Abstract
Chiral analysis of β-adrenergic blockers in human plasma is an important research area due to their different pharmaceutical activities. The solid phase extraction of alprenolol, carazolol, metoprolol, oxprenolol from human plasma was achieved on C18 cartridges using phosphate buffer (50 mM, pH 9.0) followed by elution with methanol containing 0.1% acetic acid. Chiral-HPLC was carried out on CelluCoat (250 × 4.6 mm, 5.0 μm silica particle size) column using different combinations of n-heptane–ethanol–diethylamine at 1.0 and 2.0 mL min−1 flow rates, respectively. The detection was achieved at 225 nm with 27 ± 1 °C as working temperature. The chromatographic parameters, i.e., retention (k), separation (α) and resolution (Rs) factors ranged from 0.26 to 10.64, 1.12 to 2.10 and 1.05 to 2.26, respectively. The binding differences of enantiomeric concentrations of oxprenolol, carazolol, alprenolol, and metoprolol were 0.12, 0.08, 0.05, and 0.01, respectively. These values suggest that their activities may be in the order of oxprenolol > carazolol > alprenolol > metoprolol. The reported SPE-Chiral HPLC methods may be used to study the enantiomeric concentrations of these β-adrenergic blockers in human and other animal plasmas for further studies.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
β-Adrenergic blockers are the preferred drugs for various cardio-vascular diseases such as hypertension, angina pectoris, cardiac arrhythmias, etc. [1] but, unfortunately, these occur in racemic forms [2]. It is well known that S-(−)-enantiomers of these drugs are about 50–500 times pharmaceutically more active than their antipodes, which act as ballast or other medication; leading to various side effects and, sometimes, toxicities [3–7]. It has been reported that a prolonged use of these drugs (racemic mixtures) might cause diabetes in hypertensive patients [8–10]. The various pharmaceutical activities of enantiomers are due to their different stereo-selective bindings with β-receptor sites of human plasma proteins [11]. Due to these serious side effects, the whole world is worried and scientists are working to develop optically active pure drugs. Therefore, US FDA [12], European Committee for Proprietary Medicinal Products [13], Health Canada [14] and Pharmaceutical and Medical Devices Agencies of Japan [15] have banned the marketing of all racemic drugs including β-adrenergic blockers. As mentioned above, the inactive antipodes of β-adrenergic blockers behave like other medications showing different toxicities and serious side effects. It is very important to analyze their concentrations in human plasma to control the unwanted side effects. Besides, chiral analysis of β-adrenergic blockers in human plasma is also important for enantiomeric pharmacokinetic and pharmacodynamic studies. Among various analytical techniques chiral high performance liquid chromatography (HPLC) is considered as the best one for enantiomeric analysis of various racemic drugs [13]. Literature survey indicates few papers on chiral analysis of some drugs in human plasma as reviewed by Chamsaz et al. [16]. Nowadays, solid phase extraction (SPE) is considered as an ideal technique for the extraction of drugs from human plasma as about 80% chromatographers are using this approach [17]. In spite of this, literature survey indicates only one paper describing SPE-Chiral HPLC method for analyses of metoprolol in human plasma [18]. Briefly, the development of SPE-Chiral HPLC method is the need of today for monitoring the concentrations of each enantiomer of β-adrenergic blockers. In view of these facts, attempts have been made to develop and validate SPE-Chiral HPLC method for analysis of enantiomers of some β-adrenergic blockers (alprenolol, carazolol, metoprolol, and oxprenolol). Efforts have also been made to calculate the binding concentrations of each enantiomer with human plasma proteins. The results of this study are presented herein.
Experimental
Chemicals, Reagents and Instruments
β-Adrenergic blockers (alprenolol, carazolol, metoprolol, and oxprenolol) were purchased from Sigma Chemical Co., Milky Way, USA. Methanol, ethanol, n-heptane, diethylamine of HPLC grade, and ammonium chloride were supplied by Merck, Mumbai, India. Glacial acetic acid and ammonium hydroxide solution (30% NH3) were obtained from Qualigens, Mumbai, India. Frozen Human plasma (Mfg. License No. 504) was purchased from Rotary Blood Bank, New Delhi, India. The standard solutions (0.10 mg mL−1) of β-adrenergic blockers were prepared in methanol. Purified water was prepared using a Millipore Milli-Q (Bedford, MA, USA) water purification system. The HPLC system used was ECOM (Prague, Czech Republic) consisting of solvent delivery pump (model Alpha 10), manual injector, absorbance detector (Sapphire 600 UV–vis), chromatography I/F module data integrator (Indtech. Instrument, Bombay, India) and Winchrome software. The chiral column used was CelluCoat (250 × 4.6 mm, 5.0 μm silica particle size); Kromasil, Bohus Sweden. SPE unit was purchased from Varian USA. C18 cartridges were obtained from Waters, Milky Way, USA. pH meter of Control Dynamics (model APX 175 E/C) and centrifuge of Remi (model C-30BL) were used.
