Abstract
A new, simple, selective, and robust normal-phase method for the accurate quantification of all the four stereoisomers and one geometrical isomer of pitavastatin calcium (PIT) in drug substances and drug products was developed. The method is capable of quantifying all the isomers in the presence of other related substances. Separation was achieved using immobilized amylose stationary phase (Chiralpak IA) with a mixture of n-heptane, 1-butanol, methanol, formic acid, and diethylamine. Multivariate analysis and statistical tools were used to develop this highly robust method in a short span of time. A central composite design was employed to study the main effects and interactions of the independent variables. The method exhibited consistent, high-quality recoveries [97.3 ± 1.7 to 99.3 ± 2.1 (mean ± RSD)] with a high precision for all the isomers. Linear regression analysis revealed an excellent correlation between peak responses and concentrations (r 2 values of 0.9990–0.9998) for the isomers. The method is sensitive enough to quantify any isomers above 0.02 % and detect any isomer above 0.006 % in PIT. Forced degradation studies proved that the method is specific for isomers. m/z values were determined for the major degradants and their possible structures were proposed on the basis of the known reactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the family of statins, pitavastatin calcium (PIT) plays a vital role owing to its distinctive lipid-lowering characteristics [1–5]. It is indicated for hypercholesterolemia (elevated cholesterol) and for the prevention of cardiovascular disease. It is indicated as an adjunctive therapy to diet to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) in adult patients with primary hyperlipidemia or mixed dyslipidemia. PIT completely inhibits HMG CoA reductase, the rate-determining enzyme in hepatic cholesterol synthesis. Consequently, LDL-C receptors in the liver are increased, thereby increasing the removal of LDL-C from the blood [6].
Stereoselectivity plays a major role in the drug’s action [7] and single enantiomer drugs are safer, better tolerated, and more efficient [8]. To our knowledge, no stereoselective HPLC method for the quantification of all the stereoisomers and geometrical isomers of PIT exists in the literature. The reported analytical methods that relate to PIT are two mass detection methods for determination of pitavastatin in plasma and biological fluids [9, 10], a stability-indicating related substances method by UPLC [11], two HPTLC methods for the quantification of pitavastatin in tablets [12, 13], and a capillary zone electrophoresis method for separation of one enantiomer [14]. Moreover the capillary electrophoresis technique is not routinely used in the pharmaceutical industry for impurity profiling [15]. But none of the methods either individually or combined are capable of quantifying all the stereoisomers and geometrical isomer of pitavastatin calcium.
In this paper, we describe the development of a stereoselective method for PIT. ICH Q11 guidelines describe enhanced approaches for manufacturing processes [16]. We have applied the same principles to analytical method development. Selecting stationary phases on the basis of functional groups present in the compound, applying the understanding gained from initial screening for selection of factors for design of experiments (DoE), finalizing the method conditions on the basis of a thorough understanding gained from multivariate analysis about edges of failure (design space) and inclusion of comparatively green solvents wherever possible are considered as an enhanced approach here.
Experimental
Standards and Reagents
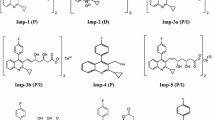
Pitavastatin Ca standard and its related impurities were synthesized at Dr. Reddy’s Laboratories, Hyderabad, India. Livalo (pitavastatin Ca) tablets (lot no. 3080532) were procured from Kowa Pharmaceuticals America, USA. n-Heptane, 1-butanol, methanol, formic acid, acetonitrile, and tetrahydrofuran were procured from Rankem, New Delhi, India. Diethylamine was procured from Merck, Mumbai, India. HPLC-grade water was obtained from a Milli-Q water purification system (Millipore, Milford, USA). Structures of PIT, its isomers, and its related substances are shown in Fig. 1.
Instruments and Software
-
LC1: Waters Alliance 2,695 separation module equipped with PDA detector (Waters Corporation, Milford, MA, USA) was used for specificity and development studies.
