS11–pollen with aphid, and aphids only) fed to P. japonica had significant effects on the development time of second, third, and fourth instar larval stages and on the entire larval stage, but not on the development time of first instar larvae, pre-pupae, or pupae, or on the percentage of larvae that pupated, percentage of pupae that eclosed, pupal or adult weight (Table 1). As compared with the aphid treatment, significantly prolonged development was observed during the second instar in the KMD1–pollen and KMD2–pollen treatments, during the third instar in the KMD1–pollen treatment, during the fourth instar in the KMD2–pollen and XS11–pollen treatments, and during the entire larval stage in each pollen treatment (P<0.05). The development time of the third and fourth instars in the KMD1–pollen treatment was significantly longer than that in the XS11–pollen treatment (P<0.05). However, when comparing the development time of the entire larval stage among the three pollen treatments, no significant difference was found (P>0.05) (Table 1).
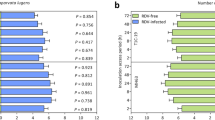
The type of diet had significant effects on female longevity, but not on preovipositional period, ovipositional period, number of eggs per egg clutch, fecundity, male longevity, or egg hatch rate (Table 2). Female longevity in the KMD1–pollen treatment was significantly shorter than that in the XS11–pollen treatment (P<0.05), but not than that in the aphid treatment (P>0.05). The χ2 test showed that flip time of young (6-h-old) male adults (Fig. 1a) was not significantly related to the type of diet that had been fed during the larval stage (df=15, χ2=20.60<χ2 0.05, 15). For adults flipping within 15 s, flip time of the individuals given KMD1–pollen or KMD2–pollen did not differ significantly from that of the individuals given XS11–pollen, or from that of the individuals given aphids only (P>0.05) (Fig. 1b).
Flip time of male P. japonica adults (6 h old, taking no food after emergence) that emerged from individuals fed different diets during the larval stage. K1P-A KMD1 pollen with the aphid M. persicae, K2P-A KMD2 pollen with aphid, XS-A XS11 with aphid, SA aphids only. a Percentage of adults in different flip-time grades. b Flip time (mean±SE) of adults flipping within 15 s. Bars with the same letter are not significantly different from each other, Tukey HSD test, P>0.05. Values in parentheses indicate the number of adults observed
Discussion
The Cry1Ab protein expressed in Bt rice is lepidopteran-specific with high activity against C. suppressalis, S. incertulas and many other lepidopteran pests (Shu et al. 2000; Wu et al. 2001; Ye et al. 2001). Although this would suggest that Bt rice would not have any negative effect on predators, suitability of rice pollen as a food source for predators may have been altered due to the introduction of cry1Ab gene. Such a potential effect requires proper assessment of the ecological risks posed by Bt rice, by carrying out a combination of laboratory tests, field experiments and longer-term monitoring, as suggested by Jepson et al. (1994). As a case study, our observations indicated that Bt rice pollen has no adverse effect on P. japonica when used as a supplemental food. This result agrees with the studies of Pilcher et al. (1997) and Sims (1995), who found that feeding Bt corn pollen that expresses Cry1Ab or feeding a diet of Cry1Ac protein (also lepidopteran-specific) at 20 μg/ml (>100 times the amount of Cry1Ac protein present in pollen and nectar of transgenic cotton) posed little risk to the coccinellids Coleomegilla maculata lengi Timberlake, and Hippodamia convergens Guerin.
The aphid M. persicae is a suitable prey for P. japonica (Wei and Ran 1983), but the amount of this aphid consumed by P. japonica during larval and adult stages is not clear. According to Song et al. (1988), when provided with suitable aphid prey, such as Aphis citricidus (Kirkaldy), A. gossypii Glover, Macrosiphum avenae (Fabricius), and A. glycines Matsumura, a P. japonica larva can consume on average 10, 20, 36 and 47 aphids per day during first, second, third and fourth instar, respectively. In the present study, nearly all of the aphids provided were consumed in the aphids-only treatment, when as many as 10, 20, 40 and 60 aphids per day were provided during the four respective P. japonica instars (personal observation). Overall, such an aphid-consumption capacity is in accordance with that reported by Song et al. (1988). Based on this, we speculated that in the aphid+pollen treatments, during which only 5, 10, 20 and 30 aphids per day were provided during the four respective P. japonica instars, pollen had been used as food source for normal development. In addition, the presence of pollen granules in larval feces verifies the ingestion of pollen. Methodology should be developed to quantify rice pollen consumed by P. japonica, although such a trial appears to be difficult.
