Abstract
A quantitative determination of six neuroactive amino acids (NAAs) was performed by capillary zone electrophoresis with amperometric detection (CZE-AD). This CZE-AD method utilized two electrolytes: the borate solution flowing in a capillary has the NAAs-separation effects, and the sodium hydroxide (NaOH) solution filled in the detection reservoir for the amperometric analysis of NAAs. The following experimental parameters were optimized: the working electrode potential, the pH value, the component, and the concentration of running buffer, the separation voltage, and the injection time on CZE-AD. Then, under the optimum conditions, the six NAAs could be completely separated in 30 min and had well-shaped AD responses at 0.75 V (versus SCE) on a copper electrode. The linear calibration range of NAAs was from 5 × 10−4 to 5 × 10−6 mol L−1 with the limits of detection (LODs) ranging from 10−6 to 10−7 mol L−1 (signal-to-noise ratio = 3), and the relative standard deviations (RSDs) of the migration time and peak area were 0.45–0.55 and 3.8–6.3 %, respectively. Moreover, this method has succeeded in human serum analysis, and the determined contents of the six NAAs in human serum were in an average recovery range of 85.3–117.9 %, which confirmed the validity and practicability of this method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As we know, there is an intensive focus on the NAAs studies, due to the important role of NAAs in the central nervous system [1–3]. The most studied NAAs are alanine (Ala), glutamic acid (Glu), aspartic acid (Asp), serine (Ser), taurine (Tau), and glycine (Gly). Asp and Glu are excitatory neurotransmitters within the central nervous system and responsible for normal synaptic neurotransmission [1]. Ala is a neurotransmitter in the optic nerve of the mammalian visual system [4]. Gly and Ala serve as an inhibitory neurotransmitter in the spinal cord and brainstem via the strychnine sensitive glycine receptor (GlyR) [5]. l-serine is the precursor for d-serine, which has a role of neurotransmitter/neuromodulator and also in neural development. Furthermore, it is also believed that d-serine is the endogenous obligatory co-agonist of the N-methyl-d-aspartate (NMDA) receptors in the human CNS [6, 7]. Tau is a mainly inhibitory neurotransmitter, membrane stabilizer, and modulator of intracellular calcium levels, and also acts as an agonist of GlyR [8]. These amino acids were proposed as having pathological roles in response to neurodegenerative conditions from Alzheimer’s disease [9], Parkinson’s disease [10], and epilepsy [11] to schizophrenia [12]. Their contents in human organisms could be considered as a biomarker to indicate the severity of these diseases.
Many methods have been developed for analyzing amino acids in biological fluids, such as fluorometry, enzymatic assay, high performance liquid chromatography (HPLC) [13], gas chromatography-mass spectrometry (GC–MS) [14, 15], and laser-induced fluorescence (LIF) [16]. Considering that these compounds lack natural chromophores or fluorophores, the amino acids cannot always be determined directly with photometric and fluorometric detection. Therefore, the most widely used methods for the analysis of amino acids has always depended on the pre- or post-column derivatization with ninhydrin, naphthalenedialdehyde (NDA), or o-phthalaldehyde (OPA) [17–19]. However, these derivatization methods of amino acids have various drawbacks, including complicated derivatization for sensitive detection, time-consuming for the sample cleaning process, and a higher background contribution [20].
Here, we introduce a method based on the separation of the different NAAs firstly with capillary zone electrophoresis (CZE) technology and then coupled with the amperometric detection (AD). As a liquid-phase separation technique, CZE has the advantages of short analysis time, ultra-small sample volume, simple experiment operation, high separation efficiency, and easy clearing of the contaminants [21]. Over the past two decades, various detection techniques have been developed for being coupled with CZE to determine amino acids, such as UV–visible absorbance [22], capacitively coupled contactless conductivity detection (C4D) [23], MS [24], AD [25], and LIF [26, 27]. Among these analytical detection methods, AD is the most promising compared to UV–vis and LIF detection, because AD is inexpensive in cost, provides good selectivity, and appropriate sensitivity.
An effective way to detect amines is to use metallic electrodes [28, 29], which have greater activity toward the amino group oxidation. Amino acids can be oxidized in strong alkaline media on transition metal electrodes such as Ni and Cu [25, 30] with the application of constant potential. NiO(OH) or CuO(OH) is formed and acts as electrocatalyst in different states, including Cu/Ni (I), Cu/Ni (II), and Cu/Ni (III) [31, 32]. The corresponding process on a copper disk electrode at a relatively high positive potential of 0.75 V is as follows [33, 34]:
By using our CZE-AD method, these six NAAs have been simultaneously detected without derivatization. Two main kinds of buffers were used in this proposed method, and the detection limits of NAAs were within 10−6 to 10−7 mol L−1. Following a simple sample preparation procedure, the detection of NAAs in human serum showed the practical applicability of this non-derivatization method. The proved method was simple and rapid for the analysis of NAAs, and the results suggested some potential to provide an effective way for early diagnosis of neurodegenerative diseases with abnormal NAAs concentrations.