-
LC2: Agilent 1,200 series equipped with VWD (Agilent Technologies, Waldron, Germany) was used for validation parameters.
-
LC1 and LC2 were monitored with Empower 2 software (Waters Corporation, Milford, MA, USA).
Design Expert version 7.1.6 (Stat-Ease Inc., Minneapolis) was used for central composite design construction and interpretation. Microsoft Excel 2007 was used for analysis of validation results.
Chromatography Conditions
Column, Chiralpak IA-3 250 × 4.6 mm × 3.0 µm (Daicel Chiral Technologies); mobile phase, n-heptane/1-butanol/methanol/formic acid/diethylamine (940:35:25:2:1, v/v/v/v/v); flow rate, 1.0 mL min−1 (isocratic); column oven temperature, 35 °C; injection volume, 20 µL; wavelength, 250 nm; sample concentration, 0.5 mg mL−1. Diluent is tetrahydrofuran (THF) and n-heptane (2:8, v/v).
Preparation of Blend Solutions
For accurate quantification, it is necessary to ensure the separation of other related substances from isomers. Hence a blend solution containing 0.5 mg mL−1 solution of PIT and 0.1 % level of all isomers (Iso-1 to Iso-4), and all impurities (Imp-1 to Imp-7) was prepared (blend solution 1) and used for development studies.
A blend solution (blend solution 2) containing 0.5 mg mL−1 solution of PIT and 0.1 % level of all isomers (Iso-1 to Iso-4) and Imp-1 (Imp-1 was included to ensure its separation from isomers as it was the only close-eluting impurity) was prepared and used for DoE.
Tablet Analysis
Twenty Livalo tablets (each tablet contains 4.18 mg of PIT) were crushed into a fine powder; an amount equivalent to 25 mg PIT was dissolved in 10 mL of tetrahydrofuran, made up to 50.0 mL with n-heptane, and the filtered solution was injected onto the column (excipients were lactose monohydrate, low substituted hydroxylpropyl cellulose, hypromellose, magnesium aluminosilicate, magnesium stearate, titanium dioxide, and triethyl citrate).
Results and Discussion
Method Development and Optimization
Selection of Column
One of the best chiral stationary phases (CSP) for the separation of aromatic compounds with functional groups such as carbonyl, alcohol, and amine (like pitavastatin) are amylose-based polysaccharide stationary phases [17]. Immobilized polysaccharide columns are compatible with a wide range of solvents and have increased the range of applications (chiral recognition mechanisms) [18]. Hence the immobilized amylose-based polysaccharide column Chiralpak IA was selected for screening.
The CSP in Chiralpak IA is amylose tris(3,5-dimethylphenyl carbamate) bonded to silica gel. The separation of isomers may be attributed to hydrogen bonding interactions between the solute (C=O and OH groups in PIT) and the polar carbamate group on the CSP. In addition, dipole–dipole interactions can occur between C=O groups of the CSP and C=O groups present in PIT. Solutes having aromatic functionalities could provide additional stabilizing effects on the solute–CSP complex by insertion of the aromatic portion into the chiral cavity [19]. This type of stabilization effect may also exist in PIT owing to the presence of aromaticity.
Selection and Optimization of Acidic/Basic Additives
As polysaccharide-based CSPs generally show a higher success rate in resolving enantiomers under normal-phase elution [20], this mode was explored first. The screening of mobile phases was started with traditional alkane/alcohol mixtures [21]. n-Heptane was given first preference as it is comparatively green [22].
For separation of most of the analytes containing basic and/or acidic functional groups, the additive plays a major role in increasing the chromatographic efficiency [23]. As PIT contains both acidic and basic functional groups it was decided to optimize the additives for good peak shape; all other chromatographic parameters were kept constant: flow rate, 1.0 mL min−1 (isocratic); column oven temperature, 25 °C; injection volume, 20 µL; wavelength, 250 nm; mobile phase, n-heptane and ethanol (90:10, v/v) to which different additives were added and used as mobile phase for screening their effect.