When provided with A. gossypii, M. avenae or A. glycines, a P. japonica adult can predate over 80 aphids during each of the 3 days after emergence; when receiving no food after emergence, adults die within 2 days (Lu et al. 1983; Yang 1983). In the present study, to determine the effect of pollen on P. japonica adult fitness, we provided only 30 aphids (together with pollen) per day to P. japonica adults during the 3 days after emergence. This suggests that the amount of Bt pollen ingested by young P. japonica adults, which was not measured, was probably great, as normal reproductive development occurred. The occurrence of normal reproductive development also supported our belief that the consumption of transgenic KMD1 and KMD2 rice pollen by larval or adult P. japonica has little adverse impact on fitness.
The lengthened development time of P. japonica larvae in pollen treatments compared to the aphids-only treatment could have been the result of insufficient nutrition. M. persicae has been reported as an optimal diet of P. japonica (Guo and Wan 2001), but apparently both Bt and non-Bt rice pollen are less suitable than M. persicae as a diet of P. japonica larvae. On the other hand, the Bt rice (KMD1 or KMD2) pollen, although posing no evident adverse effects on P. japonica fitness overall, appeared to be less suitable than the non-Bt rice pollen, as indicated by the increased time for young male adults to flip and the decreased female longevity in the KMD2–pollen and KMD1–pollen treatments, respectively.
Other studies have shown that lepidopteran-specific Bt crops could have significant effects on P. japonica through other ecological pathways than pollen. For example, larvae of the cotton bollworm, Helicoverpa armigera (Hübner) feeding on leaves of Cry1Ab-expressing Bt cotton had a significantly lower body weight than those feeding on leaves of normal cotton, and thus were predated in reater numbers by P. japonica (the study did not examine whether the mean mass of prey consumed differed significantly between the two treatments) (Cui and Xia 1999). Potential effects of Bt rice on P. japonica via trophic interactions other than consumption of pollen are to be evaluated in the future.
References
Alam MF, Datta K, Abrigo E, Oliva N, Tu J, Virmani SS, Datta SK (1999) Transgenic insect-resistant maintainer line (IR68899B) for improvement of hybrid rice. Plant Cell Rep 18:572–575
Bernal CC, Aguda RM, Cohen MB (2002) Effect of rice lines transformed with Bacillus thuringiensis toxin genes on the brown planthopper and its predator Cyrtorhinus lividipennis. Entomol Exp Appl 102:21–28
Breitler JC, Marfa V, Royer M, Meynard D, Vassal JM, Vercambre B, Frutos R, Messeguer J, Gabarra R, Guiderdoni E (2000) Expression of a Bacillus thuringiensis cry1B synthetic gene protects Mediterranean rice against the striped stem borer. Plant Cell Rep 19:1195–1202
Cheng JA (1996) Rice insect pests. China Agricultural Press, Beijing
Cheng XY, Sardana R, Kaplan H, Altosaar I (1998) Agrobacterium-transformed rice plants expressing synthetic cry1A(b) and cry1A(c) genes are highly toxic to striped stem borer and yellow stem borer. Proc Natl Acad Sci USA 95:2767–2772
Cui JJ, Xia JY (1999) Effect of transgenic Bt cotton on the population dynamics of natural enemies. Acta Gossypii Sinica 11:84–91
Dale D (1994) Insect pests of the rice plant—their biology and ecology: stem borers. In: Heinrichs EA (ed) Biology and management of rice insects. Wily Eastern, New Delhi, pp 388–408