Solid Phase Extraction
To study the interactions of β-adrenergic blockers with human plasma protein, 1.0 mL (1.0 mg mL−1) of each β-adrenergic blocker was mixed with 5.0 mL fresh frozen Human plasma, separately and, respectively. The spiked plasma samples were vortexed for 2.0 min, kept for 30.0 min, mixed with 15.0 mL acetone and centrifuged at 10,000 rpm (11,180g) for 10 min to separate the supernatant. The supernatant was evaporated to dryness and the residue was re-dissolved in 10.0 mL ammonia buffer (50 mM, pH 9.0). Sep-Pak C18 cartridges (1.0 mL Waters, Milky Way, USA) were pre-conditioned with 2.0 mL methanol followed by 5.0 mL Millipore water, separately and, respectively. About 10 mL ammonia buffer (50 mM, pH 9.0); having β-adrenergic blockers; was passed through the cartridges with 0.1 mL min−1 flow rate, followed by cartridge washing with 2.0 mL Millipore water at same flow rate. The cartridges were dried by passing hot air and β-adrenergic blockers were eluted by 10.0 mL methanol containing 0.1% acetic acid at 0.1 mL min−1 flow rate. The eluted methanol solutions for each β-adrenergic blocker were concentrated under vacuum to 0.1 mL, separately and, respectively. These samples were used for Chiral-HPLC analyses.
Chiral-HPLC
All the experiments were carried out by Chiral-HPLC system as described above. The aliquots of 5.0 μL standard solutions of each β-adrenergic blocker (0.10 mg mL−1 in methanol) were loaded on Chiral-HPLC machine, separately and, respectively. The mobile phases were prepared and degassed daily before use. All the experiments were carried out at 27 ± 1 °C temperature with detection at 225 nm. The chromatographic parameters such as retention (k), separation (α) and resolution (Rs) factors were calculated. The SPE extracted samples of β-adrenergic blockers were also analyzed as in case of standard solutions. The orders of elution of the enantiomers were determined using optically active pure form of each β-adrenergic blocker. The qualitative and quantitative chiral analyses were carried out using retention times and peak areas, respectively. The results of quantitative analyses were used to determine the amount of β-adrenergic blockers bounded with human plasma proteins. Chromatographic method was optimized by carrying out an extensive experimentation. The final optimized Chiral-HPLC conditions are given in Table 1.
Result and Discussion
Solid Phase Extraction
β-Adrenergic blockers were mixed with human plasma to carry out their biological interactions with β-adrenergic receptor sites. Some amounts of β-adrenergic blockers were reacted with proteins while the remaining amounts were extracted from plasma by SPE. The optimization of SPE was achieved by different concentrations and pHs of ammonia buffer, flow rate of plasma sample and eluting solvents. The best experimental conditions for ammonia buffer were 50.0 mM, pH 9.0, and 0.10 mL min−1 as flow rate. The elution of β-adrenergic blockers from C18 was achieved using various solvents such as methanol, ethanol, dichloromethane, ethyl acetate, and acetone. These solvents were used as pure or containing different amounts of hydrochloric acid or acetic acid or trifluroacetic acid. As a result of an extensive experimentation the best eluting solvent was methanol containing 0.1% acetic acid for all β-adrenergic blockers; with 0.1 mL min−1 flow rate. The percentage recoveries and binding with human plasma proteins of alprenolol, carazolol, metoprolol, and oxprenolol β-adrenergic blockers were calculated and given in Table 2.