Experimental
Chemicals
Glu, Asp, Ala, Gly, Ser, and Tau were purchased from Sinopharm Chemical Reagent, (Shanghai, China). Sodium tetraborate, phosphate, methanol, ethanol, and all the other chemicals were obtained from Shanghai First Reagent Factory (Shanghai, China), and all were of analytical reagent grade. Deionized water was supplied by a Milli-Q water purification system (Millipore, Milford, MA, USA).
All the stock solutions of the six analytes were prepared in deionized water with a concentration of 5 × 10−2 mol L−1, and diluted by running buffer. Before the CZE experiments, all solutions were filtered through a 0.22-μm polypropylene acrodisc syringe filter (Xinya Purification Instrument Factory, Shanghai, China) and sonicated for 5 min to remove bubbles.
Apparatus
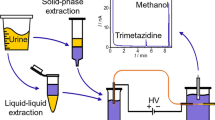
The home-built capillary electrophoresis system with wall-jet amperometric detection has been described in our previous research [31, 35]. Electrophoresis was driven by a high-voltage power supplier (±30 kV; Shanghai Institute of Nuclear Research, Shanghai, China). Separations were performed in a fused silica capillary (Yongnian Optical Fiber Factory, Hebei, China) with 25 μm inner diameter, 360 μm outer diameter, and 65 cm length. The pH value was monitored by using PHS-3C Acidometer (Shanghai Jicheng Instrument Factory, China). The electrochemical data were recorded by recorded by a CHI 660C electrochemical workstation (Shanghai Chen Hua Instrument, Shanghai, China). The three-electrode system is consisted of a copper-disk (300 μm diameter) working electrode, a saturated calomel reference electrode (SCE), and a platinum wire counter electrode. And the preparation of the copper-disk electrode has been introduced by our group [36, 37].
Electrophoresis Procedure
During the CZE-AD experiments, the three electrodes were placed in their corresponding holes of the electrochemical reservoir, and the copper-disk electrode has to be positioned accurately close to the capillary outlet with a three-dimensional locator. The copper-disk electrode was pre-treated with emery sand paper, and then sonicated in deionized water for 3–5 min. Additionally, the capillary was sequentially rinsed with 0.1 mol L−1 hydrochloric acid, deionized water, 0.1 mol L−1 NaOH, and then deionized water for 5 min, respectively, in a self-made washing system, subsequently with running buffer for 10 min to obtain a reproducible electroosmotic flow (EOF). After the first injection, the capillary was washed by these washing solutions for 2 min each successively between run cycles. All the experiments were performed at room temperature.
Preparation of Human Serum Samples
Blood samples from the healthy volunteers were collected between 0630 and 0730 hours in the infirmary of East China Normal University. Then, whole blood samples were centrifuged at 2,000 rpm for 15 min to obtain serum samples, and then stored at −20 °C until analysis. For the CZE-AD experiments, a 200 μl portion of serum sample was diluted with 400 μl of acetonitrile, and vortexed thoroughly for 5 min to precipitate out proteins. After centrifuging at 10,000 rpm for 15 min, the supernatant was transferred into a 1.5-ml vial and dried with a N2 stream [16, 38]. The supernatant fluid was stored at 4 °C for analysis and it was vortexed before injected into the CZE-AD system. Since the content of NAAs in the serum sample was at low micromolar level, and the sample was injected directly into capillary without further dilution.
Results and Discussion
Optimization of CZE-AD Parameters
Electrochemistry Experiments
Firstly, the cyclic voltammetric behaviors of NAAs were investigated in the NaOH solutions at a copper-disk working electrode (Fig. 1). Obviously, anodic peaks at about 0.65 V (vs. SCE) appears in the potential range from 0.20 to 0.90 V (red lines), while there was no anodic peak in the blank NaOH solution (black lines).
Amino acids can be electrochemically oxidized at a relatively moderate potential on the copper electrode. Fig. 2 illustrates the hydrodynamic voltammetries (HDVs) of the above analytes, which were obtained by monitoring the current responses with the applied potential varying from 0.55 to 0.75 V. Clearly, the increasing applied potential led to the stronger, current responses of these analytes. However, when the applied potential was greater than 0.75 V, the baseline noise was too large. In order to obtain signals with the highest signal-to-noise ratio, 0.75 V was selected as the most suitable detection potential in the following experiments.