Diethylamine is often used as a modifier for amine groups in order to ensure their elution from the column and peak shape in normal-phase chromatography [24]. Hence 0.1 % diethylamine was introduced into the mobile phase. PIT eluted at around 10 min and the peak was highly asymmetric (USP tailing = 2.8). Further increase in diethylamine content did not have any significant impact on the peak shape, whereas removal of diethylamine from the mobile phase significantly distorted the peak shape.
In order to further improve the peak shape, an acidic additive was used in combination with diethylamine. Formic acid was given first preference as it is comparatively green [22]. Therefore 0.1 % formic acid was introduced into the mobile phase which significantly improved the peak shape (USP tailing = 2.0). The acid probably promotes the ionization of the tertiary amine of pitavastatin and might form an ion pair, improving the selective interaction with the stationary phase and improving the peak shape. When 0.2 % formic acid was used in the mobile phase, the peak shape improved (USP tailing = 1.4); however, no further improvement was observed at higher contents. Also in the absence of diethylamine, i.e., only with formic acid, again distorted peak shape was observed (USP tailing = 2.6). Hence 0.2 % formic acid and 0.1 % diethylamine were selected as additives for the mobile phase screening.
Screening of Alcoholic Modifiers
On the basis of the retention time obtained using n-heptane/ethanol (90:10, v/v), it was decided to reduce the alcohol volume to allow better interaction with the stationary phase. In chiral separations, the alcohols play a major role in creating a selectivity difference [25]. Hence, the ratio of the mobile phase components n-heptane/alcohol/formic acid/diethylamine was fixed at 93:7:0.2:0.1 (v/v/v/v) and the effect of different alcohols on selectivity was studied.
The details of different alcohols screened (trials 1–5) and their results are given in Table 1. The results of trials 3 and 4 appeared to be complementary. In trial 4, ethanol was added to make the methanol miscible with n-heptane; ethanol alone as alcoholic component in trial 1 did not produce satisfactory resolution. Hence the improved resolution observed in trial 4 could be due to methanol. To achieve the complementary effects of trials 3 and 4, methanol and 1-butanol were tested in combination (trial 5).
Since trial 5 showed a significant improvement in selectivity, DoE was planned to optimize the volume of these alcoholic modifiers and other chromatographic parameters. The critical method parameters (CMPs) considered for optimization (DoE) were (a) methanol content in mobile phase, (b) temperature, and (c) flow.
Factors for DoE and Their Variation Limits
-
(a)
Methanol volume (15–35 mL for 1,000 mL of mobile phase): when the methanol volume was greater than that of 1-butanol it led to immiscibility. Hence the volume of 1-butanol was fixed (35 mL) and the volume of methanol (x) was varied from 15 mL (considered as minimum volume required for a significant change in selectivity) to 35 mL (the volume above which immiscibility resulted). The volume of n-heptane for each DoE run was calculated using the following formula: 1,000 − (x + 35).
-
(b)
Column oven temperature, 25–40 °C.
-
(c)
Flow, 0.7–1.2 mL min−1.
Execution of DoE
A central composite design (CCD) (alpha = 1.0) was adopted to optimize the factors for resolution of critical pairs. Centre points were taken by the design and run in six replicates. With all other chromatography conditions fixed (as indicated in Sect. 3.1.4), methanol volume, flow rate, and column temperature (CMPs) were varied in each DoE run. Blend solution 2 was injected in each run. The goal of the DoE was to establish an optimal condition in which all isomers and Imp-1 should separate from each other and from PIT. The close-eluting pairs were taken as responses for optimization and to make the method short, the run time was also taken as one of the responses. The standard run and run order along with responses measured are given in Table 2.
Data Interpretation
For an experimental design with three factors, the model including linear, quadratic, and cross terms can be expressed as
Y is the response to be modeled, β is the regression coefficient, and X 1, X 2, and X 3 represent factors A, B, and C, respectively. To obtain a simple and yet realistic model, the insignificant terms (P > 0.05) are eliminated from the model through a ‘backward elimination’ process.
