Abstract
Mesoporous materials were prepared in choline chloride deep eutectic solvents by triblock copolymer-templated hydrothermal synthesis. The mesoporous materials were characterized by thermogravimetric analysis, scanning electron microscopy and nitrogen sorption analysis. The mesoporous material-based choline chloride-ethylene glycol (1:2 mol ratio) deep eutectic solvent had a flower-type shape, but the material-based choline chloride-urea (1:2) and based choline chloride-acetic acid (1:2) had mesoporous spherical shapes. These mesoporous materials were applied to high-performance size exclusion chromatography column packing. The separation effect of the two dextrans was better from the choline chloride-acetic acid deep eutectic solvent-based mesoporous spheres packed in a high-performance size exclusion chromatography column. In addition, the three polysaccharides, dextran-1 (molecular weight: 670 KD), dextran-2 (molecular weight: 50 KD) and dextran-3 (molecular weight: 5 KD), were separated successfully using the choline chloride-acetic acid deep eutectic solvent-based mesoporous spheres packed in a high-performance size exclusion chromatography column. The mesoporous spheres-based deep eutectic solvents are a potential packing material in high-performance size exclusion chromatography. The separation conditions of more target compounds will be explored in future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increasing attention on the development of green chemistry, green chemistry has encouraged the application of more eco-friendly substances and more green products generated in more chemical processes. Ionic liquids (ILs), which consist of an organic cation paired with an organic or an inorganic anion, as a new type of green solvent, have been applied widely in chemical process by researchers over the past 20 years [1–4]. On the other hand, most ILs with hazardous toxicity and poor biodegradability, have been reported; hence, there is some doubt regarding fully eco-friendly ILs [5–7]. The discount of the green property of ILs hampers their development, and promotes new solvent generation with greener properties. Under this background, deep eutectic solvents (DESs), have emerged as eco-friendly solvents in chemical research and aroused the interest of many researchers.
A DES is generally composed of a mixture consisting of a hydrogen bond acceptor (HBA), such as a quaternary salt, with a hydrogen bond donor (HBD), such as amines, carboxylic acids, alcohol, and carbohydrates [8]. Choline chloride (ChCl) with great biodegradability and low toxicity, has been selected popularly as a HBA [9, 10]. In addition, these HBDs are also common organic compounds from organisms. Therefore, DESs have greener credentials than ILs, and have attracted considerable attention in related chemical research. From the appearance of the DESs definition in 2003 to now [8], DESs have been applied rapidly to some branches of chemistry, such as electrochemistry, nanomaterial preparation, and biochemistry [11–14]. Based on the green affiliation of DESs and the compositional flexibility, DESs have attracted interest in materials preparation [15]. In addition, mesoporous materials have been applied widely in areas, such as catalysis, sorption, ion exchange, etc., in various chemical processes. Therefore, the preparation of mesoporous materials in DESs should be examined.
Among mesoporous materials, triblock copolymer-template hydrothermal synthesis has been applied widely in the preparation of a stable mesoporous siliceous material [16, 17]. In the synthesis of mesoporous siliceous materials, surfactants with ionic hydrophilic groups and hydrophobic tails, can as template for the ordered arrays of pores in mesoporous materials [18]. Based on flexible structure of DESs containing both an organic group and an anion, DESs might be a potential surfactant in the preparation of mesoporous siliceous materials and be explored. In this study, three choline chloride DESs with different HBDs were applied to the preparation of mesoporous materials. If mesoporous materials have uniform pore structures and excellent stability, they can be applied as packing materials to high-performance size exclusion chromatography (HP-SEC) [19, 20]. Therefore, the three prepared mesoporous materials in choline chloride DESs were packed in a HP-SEC column for the separation of polysaccharides.