Effect of pH Value and Buffer Concentration
The pH value of running buffer plays an important role in CZE as it affects on the zeta potential (ζ), the EOF, as well as the overall charge of the analytes, which influences the migration time and the resolution of the analytes. The running buffer with a series of concentrations in the pH range of 9.2–13.5 was introduced to obtain the optimum separation conditions. Firstly, NaOH solution (pH > 12) was employed in the CZE separation to give good response, because the amine groups of amino acids have a pKa between 9 and 10 [22]. Under strong alkaline conditions, the charge–mass ratio of amino acids has the maximized differences and CZE separation is always achieved due to the ionized analytes. With the concentration of the NaOH solution increasing from 20 to 100 mmol L−1, the resolution has no evident improvement. However, tailing peaks appeared and resulted in declined resolution and overlapping peaks. Furthermore, the increase of both the baseline noise and background current were attributed to Joule heating from NaOH solutions with high concentrations (>100 mmol L−1).
The borate buffer system has previously been known as a suitable medium for amino acids separation. The influence of its pH value was also investigated in the range of 9.2–12.5. The current was very small with pH arranging from 9.2 to 11. Once the value was larger than 11, the signal intensity increased obviously, but the analytes’ resolution was deteriorated.
The borate solution was chosen as running buffer in the capillary for separation, while NaOH solution just filled into the detection reservoir for amperometric analysis. With the increase of borate concentrations (20–100 mmol L−1), the resolution became better, but at the same time the migration time was prolonged. When the concentration was higher than 50 mmol L−1, the six NAAs reached baseline separation in borate solution. However, when borate concentration exceeded 100 mmol L−1, the effect of Joule heat in the capillary was elevated, which is associated with some negative effects, such as decreased detection sensitivity with an increase in baseline noise. Moreover, when the ionic strength of the buffer in the capillary was lower than the detection reservoir, the baseline was unstable and the current in the capillary raised, because the NaOH solution back-filled into the capillary.
Finally, in considering the resolution, migration time, sensitivity, and stability, 50 mmol L−1 borate solution (pH = 9.2) was selected as the optimum separation medium and 50 mmol L−1 NaOH solution (pH = 12.6) filled into the detection reservoir for the amperometric determination.
Effect of Separation Voltage and Injection Time
For a given capillary length, the separation voltage determines the electric field strength, which affects both the velocity of EOF and the migration velocity of the analytes. The separation efficiency of CZE was investigated in the separation voltage range from 10 to 20 kV, as shown in Fig. 3. As expected, the analytes had a shorter migration time with higher separation voltage. However, when the separation voltage exceeded 20 kV, the baseline noise increased continuously. Finally, 15 kV was selected as the optimum separation voltage, at which a good separation could be achieved for all analytes within 30 min.
Correlation between the separation voltage and the migration time of the analytes: 5 × 10−5 mol L−1 Ala, Gly and Ser, and 2.5 × 10−4 mol L−1 Tau, Glu, and Asp. Running buffer: 50 mmol L−1 borate buffer (pH = 9.2); Electrokinetic injection: 10 s. Error bars are not shown, since they are smaller than the figure symbols
The injection time determines the amount of sampling and affects both the peak current and the peak shape. The influence of injection time was studied by varying the injection time from 4 to 14 s at 15 kV (Fig. 4). It can be seen that the peak current increased with the increasing of injection time. When the injection time was longer than 12 s, the peak heights of the analytes nearly leveled off and peak broadening became more severe. Therefore, 10 s (at 15 kV) was selected as the optimum injection time.
In summary, under the optimal conditions, the six NAAs achieved a very good baseline separation within 30 min, as shown in Fig. 5.
Electrophoregrams of a standard solution containing 5 × 10−5 mol L−1 (a) Ala, (b) Gly and (c) Ser, and 2.5 × 10−4 mol L−1 (d)Tau, (e) Glu and (f) Asp. Detection potential: 0.75 V (vs. SCE); fused-silica capillary: 25 μm i.d. × 65 cm; running buffer: 50 mmol L−1 borate buffer (pH = 9.2); electrokinetic injection: 10 s (at 15 kV)
Method Validation
Linearity, Detection Limits and Reproducibility
To explore the linearity of the analytes, NAAs with a series of concentrations ranging from 5 × 10−4 to 5 × 10−6 mol L−1 were determined. Regression analysis was used to obtain the correlation between peak area and concentration of each analyte. The linearity ranges, regression equations, correlation coefficients (R 2), RSDs, and detection limits are summarized in Table 1, in which the detection limit is mainly determined by the 3 sb/m criterion, where m is the slope of the calibration curve and sb is the standard deviation [39]. The results showed that an excellent linearity between peak area and concentration of each analyte was gained. Moreover, the results are repeatable, indicating that our method has good reproducibility.
