Abstract
The level of short-chain fatty acids (SCFAs) is related to health benefits. In this study, a static headspace (HS) gas chromatography method was developed and validated for analyzing SCFAs in fecal samples. SCFAs were injected onto a column through an HS injector through an HS procedure and salting-out technology. The limits of detection and quantification (LOQ) of this method are 0.02–0.08 µg L− 1 (0.4–1.6 µg g− 1 sample) and 0.005–0.020 µg L− 1 (0.1–0.4 µg g− 1 sample), respectively. The calibration curves show good linearity over the range (LOQ–200 µg L− 1 or LOQ–4000 µg g− 1 sample) of these compounds, and the correlation coefficient is > 0.99. The recovery of the method is between 80 and 105%, and matrix effects were not observed. The proposed method was successfully applied to analyze SCFAs in fecal samples from rats.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Short-chain fatty acids (SCFAs), also known as volatile fatty acids, are composed of one to six carbon atoms in organic fatty acid [1]. The bacterial fermentation of undigested carbohydrates and dietary fiber in colon results in the production of SCFAs, primarily acetic, propionic, butyric, and valeric acid in animals and humans [2]. SCFAs differ in terms of type, quantity, and role in the intestinal tract because of the influence of factors, such as fermentation substrates and bacterial species [3]. Approximately 95% of the produced SCFAs are rapidly absorbed by colonocytes, and residues are excreted in feces [4,5,6]. SCFAs participate in the metabolism performed by different organs in the human body and exhibit specific properties. Acetic acid is absorbed by bacteria to provide the main source of energy for the host [5], yielding 10% of the total daily energy for the human body [6]. Propionic acid, which is absorbed in the blood and catabolized in the liver, participates in pyruvate inverse transformation of glucose while possibly inhibiting fat synthesis [6]. Butyric acid is used mainly by epithelial cells as their main energy source [4]. In the last few decades, these compounds have gained increasing interest due to their key role in prevention and treatment of metabolic syndrome, bowel disorders, and certain types of cancer [7]. Developing rapid, selective, and inexpensive analytical methods for SCFA identification and quantification in complex biological matrices, such as feces, is important due to the biological relevance and potential health effects of SCFAs [8].
Many different instrumental techniques have been used to determine SCFAs in biological matrices; these techniques include gas chromatography (GC) [9], gas chromatography–mass spectrometer (GC–MS) [10], liquid chromatograph (LC) [11], capillary electrophoresis (CE) [12], ion-exchange high-performance liquid chromatography (IC) [13] and nuclear magnetic resonance (NMR) [14] Pretreatment prior to direct determination of biological samples leads to high accuracy and sensitivity of the analysis. The distinct sensitivity is probably due to the different sample preparation techniques and analytical conditions. LC, CE, and IC involve rapid sample pretreatment but exhibit poor sensitivity, resolution, and recovery [11,12,13]. Directly injecting the supernatant extracted from aqueous fecal suspensions into GC is harmful for GC instruments. In GC, different sample preparation techniques have been proposed, such as extraction with organic solvents, distillation, ultra filtration, and solid-phase microextraction (SPME) [8,9,10]. These technologies have resulted in a good cleanup effect and high response rates, but they are relatively time consuming and expensive, reduce recovery, and affect the accuracy and repeatability of the method. Moreover, organic solvents used in pretreatments are hazardous to the health of staff performing the experiment and the environment. Directly injecting the supernatant extracted from aqueous fecal suspensions into GC is harmful for GC instruments. Headspace (HS) sampling method has been applied to analyze volatile compounds in tissues and yields inspiring results [14]. This method has been increasingly applied because of easy preparation of samples under water [15, 16]. Different salting-out technologies are also used to improve the volatile efficiency of analytes by increasing the concentration of non-polar analytes in HS during HS sampling [8, 17]. The addition of salting-out agent increases the ionic strength of the solution and decreases the solubility of SFCAs. The application of bivalent salts will further decrease SFCA solubility because they increase the ionic strength more than monovalent ions do [8].