The regression equations for the reduced models for each response were
-
Response 1 = 1.47 − 1.91A + 0.21B − 0.25AB + 0.40A 2
-
Response 2 = 2.11 − 0.74A + 0.53B − 0.21AB + 0.12AC
-
Response 3 = 1.28 − 0.037A − 0.038B − 0.15C − 0.047A 2 − 0.032B 2 − 0.072C 2
-
Response 4 = 3.08 − 0.059A + 0.081B − 0.32C − 0.13AB − 0.34A 2
-
Response 5 = 22.46 − 8.10A − 2.49 B − 6.60C + 0.72AB + 0.60AC + 2.79A 2 + 0.67B 2 + 1.56C 2
For all the responses, R 2 > 0.90, showing that the experimental data shows a good fit with the polynomial equations; P < 0.05, implying that these models are significant; adequate precision > 4 (27–107), indicating an adequate signal for the optimization process; and coefficient of variation <10 %, indicating good reproducibility.
Study of Effects Graph
The effects graph (Fig. 2) for each response was plotted and the following conclusions were made:
-
(a)
A decrease in methanol content and increase in temperature increased the resolution between Imp-1 and Iso-4. Flow did not show a significant effect on this resolution.
-
(b)
A decrease in methanol content and increase in temperature increased the resolution between Iso-4 and Iso-1. Flow did not show a significant effect on this resolution.
-
(c)
A decrease in flow increased the resolution between Iso-1 and Iso-2. Methanol content and temperature did not show a significant effect on this resolution.
-
(d)
A decrease in flow increased the resolution between PIT and Iso-3. Methanol content and temperature did not show a significant effect on this resolution.
-
(e)
Any increase in flow, methanol content, and temperature decreased the retention time of PIT.
Desirability and Design Space
To optimize the method for five responses with different targets, Derringer’s desirability function (D) was used [26], which is defined as the geometric mean, weighted or otherwise, of the individual desirability functions according to Eq. 2. Equal importance was given to all five responses and the optimum condition was obtained with Derringer desirability function.
pi is the weight of the response, n is the number of responses, and di is the individual desirability function of each response. A contour plot of the desirability obtained is given in Fig. 3.
The high desirability region was chosen for normal operation. Within this region, the point chosen for normal operation was methanol volume, 25 mL; temperature, 35 °C; and flow, 1.0 mL min−1. In order to confirm the optimal conditions suggested by multivariate analysis, the blend solution containing all four isomers and close-eluting impurity (Imp-1) was run in six replicates. The chromatogram obtained showed the desired separation of all isomers and Imp-1. Percentage relative standard deviation (% RSD) values for each isomer and Imp-1 were within 5 %.
To further verify the obtained design space, to better understand the edges of failures, and to verify the robustness of the method, two verification trials were done: one with all CMPs at significantly higher ranges and another with significantly lower ranges than the optimized condition. The obtained results were very close to the design space’s prediction and proved the inbuilt robustness of the method.
The design space for a chiral method (LC) can be defined as a “multidimensional combination and interaction of mobile phase variables (such as alkane and alcohol ratio, additives concentration, etc.) and chromatographic parameters (like flow rate, column oven temperature, etc.) that have been demonstrated to provide assurance of [a] result obtained with the method” [27]. The proposed design space is illustrated in Fig. 3. The design space around normal operation indicates the robustness of the method.
Induced Degradation Studies
Degradation impurity investigations must be initiated in the early stage of product development to provide a clear understanding of the stability issues and a safe product.
For this stereoselective method, it is necessary to ensure that no degradant which may form under various stress conditions interferes with any of the analytes. The details of the induced degradation study performed and the outcome of the degradation studies are presented in Table 3 and Fig. 4.