Chromatography
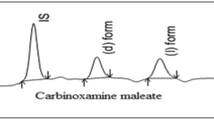
The Chiral-HPLC parameters such as capacity (k), separation (α) and resolution (Rs) factors for the enantiomeric separation of β-adrenergic blockers (alprenolol, carazolol, metoprolol, and oxprenolol) were calculated and given in Table 3. All (R)-(+)-enantiomers eluted first followed by S-(−)-enantiomers except in carazolol. The typical chromatograms of enantiomers of alprenolol, carazolol, metoprolol, and oxprenolol are shown in Figs. 1 and 2 for standard and extracted from human plasma samples, respectively. A perusal of Table 3 and Figs. 1 and 2 clearly indicates a good base lined chiral separations of β-adrenergic blockers. To optimize the chromatographic conditions, various combinations of alkane, alcohols and some additives were tested and the optimized chromatographic conditions were developed and reported herein. The values of separation and resolution factors are >1.0 indicating the complete resolution; with sharp peaks of all β-adrenergic blockers. The limit of detection of all β-adrenergic blockers were calculated and ranged from 0.10 to 0.12 μg L−1. On the other hand, the limit of quantification ranged from 0.10 to 0.13 μg L−1 for the reported β-adrenergic blockers.
All β-adrenergic blockers were separated using 1.0 mL min−1 flow rate except carazolol, which was resolved using 2.0 mL min−1 flow rates indicating strong bindings of carazolol with the CelluCoat column. Furthermore, the maximum (Rs = 2.26) and minimum (Rs = 1.05) resolutions of oxprenolol and metoprolol were achieved at 1.0 mL min−1 flow rate, respectively. It is due to high enantio-selective bondings of oxprenolol enantiomers in comparison to metoprolol bonding. These results can be explained on the basis of the chiral recognition mechanisms as described by various workers [1, 19, 20]. Basically, cellulose has well defined grooves providing the chiral surface to the enantiomers. The different retention of enantiomers is controlled by π–π interactions, hydrogen bondings, dipole-induced dipole interactions, and steric effects. The order of elution of β-adrenergic blockers was carazolol > oxprenolol > metoprolol > alprenolol. This can be explained on the basis of different bondings of β-adrenergic blockers with tris-3,5-(dimethylphenylcarbamate) cellulose CSP at supera-molecular levels. A look of the structures of β-adrenergic blockers (Fig. 3) shows, three aromatic rings with two oxygen atoms and one extra amine group in carazolol, one aromatic ring with three oxygen atoms in oxpranolol and metopralol and one aromatic ring with two oxygen atoms in alprenolol. The above-mentioned order of elution of these β-adrenergic blockers can be explained by considering π–π interactions and hydrogen bondings as the significant interactions. Carazolol is eluted late due to strong π–π bondings (three aromatic rings) and strong hydrogen bonding owing to one extra amine group in comparison to others. Oxpronalol and metopralol both have same aromatic rings and number of oxygen atom but metoprolol is eluted first. It may be due to poor aromaticity of metoprolol due to the attachment of oxygen atoms on both side of aromatic ring; making weak π–π bondings in comparison to oxpranolol. Alpranolol eluted first due to poor π–π interactions and hydrogen bonding because of the presence of one aromatic ring and only two oxygen atoms. The different structures of β-adrenergic blockers are responsible for their elution at different retention times. Moreover, the above-cited explanation indicates that the separation is controlled by mainly π–π interactions and hydrogen bondings. Besides, other forces such as van der Waals forces, dipole-induced dipole attractions and steric effects are also playing quite good role in the chiral separations [13]. Briefly, CSP grooves provide the chiral environment to β-adrenergic blockers in which enantiomers get fitted in stereo-selective fashion. The binding stabilities of enantiomers on this chiral environment are provided by the above-cited forces and interactions. As a result of combined effect bindings and mobile flow pressure enantiomers get separated under the reported Chiral-HPLC conditions.
Binding of β-Adrenergic Blockers with Human Plasma Proteins
It is well known that both the enantiomers of β-adrenergic blockers show different pharmaceutical activities due to their stereo-selective binding with human plasma proteins [18, 21]. Therefore, efforts were made to determine the binding concentrations of the reported β-adrenergic blockers, which are given in Table 2. The binding concentration differences of S-(−) and R-(+) enantiomers of oxprenolol, carazolol, alprenolol, and metoprolol are 0.12, 0.08, 0.05, and 0.01, respectively. Therefore, it may be assumed that the order of their activities may be oxprenolol > carazolol > alprenolol > metoprolol. The above-described order of their activities is due to non-selective nature and intrinsic sympathomimetic activity of oxprenolol and only non-selective nature of alprenolol. Contrarily, metoprolol is of least activity owing to its β1 selective nature. Therefore, oxprenolol is the most active β-adrenergic blocker among the reported ones.