Therefore, a HS sampling method involving minor sample pretreatment and major sensitivity would be desirable for analyzing SCFAs in biological samples. This work aims to develop an HS-GC method for rapid identification and quantification of SCFAs in rat feces. Results will provide a reference for SCFA analysis in other biological samples.
Experimental
Chemicals and Reagents
Acetic acid (C2), propionic acid (C3), isobutyric acid (iC4), butyric acid (C4), isovaleric acid (iC5), valeric acid (C5), and 4-methyl valeric acid (internal standard, IS) were purchased from Shanghai Aladdin Bio-Chem Technology Co., LTD (Shanghai, China). NaH2PO4, (NH4)2SO4, and H3PO4 were purchased from Tianjin chemical reagent factory (Tianjin, China). All HPLC-grade water was obtained from a Millipore Milli-Q ultrapure water system (Millipore, USA). All solvents used in gas chromatography were of chromatographic reagent grade and other chemicals were of analytical regent grade.
Fecal Samples
Rat fecal samples were randomly collected and used to develop and validate the proposed method. Ten ileocecal samples and 17 fecal samples were obtained from male rats (weighing 160–200 g) and analyzed as actual samples to test the application of the method. The fecal samples were treated immediately and stored at − 20 °C after collection.
Sample Preparation Procedure
Each sample (about 100 mg) was weighted in a 20 mL screw cap vial (Agilent Technologies, USA), to which 5 mL of salt solution, which contains 882 g L− 1 of (NH4)2SO4, and 238 g L− 1 of NaH2PO4 [18], and 4-methyl valeric acid were added as IS at a final concentration of 200 µg L− 1. The solution was then adjusted to a pH of 2.5 by H3PO4. The prepared solutions were stored in − 20 °C until analysis. The solutions were placed in an HS injector for analysis in three independent replicates per sample.
HS Protocol
Samples were placed in the HS injector with the following conditions: 14 psi vial pressure, sample shaker set to high speed, two extractions per vial, 50 °C oven temperature, 30 min vial equilibration time, 0.1 min vial pressurization time, 0.15 min loop fill time, 0.05 min loop equilibration time, 1 min sample injection time, 75 °C loop temperature, 85 °C transfer line temperature, and 15 min GC cycle time.
GC Analysis
GC analysis was determined by Agilent 7890A gas chromatograph equipped with a G1888 HS injector and a flame ionization detector (FID) (Agilent Technologies, USA). The capillary chromatographic column used was an HP-innowax capillary column with polyethylene glycol as stationary phase (30 m × 0.32 mm i.d. × 0.50 µm film thickness; Agilent Technologies, USA). Carrier gas was nitrogen at constant pressure (50 °C, 33 cm s− 1). The temperature of the detector was 250 °C. The injection was performed in splitless mode, and the injection port temperature was set to 200 °C. Oven temperature was initially at 150 °C, raised to 190 °C at 5 °C min− 1, and finally to 210 °C at 20 °C min− 1 and held for 1 min. The total analysis time was 10 min. Data acquisition and operation processing were conducted using ChemStation software (version B.0403, Agilent Technologies). SCFA identification was based on the retention time of standard compounds.
Linearity and Sensitivity
The standard solutions of SCFAs were prepared at gradient concentrations of 1, 5, 10, 20, 50, and 200 µg L− 1 according to sample preparation, to which IS was added at a final concentration of 200 µg L− 1 and then adjusted to pH 2.5 using H3PO4. The calibration graphs were built through the internal standard curve method. The variability in pretreatment procedure and instrument response was modified by calibrating the peak area with that of IS.
The standard solution was gradually diluted and injected into the GC. The limit of detection (LOD) and limit of quantification (LOQ) of each individual SCFA were obtained when the signal-to-noise ratio (S/N) is about 3 and 10, respectively.