To explore the synthesis of mesoporous siliceous materials in the green solvent of DESs, three choline chloride DESs, ChCl-Urea, ChCl-Ethylene glycol and ChCl-Malonic acid, were applied to hydrothermal synthesis, as shown in Table 1. After the syntheses, the prepared materials were characterized by thermogravimetric analysis (TGA), scanning electron microscopy (SEM) and nitrogen sorption analysis. All the mesoporous materials were packed in HP-SEC columns and applied to the separation of polysaccharides.
Experimental
Materials
Choline chloride (ChCl) (>98.0 %), ethylene glycol (>99.5 %), urea (>98.0 %) and 1,3,5-trimethylbenzene (TMB, 99 %) were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). Acetic acid (>99.0 %) was supplied by Duksan Pure Chemicals Co., Ltd. (Ansan, Korea). Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEG-PPG-PEG), and three polysaccharides standards, dextran-1 (analytical standard, for GPC, molecular weight: 670 KD), dextran-2 (analytical standard, for GPC, molecular weight: 50 KD) and dextran-3 (analytical standard, for GPC, molecular weight: 5 KD), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Teraethoxysilane (TEOS, 98 %) was supplied by Alfa Aesar (Heysham, England). Hydrochloric acid (HCl, 36 %) was obtained from Kosdaq Co., Ltd. (Siheung, Korean). All other organic solvents and inorganic reagents were acquired from Duksan Pure Chemicals Co., Ltd. (Ansan, Korea). Distilled water was filtered using a vacuum pump and filter (HA-0.45, both from Millipore, USA) prior to use. All samples were filtered (MFS-25, 0.2 μm TF, Whatman, USA) before being injected into the HPSEC system.
Preparation of DESs
Three DESs were prepared by heating ChCl salt and a HBD, such as urea, ethylene glycol and malonic acid, at a ratio of 1:2 (mole ratio) to 60 °C for 30 min with constant stirring until a homogeneous liquid had formed. Table 1 lists the compositions of the prepared DESs.
Synthesis of Mesoporous Siliceous Materials in DESs
The mesoporous materials were prepared using the hydrothermal polymerization method shown in Fig. 1 and synthesized using the following synthetic scheme. 4.0 g of PEG-PPG-PEG as a copolymer was dissolved in 65.0 mL of H2O and 10.0 mL of HCl solution in a reactor, which was followed by the addition of 5.0 g of TMB. The mixture was stirred at 40 °C for 2 h. Subsequently, 9.0 mL of TEOS was added to the reaction mixture, and the mixture was transferred to an autoclave at 40 °C for 20 h. The DES was then added to the autoclave, and the temperature was increased to 150 °C and held at that temperature for 24 h. After aging, the resulting white precipitate was filtered, washed sequentially with water and ethanol, and dried at 60 °C. The white powder was then calcined at 900 °C in a muffle furnace for 6 h. After cooling, the desired DES-based mesoporous materials were obtained as white particles [21].
Characterization of the Mesoporous Materials
The mesoporous materials were characterized by TGA (SCINCO thermal gravimeter S-1000), field emission-scanning electron microscopy (FE-SEM, S-4200, Hitachi, Ontario, Canada) and Brunauer-Emmett-Teller (BET) analysis. The TGA data was obtained at a heating rate of 10 °C min−1 under N2. The BET surface area (N2 atmosphere at −195.8 °C) was measured using an ASAP2020 surface area analyzer (Micromeritics, Norcross, GA, USA).
Packing HP-SEC and Analysis
The mesoporous material was packed in a 250 × 4.6 mm stainless steel column using a dry packing method. The HPSEC system was comprised of an M930 solvent delivery pump (Younglin, Korea) and a refractive index detector (RID, Younglin, Korea). To prepare the standard solutions, a series of aqueous dextran-1, dextran-2 and dextran-3 solutions at accurate concentrations (5.0 mg mL−1, respectively) were obtained to characterize the prepared column, and 10.0 µL of each standard solution was injected. The mobile phase was methanol (flow rate: 0.5, 1.0 and 1.5 mL min−1).