Real Sample Analysis and Recovery
The determination of NAAs in the healthy human serum samples were performed with this CZE-AD method. Compared to traditional techniques, the sample preparation for this method was considerably shorter, less laborious, and more cost-efficient, and required 200 μl of urine at most, as mentioned in the section on sample preparation.
The electropherograms of the healthy human serum (a) and spiked (b) samples are shown in Fig. 6. Because some of these NAAs were at trace level, the standard addition method, explained in Fig. 6b, was applied to distinguish the signals of the target analytes. There is no doubt that all the six analytes were determined (Table 2), in spite of some coexisting interference substances in human serum samples, such as amino acids, organic acids, and carbohydrates, showing that the sensitivity of this method can meet the needs of real sample analysis. Average recovery of the six analytes in serum samples was in the range of 85.3–117.9 %. The determined contents of NAAs in the healthy human serum samples were consistent with literature values [40], except Gly. Its basal level was 675.8 ± 38 μM, which is more than the reported value, 248 ± 13 μM. The unexpected high level of Gly is probably caused by the interference of some electroactive substances.
Typical electropherograms of serum (a) and spiked (b) samples from healthy volunteers obtained with CZE-AD. Experimental conditions and peak labels were the same as those in Fig. 5
Concluding Remarks
A simple and convenient CZE-AD method for the determination of NAAs in the healthy human serum samples has been developed by using two kinds of electrolytes. Within 30 min, the six analytes were separated completely, and the detection limits of NAAs are in the range of 5.5 × 10−7 to 2.8 × 10−6 mol L−1 (signal-to-noise ratio = 3). The results illustrate that this CZE-AD method succeed in the identification and quantification of NAAs. Additionally, the results suggested some potential alternative methodologies for the prevention and treatment of neurodegenerative diseases when statistical data can be obtained. Further work will focus on the improvement of the sensitivity through online preconcentration techniques, and quantitative determination of total amino acids, as well as the extension of this method to other biological fluids.
References
Curtis, Johnston GA (1974) Ergeb Physiol, Biol Chem Exp Pharmakol 69:97–188. doi:10.1007/3-540-06498-2-3
Perry M, Li Q, Kennedy RT (2009) Anal Chim Acta 653(1):1–22. doi:10.1016/j.aca.2009.08.038
Poinsot V, Gavard P, Feurer B, Couderc F (2010) Electrophoresis 31(1):105–121. doi:10.1002/elps.200900399
Tiedje KE, Stevens K, Barnes S, Weaver DF (2010) Neurochem Int 57:177–188. doi:10.1016/j.neuint.2010.06.001
Zinellu A, Sotgia S, Deiana L, Carru C (2013) Methods Mol Biol 919:35–42. doi:10.1007/978-1-62703-029-8-4
Fuchs SA (2010) d-serine in health and disease. Utrecht University, Utrecht
Wolosker H (2007) Mol Neurobiol 36(2):152–164. doi:10.1007/s12035-007-0038-6
Zinellu A, Sotgia S, Bastianina S, Chessa R, Gaspa L, Franconi F, Deiana L, Carru C (2009) Amino Acids 36(1):35–41. doi:10.1007/s00726-007-0022-5
Paik M-J, Cho I-S, Mook-Jung I, Lee G, Kim K-R (2008) BMB Rep. 41(1):23–28. doi:10.5483/BMBRep.2008.41.1.023
Engelborghs S, Marescau B, De Deyn PP (2003) Neurochem Res 28(8):1145–1150. doi:10.1023/A:1024255208563
Kirchner A, Breustedt J, Rosche B, Heinemann UF, Schmieden V (2003) Epilepsia 44(9):1145–1152. doi:10.1046/j.1528-1157.2003.01603.x
Labrie V, Wong AHC, Roder JC (2012) Neuropharmacology 62(3):1484–1503. doi:10.1016/j.neuropharm.2011.01.030