Recovery and Precision
Recovery was obtained by adding standards into the real samples because finding fecal samples completely free of SCFAs was impossible. The same fecal sample was divided into four parts, one of which was treated according to sample preparation. The others were dissolved using 10, 50, and 200 µg L− 1of standard solutions instead of salt solution, as described in sample preparation procedure. Three replicates were performed. The recovery of each analyte during sample preparation was calculated using the following formula. In the equation, R recovery, %; A1 area of SCFA in adding standard into fecal sample; A2 area of SCFA in fecal sample; A3 area of SCFA in standard solution.
Results and Discussion
Optimization of Sample Preparation
The efficiency of volatile compound extraction from solution was enhanced by adding a salting-out agent, which increases the ionic strength of the solution and decreases the solubility of these compounds in the solutions. The effect of various salting-out agents is different. Some researchers recommend (NH4)2SO4/NaH2PO4 (3.7/1, w/w) as salting-out agent for analyzing fecal samples, demonstrating better salting-out efficiency than others [8, 17]. Each sample was divided into two parts, comprising with and without added salt. The sample analysis involved comparison of the peak area of two samples. The addition of (NH4)2SO4/NaH2PO4 increased the SCFA concentration in HS in aqueous fecal suspensions to about 30% (Table 1). This result was consistent with that of Fiorini [8].
Decreasing the loss of SCFA due to their easy volatility must be considered. The real-time treatment procedure to decrease loss from volatilization was adopted in this paper. In previous research, samples were immediately stored in − 20 °C after collection and then treated before analysis [9]. SCFA loss due to volatilization will occur during treatment processing, including unfreezing and weighting. A sample (100 mg) of feces was weighed and treated according to sample preparation procedure, and the remainder was stored in a sealed centrifuge tube at − 20 °C. After 1 month, the remainder was taken out from − 20 °C and a 100 mg sample was obtained from it, which was treated. The two samples were analyzed through GC. The results indicated that real-time treatment was superior to post-treatment, as shown by an increasing in the relative area by 2.5–9.8% (Table 1). Li also obtained high recovery by adopting the method of real-time treatment to detect SCFA in plasma [19].
The six standard solutions at the same concentration (50 µg L− 1) were placed in the − 20 °C refrigerator, and a sample analysis was performed every other week to investigate sample stability. The results showed that six repeated injections of a mixture of standard solution at different time points within 5 weeks resulted in very low variability (Table 1). Thus, the SCFAs in saline solution were stable at − 20 °C, and the content changes was not significant (RSD < 5%) for long-term storage (at least 5 weeks).
Optimization of HS Protocol and GC Analysis
The oven temperature and vial equilibration time of the HS injector were selected as tuning objectives for optimal SCFA volatilization from the aqueous standard mixtures. Nine samples were treated according to sample preparation procedure. The three oven temperatures 40, 50, and 60 °C and the three vial equilibration times 15, 30, and 45 min were selected as HS injector parameters for investigation. The best result on volatilization was obtained at 50 °C for 30 min (results not shown).
A mixture of the SCFA standards was dissolved in salt solution according to sample preparation procedure and used to optimize the GC conditions. Figure 1a shows the chromatogram of the standard mixture containing C2, C3, C4, iC4, C5, iC5, and IS with retention times of 4.13, 4.795, 5.009, 5.615, 6.018, 6.802, and 7.248 min, respectively. As displayed in Fig. 1a, the resolution factor between the peaks was > 2 and peak shape and symmetry were good. Moreover, the GC analysis time was only 10 min, which was faster than that in previous studies using other methods; however, the total run time (including HS time) was longer [8,9,10, 20]. The chromatograms of ileocecal sample and fecal sample from rats are illustrated in Fig. 1b, c, respectively. Figure 1 demonstrates that the SCFAs were well extracted and separated from other compounds present in the matrix. The retention times of SCFAs in fecal samples were determined and compared with those of the standards.