Results and Discussion
Synthesis in DESs
Previous studies reported the preparation of mesoporous materials using block copolymers as templates in hydrothermal synthesis and the mesoporous structure induced by each reagent in the synthesis [22, 23]. Because inorganic salts are a popular reagent in the preparation of mesoporous materials, DESs containing a quaternary salt with a HBD have attracted attention and were introduced in the synthesis [24, 25]. In this study, three ChCl-based DESs were selected for the preparation of mesoporous materials, as shown in Table 1. The three DESs with hydrogen-bonds can interact with the copolymer PEG-PPG-PEG, and those with an ionic group can interact with TEOS as a surfactant template. Compared to previous prepared mesoporous materials, these prepared materials have ionic groups on the surface and have an ion exchange property, as shown in Fig. 1. The selected DESs with three different HBDs, including alcohols, organic base and organic acid, were explored to determine their influence on the preparation of mesoporous materials. All the structural features, including shape, particle size and pore size, of the three prepared materials were characterized and compared in the following text.
TGA Analysis
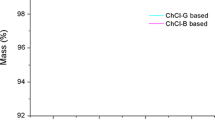
To check the stability of the mesoporous materials, these materials were characterized by TGA. The total weight loss of each of these materials from 200 to 700 °C was less than 2 %, as shown in Fig. 2. For the little loss, the desorption of water, the evaporation of TMB and the decomposition of organics were already performed during the calcination process. Therefore, only a little of the fly ash was removed from the prepared materials under the TGA conditions.
SEM Analysis
Figure 3 shows SEM images of the three mesoporous materials; an obvious difference in their structure was observed. The materials synthesized with DES-1 and DES-3 had uniform spheres, but DES-2 based materials did not. The results showed that different DESs affect the particle morphologies of these mesoporous materials. The ChCl DESs of DES-1 with urea and DES-3 with acetic acid interacted with PEG-PPG-PEG to shape the uniform mesoporous spheres. On the other hand, the material-based DES-2 had a cauliflower-type morphology and was not spherical. The DES-1 or DES-3 based mesoporous spheres were larger in size than the other ChCl DES-based materials, as shown in Table 2. For the morphologies of ChCl DES-based mesoporous materials, the spherical particles were very convenient to pack a HP-SEC column with the ChCI DES-based mesoporous materials, which also had an excellent performance at macromolecule separation.
BET Analysis
The BET data was used to examine the porosity of these mesoporous materials by nitrogen sorption and desorption. The popular sorption and desorption isotherms at the relative pressures were used to characterize the surface area, pore size and pore volume of the materials, as shown in Fig. 4. The isotherms had larger quantity adsorption and desorption values with the same relative pressures in Fig. 4a, c than in Fig. 4b. The isotherms in Fig. 4a, c show that DES-1-based and DES-3-based mesoporous spheres have more absorptive capacity and evaporation with a larger porous volume than DES-2-based materials. In addition, the data of the porous volume of the three mesoporous materials are agreement with the isotherms listed in Table 2. The porous volumes of DES-1 and DES-3 were larger than 0.8 cm3 g−1, but the porous volumes of DES-2 was less than 0.8 cm3 g−1. In addition, the pore size of all the DES-based materials was larger than 8 nm and suitable for the separation of large molecules based on the SEC principle [26–28]. Therefore, the mesoporous materials were packed in the HP-SEC column for the separation of macromolecules.
Separation of Dextran
To evaluate the three DES-based materials as HP-SEC packing materials, dextran-1 and dextran-2 were first selected as the target compounds to evaluate the properties of their packing columns.
Figure 5 clearly shows the separation effect of the three DES-based HP-SEC, and two peaks of the targets compounds only appeared in the DES-3-based HP-SEC chromatogram. Based on the results, the shape of the materials is a key point and the DES-3-based spheres are suitable for forming a uniform track in the column. The other anomalous materials were packed disorderly in the column and could not separate the target compounds according to their size.