Datta K, Vasquez A, Tu J, Torrizo L, Alam MF, Oliva N, Abrigo E, Khush GS, Datta SK (1998) Constitutive and tissue-specific differential expression of the cry1A(b) gene in transgenic rice plants conferring resistance to rice insect pest. Theor Appl Genet 97:20–30
Duan JJ, Head G, McKee MJ, Nickson TE, Martin JW, Sayegh FS (2002) Evaluation of dietary effects of transgenic corn pollen expressing Cry3Bb1 protein on a non-target ladybird beetle, Coleomegilla maculata. Entomol Exp Appl 104:271–280
Fujimoto H, Itoh K, Yamamoto M, Kyozuka J, Shimamoto K (1993) Insect resistant rice generated by introduction of a modified delta-endotoxin gene of Bacillus thuringiensis. Biotechnology 11:1151–1155
Ghareyazie B, Alinia F, Menguito CA, Rubia LG, Palma JM de, Liwanag EA, Cohen MB, Khush GS, Bennett J (1997) Enhanced resistance to two stem borers in an aromatic rice containing a synthetic cryIA(b) gene. Mol Breeding 3:401–414
Guo JY, Wan FH (2001) Effect of three diets on development and fecundity of the ladybeetles Harmonia axyridis and Propylaea japonica. Chin J Biol Control 17:116–120
Jepson PC, Croft BA, Pratt GE (1994) Test systems to determine the ecological risks posed by toxin release from Bacillus thuringiensis genes in crop plants. Mol Ecol 3:81–89
Khanna HK, Raina SK (2002) Elite indica transgenic rice plants expressing modified Cry1Ac endotoxin of Bacillus thuringiensis show enhanced resistance to yellow stem borer (Scirpophaga incertulas). Transgenic Res 11:411–423
Li KS, Chen XD, Wang HZ (1992) New discovery of feeding habitats of some ladybirds. Shanxi Forest Sci Techn 2:84–86
Liu ZC, Ye GY, Hu C, Datta SK (2002) Effects of Bt transgenic rice on population dynamics of main non-target insect pests and dominant spider species in rice paddies. Acta Phytophylacica Sinica 29:138–144
Liu ZC, Ye GY, Fu Q, Zhang ZT, Hu C (2003a) Indirect impact assessment of transgenic rice with cry1Ab gene on predations by the wolf spider, Pirata subpiraticus. Chin J Rice Sci 17:175–178
Liu ZC, Ye GY, Hu C, Datta SK (2003b) Impact of transgenic indica rice with a fused gene of cry1Ab/cry1Ac on the rice paddy arthropod community. Acta Entomol Sin 46:454–465
Lu YX, Zhang WJ, Pei Q, Liao YZ (1983) On the biology and rearing of Propylaea japonica (Thunb.). Nat Enemies Insects 5(1):33–38
Lundgren JG, Wiedenmann RN (2002) Coleopteran-specific Cry3Bb toxin from transgenic corn pollen does not affect the fitness of a nontarget species, Coleomegilla maculata DeGeer (Coleoptera: Coccinellidae). Env Entomol 31:1213–1218
Ma XY, Liu Z (1985) Propylaea japonica (Thumberg) and its seasonal fluctuation in cotton fields. Plant Protect 11(2):12–13
Nayak P, Basu D, Das S, Basu A, Ghosh D, Ramakrishnan NA, Ghosh M, Sen SK (1997) Transgenic elite indica rice plants expressing CryIAc delta-endotoxin of Bacillus thuringiensis are resistant against yellow stem borer (Scirpophaga incertulas). Proc Natl Acad Sci USA 94:2111–2116
Obrycki JJ, Kring TJ (1998) Predaceous Coccinellidae in biological control. Annu Rev Entomol 43:295–321
Pilcher CD, Obrycki JJ, Rice ME, Lewis LC (1997) Preimaginal development, survival, and field abundance of insect predators on transgenic Bacillus thuringiensis corn. Env Entomol 26:446–454
Riddick EW, Barbosa P (1998) Impact of Cry3A-intoxicated Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) and pollen on consumption, development, and fecundity of Coleomegilla maculata (Coleoptera: Coccinellidae). Ann Entomol Soc Am 91:303–307