Each degraded sample was injected as such and spiked with all isomers. Peak purities for all the isomers were ensured with a PDA detector. PIT and its isomers were well separated from the obtained degradation products. The chromatogram of the water hydrolysis sample spiked with all isomers is shown in Fig. 5.
Insight into Degradation Products
As some of the degradation products were eluted in the void volume in the normal-phase method, an LC–MS-compatible method was developed using a Zorbax SB C-18, 150 × 4.6 mm, 3.5 μm (Agilent Technologies) to gain insight into their identities. The chromatography conditions used were column temperature, 40 °C; flow rate, 1.0 mL min−1; injection volume, 20 µL; mobile phase, 0.1 % formic acid (solvent A) and acetonitrile (solvent B); gradient elution, 30 % solvent B, increasing linearly to 100 % over 30 min, and then maintained for 10 min. The impurities were identified by LC–MS in positive electrospray/positive ionization mode with an ion source voltage of 5,000 V and a source temperature of 450 °C. GS1 and GS2 were optimized to 30 and 35 psi, respectively. The curtain gas flow was 20 psi.
The % degradation achieved for each degradation study was calculated using this method. The mass numbers were generated for each degradation study and the degradation pathways were proposed (Fig. 4) on the basis of available known impurities, m/z values, and literature [28].
Validation [29]
Specificity
Specificity was demonstrated by spiking the test PIT concentration with 0.15 % impurities and isomers. An equivalent placebo concentration was prepared and injected into the chiral system to evaluate the interference with the analyte peaks. The diluent and placebo spiked solution showed no peak interference with isomers and PIT; moreover, the purity of all the isomers and PIT were very much less than the purity threshold values, indicating the high specificity and selectivity of the method.
Sensitivity
The LOD and LOQ values for all the isomers and PIT were estimated by the signal to noise ratio method. The precision study was also determined at the LOQ level by injecting six individual preparations of isomers and PIT at the estimated LOQ level and calculating the % RSD for area. Accuracy at the LOQ level was determined in triplicate for the isomers by spiking the isomers at the estimated LOQ level. The concentrations were in the range of 0.022–0.025 µg mL−1 (LOD) and 0.088–0.098 µg mL−1 (LOQ). The obtained LOD, LOQ, LOQ precision, and accuracy are given in Table 4.
Precision
The repeatability of the method was ensured by injecting six individual preparations of PIT spiked with 0.15 % of Iso-1, Iso-2, Iso-3 and Iso-4. % RSD for the content of each isomer was calculated. The reproducibility of the method was also determined by repeating the same experiment on different days by different analysts using different equipment. A precision study was also carried out for the isomers and drug in the presence of placebo, and the % RSD was calculated (Table 4). The % RSD for the content of each isomer was found to be less than 4 % in all the studies. The results confirmed the high precision of the method.
Linearity
Linearity test solutions were prepared in triplicate at eight concentrations (LOQ, 0.18, 0.37, 0.55, 0.74, 0.92, 1.10, and 1.47 µg mL−1). The calibration curves were drawn by plotting the average analyte peak area for triplicate injections against the concentration. The results for the correlation coefficient (r) and coefficient of determination (r 2) are shown in Table 4. The plot of residuals ensured the absence of systematic error.
Accuracy
To determine the accuracy of the stereoselective method, standard addition and recovery experiments were conducted by spiking the placebo-based solution of test sample (500 µg mL−1) with known amounts of isomers (0.375, 0.75, and 1.125 µg mL−1) in triplicate. Individual and average recoveries of three preparations and at three concentrations for all the isomers were within 100 ± 5 %. The 150 % spiked accuracy chromatogram is shown in Fig. 5.
Solution Stability and Mobile Phase Stability
The solution stability and mobile phase stability at controlled room temperature (25 °C) were evaluated by injecting the test solutions spiked with isomers (for 5 days). The % RSD values for the isomer contents during the solution and mobile phase stability experiments were within 3.5 %. No significant changes in the amounts of impurities were observed during the study (for 5 days). These results confirmed that the solutions were stable in the diluent for up to 5 days at ambient temperature.