Linearity and Sensitivity
The solutions containing SCFA mixture of standards were prepared and analyzed to obtain a calibration curve ranging from 1 to 200 µg L− 1, to which IS at a final concentration of 200 µg L− 1 was added. Gradually, diluted standard solutions were injected into the GC through the HS injector, and the LOD and LOQ of individual SCFAs were achieved by calculating the S/N. The LOQ was 0.02–0.08 µg L− 1 at 0.4–1.6 µg g− 1sample, and the LOD was 0.005–0.020 µg L− 1 at 0.1–0.4 µg g− 1sample. The calibration curve and range, linearity, LOD, and LOQ of the compounds are summarized in Table 2. The linearity of all calibration curves was good in the detection range and the linear correlation coefficient was all > 0.99. This method was used to analyze SCFAs in fecal samples with the compounds at a very wide range, as exhibited by LOQ–200 or LOQ– 4000 µg g− 1sample.
Recoveries
The added standard recoveries for each SCFA were 80–105% (Table 3), indicating good recovery of these analytes from rat fecal samples. The results were similar to previously reported recoveries using different treatments [15,16,17]. In conclusion, the proposed method was sensitive and precise enough to quantify SCFA amounts in fecal samples.
Method Application
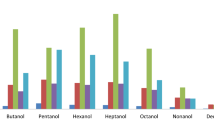
The efficiency of the developed method was investigated by analyzing the SCFAs in ileocecal and fecal samples from rat. Ten ileocecal and 17 fecal samples were treated as described above. Figure 1b, c illustrates the chromatograms of ileocecal and fecal samples, respectively. All targets, including six SCFAs and the IS, were detected and identified in the samples. The concentrations of SCFAs in all analyzed samples were all within the linear range. The results are shown in Table 4. The data demonstrate that the SCFA levels are distinct in different parts in rat. The results were similar to previous reports [1, 10, 18, 21].
Conclusions
In this study, a sensitive and specific HS-GC method, which can accurately identify and quantify SCFAs in fecal samples, has been developed and validated. Compared with existing methodologies, this method utilizes HS as a pretreatment method. This HS protocol is simple, safe as no solvent is required, allows handling a large number of samples, and requires very little manpower. The salting-out technology improves the sensitivity of this method. Moreover, this method is sensitive enough to detect 0.005–0.020 µg L− 1 SCFAs or 0.1–0.4 µg g− 1 fecal sample and quantify 0.02–0.08 µg L− 1 SCFAs or 0.4–1.6 µg g− 1 fecal sample. The total analysis took only 10 min. Good recovery and repeatability and high sensitivity make this method suitable for the analysis of biological samples with low SCFA concentration.
References
Wong JM, De SR, Kendall CW (2006) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40(3):235–243
Marette A, Jobin C (2015) SCFAs take a toll en route to metabolic syndrome. Cell Metab 22(6):954–956
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81(3):1031–1064
Ruppin H, Barmeir S, Soergel KH, Wood CM Jr SM (1980) Absorption of short-chain fatty acids by the colon. Gastroenterology 78:1500–1507
Stumpff F (2018) A look at the smelly side of physiology: transport of short chain fatty acids. Pflügers Archiv Eur J Physiol 470(4):571–598
Hu GX, Chen GR, Xu H, Ge RS, Lin J (2010) Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med Hypotheses 74:123–126
Tan J, Mckenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L (2014) The role of short-chain fatty acids in health and disease. Adv Immunol 121:91–119
Fiorini D, Pacetti D, Gabbianelli R, Gabrielli S, Ballini R (2015) A salting out system for improving the efficiency of the headspace solid-phase microextraction of short and medium chain free fatty acids. J Chromatogr A 1409:282–287