To a certain station phase of the DES-3-based HP-SEC column, the velocity of the mobile phase is a key factor for the effective separation of samples in the prepared HP-SEC. In this case, three velocities of the mobile phase were selected for the separation of dextran-1 and dextran-2 in the DES-3-based HP-SEC. Figure 6 illustrates their ability to separate the target compounds through the DES-3-based HP-SEC column. In addition, the resolution (R) is a key factor for evaluating the efficiency of a HP-SEC column. R shows how well the two target compounds are separated in the chromatograph, and is defined as:
where t R2 and t R1 are the retention times of the target compounds, 1 and 2, respectively, and W 1 and W 2 are the peak widths of the target compounds 1 and 2, respectively.
Based on the R definition, Table 3 lists the resolution data of dextran-1 to dextran-2 in three different velocities of the mobile phase. When the velocity was increased from 0.5 to 1.0 and 1.5 mL/min, the retention time of dextran-1 decreased from 8.81 to 5.47 and 0.86 min respectively. On the other hand, the retention time of dextran-2 did not always decrease with increasing velocity. Normally, R decreases with increasing mobile phase velocity. In this study, with a mobile phase velocity of 0.5 mL/min, the R of dextran-1 to dextran-2 was 2.12 in DES-3-based HP-SEC, as listed in Table 3. In addition, the R was 6.39 with 1.0 mL/min and the R was 2.04 with 1.5 mL/min. In any case, R of each velocity was more than 1.5 for clear separation of the two targets compounds. Based on the three velocities, the R with 1.0 mL/min was the largest and the data was consistent with the chromatograms shown in Fig. 6.
To further evaluate the DES-3-based HP-SEC, three dextrans were selected as the target compounds to be separated. Figure 7 presents the chromatograms of the three dextrans. Table 3 lists the retention time of the target compounds and the R between the different dextrans. Although the R of dextrans in the DES-3-based HP-SEC was less than or similar to 1.5, the prepared DESs-based mesoporous spheres can be applied as HP-SEC column packing for further exploration.
Conclusions
A series of ChCl DESs were applied to the synthesis of mesoporous materials. Among these mesoporous materials, the DES-1 and DES-3-based materials were uniform spheres, and DES-2-based materials was cauliflower-type particles. The stability of these mesoporous materials were characterized by TGA, and the mesoporous structures were characterized in detail by SEM and BET. These DES-based materials were packed in a HP-SEC column and their separation efficiencies were examined according to their mesoporous structures. By evaluating the effects of the prepared HP-SEC, the DES-3-based mesoporous spheres were suitable as packing materials. Nevertheless, the separation conditions of the DES-3-based HP-SECs require further exploration.
References
Duchet L, Legeay JC, Carrié D, Paquin L, Vanden Eynde JJ, Bazureau JP (2010) Synthesis of 3,5-disubstituted 1,2,4-oxadiazoles using ionic liquid-phase organic synthesis (IoLiPOS) methodology. Tetrahedron 66:986–994
Zanoni MVB, Rogers EI, Hardacre C, Compton RG (2010) The electrochemical reduction of the purines guanine and adenine at platinum electrodes in several room temperature ionic liquids. Anal Chim Acta 659:115–121
Fontanals N, Ronka S, Borrull F, Trochimczuk AW, Marcé RM (2009) Supported imidazolium ionic liquid phases: a new material for solid-phase extraction. Talanta 80:250–256
Kubisa P (2009) Ionic liquids as solvents for polymerization processes—progress and challenges. Prog Polym Sci 34:1333–1347
Deetlefs M, Seddon KR (2010) Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem 12:17–30
Kareem MA, Mjalli FS, Hashim MA, AlNashe IM (2010) Phosphonium-based ionic liquids analogues and their physical properties. J Chem Eng Data 55:4632–4637