Robustness
The robustness of the method was evaluated from CCD experiments data and the effects graphs. When the methanol content of mobile phase was increased above 3.2 %, a significant decrease in the resolution of Imp-A and Iso-4 was observed. Increasing the temperature to 40 °C and increasing the flow to 1.2 mL min−1 negatively affected the resolution between Iso-1 and Iso-2. However, the method was more robust within the normal operating range, i.e., % methanol, 2.5 ± 0.7 % (factor A); column oven temperature, 35 ± 5 °C (factor B); and flow rate, 1.0 ± 0.2 mL min−1 (factor C), demonstrating the robustness of the method.
Sample Analysis
Three batches of PIT lab samples and Livalo® tablets were analyzed for isomer contents. The results from test and stability samples indicated that all the isomers were present at less than 0.15 % in the drug substance and product.
Stability Study
Two batches of each drug substance and drug product were subjected to accelerated degradation conditions (40 °C, 60 % RH) for 6 months and analyzed using this method. There was no variation in the content of isomers during this study.
Conclusion
The developed method is simple, rugged, and selective for quantification of all stereoisomers and PIT. Critical method parameters like methanol volume, column temperature, and flow rate were successfully optimized by multivariate analysis. The optimization of the method through statistical tools and the resultant design space ensured the robustness of the method. The method can be used for the quantification of all the isomers in drug substances as well as drug products. The method can be used to monitor the stability of the drug substances and drug products. The method is user-friendly and robust to operate.
References
Stender S, Budinski D, Hounslow N (2013) Pitavastatin shows greater lipid lowering efficacy over 12 weeks than pravastatin in elderly patients with primary hypercholesterolemia or combined dyslipidaemia. Eur J Prev Cardiol 20:29–39
Stender S, Budinski D, Hounslow N (2013) Pitavastatin demonstrates long-term efficacy, safety and tolerability in elderly patients with primary hypercholesterolaemia or combined (mixed) dyslipidaemia. Eur J Prev Cardiol 20:40–53
Gumprecht J, Gosho M, Budinski D, Hounslow N (2011) Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20–40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab 13:1047–1055
Eriksson M, Budinski D, Hounslow N (2011) Long-term efficacy of pitavastatin versus simvastatin. Adv Ther 28:799–810
Ose L, Budinski D, Hounslow N, Arneson V (2010) Long-term treatment with pitavastatin is effective and well tolerated by patients with primary hypercholesterolemia or combined dyslipidemia. Atherosclerosis 210:202–208
FDA/Center for Drug Evaluation and Research (2009) Label Information—drugs at FDA, MD-20993. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022363s010lbl.pdf. Accessed Oct 2012
Patrizia G, Maria CP, Giuseppina G, Anna MM, Elena C, Simona P, Antonietta S, Chiara L, Maurizio B (2012) Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev 64:102–146
Pharma Book Syndicate (2009) Hyderabad, India. http://www.pharmabooksyndicate.com/issues/309.pdf. Accessed Mar 2009
Ramakrishna N, Koteshwara M, Vishwottam K (2007) Chromatography-mass spectrometry methods for the quantification of statins in biological samples. J Pharm Biomed Anal 44:379–387
Jianzhong ST, Xiao X, Jian L, Xingjiang H, Junchun C, Lihua W, Mingzhu H, Huili Z (2009) Determination of pitavastatin in human plasma by LC–MS–MS. Chromatographia 69:1041–1047
Antony RG, Pannala RR, Nimmakayala S, Jadi S (2010) Degradation pathway for pitavastatin calcium by validated stability indicating UPLC method. Am J Anal Chem 1:83–90