Zhao G, Nyman M, Jönsson JA (2006) Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biome Chromatogr 20(8):674–682
Hoving LR, Heijink M, Van HV, van Dijk KW, Giera M (2018) GC–MS analysis of short-chain fatty acids in feces, cecum content, and blood samples. Methods Mol Biol 1730:247–256
Destandau E, Vial J, Jardya A, Henniona MC, Bonnet D, Lancelin P (2005) Development and validation of a reversed-phase liquid chromatography method for the quantitative determination of carboxylic acids in industrial reaction mixtures. J Chromatogr A 1088(1–2):49–56
Saavedra L, Garcia A, Barbas C (2000) Development and validation of a capillary electrophoresis method for direct measurement of isocitric, citric, tartaric and malic acids as adulteration markers in orange juice. J Chromatogr A 881(1–2):395–401
Horspool LJ, Mckellar QA (1991) Determination of short-chain fatty acids in equine caecal liquor by ion exchange high performance liquid chromatography after solid phase extraction. Biome Chromatogr 5(5):202–206
Wu Z, Zhang Q, Li N, Pu Y, Wang B, Zhang T (2017) Comparison of critical methods developed for fatty acid analysis: a review. J Sep Sci 40(1):288–298
Chen ND, You T, Li J, Bai LT, Hao JW, Xu XY (2016) A comparative study of three tissue-cultured Dendrobium species and their wild correspondences by headspace gas chromatography–mass spectrometry combined with chemometric methods. J Food Drug Anal 24(4):839–847
Tan L, Zhao XP, Liu XQ, Ju HX, Li JS (2005) Headspace liquid-phase microextraction of short-chain fatty acids in plasma, and gas chromatography with flame ionization detection. Chroma 62(5–6):305–309
Rincón AA, Pino V, Ayala JH, Afonso AM (2014) Multiple headspace solid-phase microextraction for quantifying volatile free fatty acids in cheeses. Talanta 129:183–190
Fiorini D, Boarelli MC, Gabbianelli R, Ballini R, Pacetti D (2016) A quantitative headspace-solid-phase microextraction-gas chromatography-flame ionization detector method to analyze short chain free fatty acids in rat feces. Anal Biochem 508:12–14
Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, Gao Y, van den Heuvel JK, Meijer OC, Berbée JFP, Heijink M, Giera M, Willems van Dijk K, Groen AK, Rensen PCN, Wang Y (2017) Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 67(7):1269–1279
Trujillo-Rodríguez MJ, Pino V, Psillakis E, Anderson JL, Ayala JH, Yiantzi E, Afonso AM (2017) Vacuum-assisted headspace-solid phase microextraction for determining volatile free fatty acids and phenols. Investigations on the effect of pressure on competitive adsorption phenomena in a multicomponent system. Anal Chim Acta 962:41–51
Wang LL, Guo HH, Huang S, Feng CL, Han YX, Jiang JD (2017) Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J Chromatogr B Analyt Technol Biomed Life Sci 1057:70–80
Acknowledgements
This work has been supported by the Foundation (No. 2017KF001) of Key Laboratory of Industrial Fermentation Microbiology of Ministry of Education and Tianjin Key Lab of Industrial Microbiology (Tianjin University of Science & Technology) and the Foundation (No. ZXKF20180304) of Tianjin Engineering Research Center of Microbial Metabolism and Fermentation Process Control.
Funding
This work has been supported by the Foundation (No. 2017KF001) of Key Laboratory of Industrial Fermentation Microbiology of Ministry of Education and Tianjin Key Lab of Industrial Microbiology (Tianjin University of Science & Technology) and the Foundation (No. ZXKF20180304) of Tianjin Engineering Research Center of Microbial Metabolism and Fermentation Process Control.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical guidelines
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Zhang, C., Tang, P., Xu, H. et al. Analysis of Short-Chain Fatty Acids in Fecal Samples by Headspace-Gas Chromatography. Chromatographia 81, 1317–1323 (2018). https://doi.org/10.1007/s10337-018-3572-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3572-7