Romero A, Santos A, Tojo J, Rodriguez A (2008) Toxicity and biodegradability of imidazolium ionic liquids. J Hazard Mater 151:268–273
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun 7:70–71
Tang B, Row KH (2013) Recent developments in deep eutectic solvents in chemical sciences. Monatshefte Fur Chemie 144:1427–1454
Tang B, Row KH (2015) Exploration of deep eutectic solvent-based mesoporous silica spheres as high-performance size exclusion chromatography packing materials. J Appl Polym Sci 132:42203–42209
Nkuku CA, LeSuer RJ (2007) LeSuer, electrochemistry in deep eutectic solvents. J Phys Chem B 111:13271–13277
Figueiredo M, Gomes C, Costa R, Martins A, Pereira CM, Silva F (2009) Differential capacity of a deep eutectic solvent based on choline chloride and glycerol on solid electrodes. Electrochim Acta 54:2630–2634
Ilgen F, Konig B (2009) Organic reactions in low melting mixtures based on carbohydrates and L-carnitine—a comparison. Green Chem 11:848–854
Gore S, Baskaran S, Koenig B (2011) Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures. Green Chem 13:1009–1013
Tang B, Park HE, Row KH (2014) Preparation of chlorocholine chloride/urea deep eutectic solvent-modified silica and an examination of the ion exchange properties of modified silica as a Lewis adduct. Analy Bioanal Chem 406:4309–4313
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Lettow JS, Lancaster TM, Glinka CJ, Ying JY (2005) Small-angle neutron scattering and theoretical investigation of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) stabilized oil-in-water microemulsions. Langmuir 21:5738–5746
Lettow JS, Han YJ, Schmidt-Winkel P, Yang P, Zhao D, Stucky GD, Ying JY (2000) Hexagonal to mesocellular foam phase transition in polymer-templated mesoporous silicas. Langmuir 16:8291–8295
Han Y, Lee SS, Ying JY (2007) Spherical siliceous mesocellular foam particles for high-speed size exclusion chromatography. Chem Mater 19:2292–2298
Han Y, Lee SS, Ying JY (2010) Siliceous mesocellular foam for high-performance liquid chromatography: effect of morphology and pore structure. J Chromatogr A 1217:4337–4343
Li X, Row KH (2015) Exploration of mesoporous stationary phases prepared using deep eutectic solvents combining choline chloride with 1,2-butanediol or glycerol for use in size-exclusion chromatography. Chromatographia 78:1321–1325
Velev OD, Jede TA, Lobo RF, Lenhoff AM (1998) Microstructured porous silica obtained via colloidal crystal templates. Chem Mater 10:3597–3602
Antonietti M, Berton B, ltner CG, Hentze HP (1998) Synthesis of mesoporous silica with large pores and bimodal pore size distribution by templating of polymer latices. Adv Mater 10:154
Jain NJ, George A, Bahadur P (1999) Effect of salt on the micellization of pluronic P65 in aqueous solution. Colloids Surf A 157:275–283
Yu CZ, Tian BZ, Fan B, Stucky GD, Zhao DY (2001) Salt effect in the synthesis of mesoporous silica templated by non-ionic block copolymers. Chem Commun 24:2726–2727
Tian R, Sun J, Zhang H, Ye M, Xie C, Dong J, Hu J, Ma D, Bao X, Zou H (2006) Large-pore mesoporous SBA-15 silica particles with submicrometer size as stationary phases for high-speed CEC separation. Electrophoresis 27:742–748
Zhao J, Gao F, Fu Y, Jin W, Yang P, Zhao D (2002) Biomolecule separation using large pore mesoporous SBA-15 as a substrate in high performance liquid chromatography. Chem Commun 7:752–753
Ma YR, Qi LM, Ma JM, Wu YQ, Liu O, Cheng HM (2003) Large-pore mesoporous silica spheres: synthesis and application in HPLC. Colloid Surf A 229:1–8
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant number: 2015042434).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Li, X., Lee, Y.R. & Row, K.H. Synthesis of Mesoporous Siliceous Materials in Choline Chloride Deep Eutectic Solvents and the Application of These Materials to High-Performance Size Exclusion Chromatography. Chromatographia 79, 375–382 (2016). https://doi.org/10.1007/s10337-016-3051-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3051-y