Satheesh NK, Baghyalakshmi J (2007) Determination and quantification of pitavastatin calcium in tablet dosage formulation by HPTLC method. Anal Lett 40:2625–2632
Hiral JP, Bhanubhai NS, Natubhai JP, Bhavesh HP (2008) A simple and sensitive HPTLC method for quantitative analysis of pitavastatin calcium in tablets. J Planar Chromatogr 21:267–270
Cheng X, Wang L, Yang G, Cheng J, Zhang Y (2010) Chiral separation of pitavastatin calcium enantiomers by capillary zone electrophoresis. Se Pu 28:1089–1093
Vishnu MM, Krishnaiah C, Kodithyala J, Katkam S, Mukkanti K, Ramesh K, Gautam S (2011) Enantioseparation of palonosetron hydrochloride and its related enantiomeric impurities by computer simulation and validation. Am J Anal Chem 2:437–446
ICH (2012) Development and manufacture of drug substances Q11. ICH harmonised tripartite guideline. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Step_4.pdf. Accessed 1 May 2012
LCGC (2008) A strategy for developing HPLC methods for chiral drugs. http://www.chromatographyonline.com/lcgc/data/articlestandard/lcgc/122008/504160/article.pdf. Accessed Feb 2008
Al-Othman Z, Ali I, Asim M, Khan TA (2012) Recent trends in chiral separations on immobilized polysaccharides CSPs. Comb Chem High Throughput Screen 15(4):339–346
Irving WW, Rose MS (1987) Resolution of enantiomeric aromatic alcohols on a cellulose tribenzoate high-performance liquid chromatography chiral stationary phase: a proposed chiral recognition mechanism. J Chromatogr A 411:139–151
Brian LH (2010) In: Alain B (ed) Chiral recognition in separation methods: mechanisms and applications. Springer, New York
Chiral Technologies Europe (2004) Instruction manual for Chiralpak-IA column. http://www.mz-at.de/pdf/Chiralpak_IA_Manual.pdf. Accessed April 2004
Paul F, Mark H, Jennifer Y (2012) In: Wei Z, Berkeley C (eds) Green techniques for organic synthesis and medicinal chemistry. Wiley, West Sussex
Zhang T, Franco P, Nguyen D, Hamasaki R, Miyamoto S, Ohnishi A, Murakami T (2012) Complementary enantiorecognition patterns and specific method optimization aspects on immobilized polysaccharide-derived chiral stationary phases. J Chromatogr A 1269:178–188
Toussaint B, Duchateau AL, Vanderwal S, Albert A, Hubert P, Crommen J (2000) Determination of the enantiomers of 3-tert-butylamino-1,2-propanediol by high-performance liquid chromatography coupled to evaporative light scattering detection. J Chromatogr A 890:239–249
Tang Y (1996) Significance of mobile phase composition in enantioseparation of chiral drugs by HPLC on a cellulose-based chiral stationary phase. Chirality 8:136–142
Parajo JC, Alonso JL, Lage MA, Vazquez D (1992) Empirical modeling of eucalyptus wood processing. Bioprocess Eng 8:129–136
Suresh KR, Hariram B, Divya G, Srinivasu MK, Srinivas K, Sagyam RR (2012) Development of a RP-LC method for a diastereomeric drug valganciclovir hydrochloride by enhanced approach. J Pharm Biomed Anal 70:101–110
Grobelny P, Viola G, Vedaldi D, Dallacqua F, Gliszczynska S A, Mielcarek J (2009) Photo stability of pitavastatin—a novel HMG-CoA reductase inhibitor. J Pharm Biomed Anal 50:597–601
ICH (2005) Validation of analytical procedures: text and methodology Q2 (R1). ICH harmonised tripartite guideline. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. Accessed Nov 2005
Acknowledgments
The authors wish to thank Dr. L. Kalyanaraman, Dr. Vyas, and the management of Dr. Reddy’s Laboratories for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hariram, B., Kumar, R.S., Jayashree, A. et al. Development of Stereoselective Method for the Quantification of Stereoisomers and Geometrical Isomer of Pitavastatin Calcium by Enhanced Approach. Chromatographia 77, 901–912 (2014). https://doi.org/10.1007/s10337-014-2693-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2693-x