Schoenly KG, Cohen MB, Barrion AT, Zhang WJ, Gaolach B, Viajante VD (2003) Effects of Bacillus thuringiensis on non-target herbivore and natural enemy assemblages in tropical irrigated rice. Env Biosafety Res 2:181–206
Shu QY, Ye GY, Cui HR, Xiang YB (1998) Development of transgenic Bacillus thuringiensis rice resistant to rice stem borers and leaf folders. J Zhejiang Agric Univ 24:579–580
Shu QY, Ye QY, Cui HR, Cheng XY, Xiang YB, Wu DX, Gao MW, Xia YW, Hu C, Sardana R, Altosaar I (2000) Transgenic rice plants with a synthetic cry1Ab gene from Bacillus thuringiensis were highly resistant to eight lepidopteran rice pest species. Mol Breeding 6:433–439
Sims SR (1995) Bacillus thuringiensis var. kurstaki (CryIA (C)) protein expressed in transgenic cotton: effects on beneficial and other non-target insects. SW Entomol 20:493–500
Smith SF, Krischik VA (1999) Effects of systemic imidacloprid on Coleomegilla maculata (Coleoptera: Coccinellidae). Env Entomol 28:1189–1195
Song HY, Wu LY, Chen GF, Wang ZC, Song QM (1988) Biological characters of lady-beetle, Propylaea japonica (Thunberg). Nat Enemies Insects 10(1):22–33
SPSS (2002) SPSS 11.5 for Windows. SPSS Inc.
Wei JH, Ran RB (1983) A study on Propylaea japonica (Thunb.) Nat Enemies Insects 5(2):89–93
Wu C, Fan Y, Zhang C, Oliva N, Datta SK (1997) Transgenic fertile japonica rice plants expressing a modified cryIA(b) gene resistant to yellow stem borer. Plant Cell Rep 17:129–132
Wu G, Cui HR, Shu QY, Ye GY, Xie XB, Xia YW, Gao MW, Altosaar I (2001) Expression patterns of cry1Ab gene in progenies of “Kemingdao” and the resistance to striped stem borer. Sci Agric Sin 34:496–501
Wünn JA, Kloti A, Burkhardt PK, Biswas GCG, Launis K, Iglesias VA, Potrykus I (1996) Transgenic indica rice breeding line IR58 expressing a synthetic cryIA(b) gene from Bacillus thuringiensis provides effective insect pest control. Biotechnology 14:171–176
Yang JH (1983) Preliminary observations on the habits of Propylea japonica. Insect Knowl 20:215–217
Ye GY, Shu QY, Yao HW, Cui HR, Cheng XY, Hu C, Xia YW, Gao MW, Altosaar I (2001) Field evaluation of resistance of transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner to two stem borers. J Econ Entomol 94:271–276
Zhou KJ, Xiang JB (1987) Observations on the efficacy of spiders and ladybirds against aphids in the seedling stage of cotton in the cotton fields. Nat Enemies Insects 9(1):17–20
Zou, YD, Wang HF, Tao QZ, Liu DK, Yu W, Holling CS (1986) Studies on predation of Propylaea japonica (Thung) larvae on Aphis gossypii Glover. Insect Knowl 23:219–222
Acknowledgements
We thank Prof. Qingyao Shu (Institute of Nuclear Agricultural Sciences, Zhejiang University) for his donation of rice seeds, and Huimei Shen (College of Plant Protection, Nanjing Agricultural University), Xianjun Gu and Leshun Xia (Department of Plant Protection, Zhejiang University) for their great assistance in data collection and careful attention to details in the laboratory. This manuscript is based on work supported by the National Science Foundation of China under Agreement No. 30070500 awarded to J.A. Cheng.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, Y.Y., Jiang, M.X. & Cheng, J.A. Effects of transgenic cry1Ab rice pollen on fitness of Propylea japonica (Thunberg). J Pest Sci 78, 123–128 (2005). https://doi.org/10.1007/s10340-004-0078-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-004-0078-x