Han H, Miyoshi Y, Ueno K, Okamura C, Tojo Y, Mita M, Lindner W, Zaitsu K, Hamase K (2011) J Chromatogr B 879(29):3196–3202. doi:10.1016/j.jchromb.2011.01.023
Fuchs SA, de Sain-van der Velden MGM, de Barse MMJ, Roeleveld MW, Hendriks M, Dorland L, Klomp LWJ, Berger R, De Koning TJ (2008) Clin Chem 54(9):1443–1450. doi:10.1373/clinchem.2007.100412
Patzold R, Schieber A, Bruckner H (2005) Biomed Chromatogr 19(6):466–473. doi:10.1002/bmc.515
Zinellu A, Sotgia S, Pisanu E, Scanu B, Sanna M, Usai MF, Chessa R, Deiana L, Carru C (2010) Anal Bioanal Chem 398(5):1973–1978. doi:10.1007/s00216-010-4134-5
Friedman M (2004) J Agric Food Chem 52(3):385–406. doi:10.1021/jf030490p
Roach MC, Harmony MD (1987) Anal Chem 59(3):411–415. doi:10.1021/ac00130a007
Lindroth P, Mopper K (1979) Anal Chem 51(11):1667–1674. doi:10.1021/ac50047a019
Casella IG, Gatta M (2002) J Agric Food Chem 50(1):23–28. doi:10.1021/jf010557d
Liu Z, Niwa O, Kurita R, Horiuchi T (2000) J Chromatogr A 891(1):149–156. doi:10.1016/S0021-9673(00)00632-4
Henchoz Y, Schappler J, Geiser L, Prat J, Carrupt P-A, Veuthey J-L (2007) Anal Bioanal Chem 389(6):1869–1878. doi:10.1007/s00216-007-1568-5
Strieglerová L, Kubáň P, Boček P (2011) J Chromatogr A 1218(37):6248–6255. doi:10.1016/j.chroma.2011.07.011
Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW (2011) J Chromatogr A 1218:7130–7136. doi:10.1016/j.chroma.2011.07.087
Ye J, Baldwin RP (1994) Anal Chem 66(17):2669–2674. doi:10.1021/ac00089a012
Klinker CC, Bowser MT (2007) Anal Chem 79(22):8747–8754. doi:10.1021/ac071433o
Denoroy L, Parrot S, Renaud L, Renaud B, Zimmer L (2008) J Chromatogr A 1205(1–2):144–149. doi:10.1016/j.chroma.2008.07.043
Welch LE, LaCourse WR, Mead DA Jr, Johnson DC, Hu T (1989) Anal Chem 61(6):555–559. doi:10.1021/ac00181a011
Johll ME, Williams DG, Johnson DC (1997) Electroanalysis 9(18):1397–1402. doi:10.1002/elan.1140091805
Luo P, Zhang F, Baldwin RP (1991) Anal Chem 63(17):1702–1707. doi:10.1021/ac00017a010
Dong S, Zhang S, Chi L, He P, Wang Q, Fang Y (2008) Anal Biochem 381(2):199–204. doi:10.1016/j.ab.2008.05.011
Xie Y, Huber CO (1991) Anal Chem 63(17):1714–1719. doi:10.1021/ac00017a012
Hampson N, Lee J, Macdonald K (1972) J Electroanal Chem 34(1):91–99. doi:10.1016/S0022-0728(72)80505-9
Mho S, Johnson DC (2001) J Electroanal Chem 495(2):152–159. doi:10.1016/S0022-0728(00)00417-4
Wang Q, Ding F, Zhu N, Li H, He P, Fang Y (2003) J Chromatogr A 1016(1):123–128. doi:10.1016/S0021-9673(03)01294-9
Dong S, Chi L, Zhang S, He P, Wang Q, Fang Y (2008) Anal Bioanal Chem 391(2):653–659. doi:10.1007/s00216-008-2053-5
Yang Z, Wang H, Zhang W, Wang Q, He P, Fang Y (2012) Chromatographia 75:297–304. doi:10.1007/s10337-012-2197-5
Zhao S, Lan X, Liu Y-M (2009) Electrophoresis 30(15):2676–2680. doi:10.1002/elps.200900115
Chicharro M, Sanchez A, Zapardiel A, Rubianes M, Rivas G (2004) Anal Chim Acta 523(2):185–191. doi:10.1016/j.aca.2004.07.039
Bergström J, Stehle P, Fürst P (1997) Clin Nutr 16(6):299–305. doi:10.1016/S0261-5614(97)80015-5
Acknowledgments
This work was financially supported by the Program New Century Excellent Talents in University (Grant NCET-08-0191) and the National Program on the Development of Scientific Instrument and Equipment (Grant 2011YQ150072).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, S., Wang, H., Wang, Z. et al. Simultaneous Determination of Neuroactive Amino Acids in Serum by CZE Coupled with Amperometric Detection. Chromatographia 76, 149–155 (2013). https://doi.org/10.1007/s10337-012-2378-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-012-2378-2