Validation of the Methods
The developed SPE and Chiral-HPLC methods were validated by carrying out five sets (n = 5) of the extraction and chromatographic procedures under identical experimental conditions. It is interesting to note that the values of chromatographic parameters for β-adrenergic blockers were almost equal when analyzing standard solutions and plasma extracts, which indicates the robustness of the method. The validation of the developed method was ascertained by regression analysis using Excel Microsoft program. The values of SD, CC, and CL for SPE experiments were ±0.06, 0.9998, and 98.5, respectively. On the other hand, the values of standard deviation (SD), correlation coefficient (CC) and confidence limit (CL) vary from ±0.06, 0.9999, and 99.6 for Chiral-HPLC, respectively. The validation data values are given in Table 4.
Conclusion
The results presented in this paper indicate that the reported SPE-Chiral HPLC methods are effective, efficient, and reproducible. The values of separation and resolution factors are >1.0 indicating a good resolution. The different binding concentrations of the enantiomers of oxprenolol, carazolol, alprenolol, and metoprolol with human plasma proteins confirm their different pharmaceutical activities. The limits of detection are quite good and no extra peak appears in the Chiral-HPLC chromatograms, showing the selective nature of SPE method. It is the first paper describing chiral analyses of oxprenolol, carazolol, alprenolol, and metoprolol in human plasma, which may be useful in enantiomeric pharmacokinetic and dynamic studies and chiral drug development (β-adrenergic blockers). Briefly, the described SPE-Chiral HPLC methods may be used to ascertain the enantiomeric concentration ratio of the reported β-adrenergic blockers in human and other animal plasmas.
References
Ali I, Gaitonde VD, Aboul-Eneinc HY, Hussaina A (2009) Talanta 78:458–463
Meyers EH, Jawetz E, Goldheim AA (1980) Review of medical pharmacology. Lange Medical Publication, USA
Walle T, Walle UK, Wilson MJ, Fagan TC, Gaffney TE (1984) Br J Clin Pharmacol 18:741–748
Schmid MG, Gecse O, Szabo Z, Kilar F, Gubitz G, Ali I (2001) J Liq Chromatogr Relat Technol 24:2493–2504
Lee EJ, William KM (1990) Clin Pharmacokinet 18:339–345
Nelson WL, Burke TR (1978) Res Commun Chem Pathol Pharmacol 21:77–85
Barrett AM, Cullum VA (1968) J Pharmacol 20:911–915
Balmer K, Lagerstrom PO, Persson BA, Schill G (1992) J Chromatogr A 592:331–337
Ram CV (2008) Am J Cardiol 102:242–244
Belknap S (2008) Evid Based Med 13:50
Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SGF, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis W, Kobilka BK, Stevens RC (2007) Science 318:1258–1265
FDA policy statement for the development of new stereoisomeric drugs, Publication date: 05/01/1992
Aboul-Enein HY, Ali I (2003) Chiral separation by liquid chromatography and related technologies. Marcel Dekker, USA
Health Canada (2000) stereochemical issues in chiral drug development. Health Canada guidance documents, http://www.hc-sc.gc.ca
Shimazawa R, Nagai N, Toyoshima S, Okuda H (2008) J Health Sci 54:23–29
Chamsaz M, Asadpour S, Yazdi AS, Ghasemi J (2009) J Iran Chem Res 2:1–21
Ali I, Gupta VK, Aboul-Enein HY, Hussain A (2008) J Sep Sci 31:2040–2053
Cerqueira PM, Cesarino EJ, Bertucci C, Bonato PS, Lanchote VL (2003) Chirality 15:542–549
Okamoto Y, Yashima E (1998) Angew Chem Int 37:1020–1043
Ali I, Kummerer K, Aboul-Enein HY (2006) Chromatographia 63:295–307
Albani F, Riva R, Contin M, Baruzzi A (1984) Br J Clin Pharmacol 18:244–246
Acknowledgments
The authors are thankful to University Grant Commission, New Delhi for providing fellowship to one of the authors (A.H.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ali, I., Al-Othman, Z.A., Hussain, A. et al. Chiral Separation of β-Adrenergic Blockers in Human Plasma by SPE-HPLC. Chromatographia 73, 251–256 (2011). https://doi.org/10.1007/s10337-010-1891-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-010-1891-4