Abstract
Increasing rates of nest predation is one of several hypotheses proposed to explain observed declines of Wood Warbler Phylloscopus sibilatrix populations in the UK. Nest predation may be influenced by how nest concealment is affected by vegetation structure, which may vary between breeding sites. Detailed nest monitoring within three study areas with varying population trends identified the main nest predators and predation as the main cause of nest failure. Comparison of habitat structure surrounding nests with non-nest locations within territories showed that nests were placed on steeper slopes, in areas with a taller canopy, intermediate understorey density, greater cover of bramble and intermediate cover of tall field layer vegetation. Predation rates were related to field layer structure with lower predation rates associated with greater cover of medium-height vegetation. However, the size of the effect of vegetation on predation rates was small, and vegetation management is unlikely to have a strong impact on predation rates except in extreme cases of very low ground cover.
Zusammenfassung
Nestraub und der Einfluss der Habitatstruktur auf die Nestprädation beim Waldlaubsänger Phylloscopus sibilatrix , einem bodenbrütenden Waldsingvogel
Ein Anstieg der Nestprädationsraten ist eine von mehreren Hypothesen, mit denen versucht wird, den zu beobachtenden Populationsrückgang beim Waldlaubsänger Phylloscopus sibilatrix im Vereinigten Königreich zu erklären. Die Nestprädation kann dadurch beeinflusst werden, inwieweit die Vegetationsstruktur, welche sich von Neststandort zu Neststandort unterscheidet, Auswirkungen auf die Nesttarnung hat. Durch ausführliches Nestmonitoring in drei Untersuchungsgebieten mit verschiedenen Populationstrends konnten die primären Nestprädatoren ermittelt und Nestraub als die Hauptursache für Brutverluste ausgemacht werden. Durch den Vergleich der Habitatstruktur in Nestumgebung mit der an Stellen ohne Nester innerhalb der Reviere zeigte sich, dass sich Nester an steileren Hängen befanden, in Gegenden mit höherem Kronendach, mittlerer Unterwuchsdichte, stärkerem Bewuchs mit Brombeere und einem mittleren Bedeckungsgrad durch hohe krautige Vegetation. Die Prädationsraten waren von der Krautschicht abhängig, insofern als niedrigere Prädationsraten in Verbindung mit einem stärkeren Bewuchs durch Pflanzen mittlerer Höhe auftraten. Allerdings war das Ausmaß des Vegetationseinflusses auf die Prädation nur schwach, und das Vegetationsmanagement wird außer in extremen Fällen mit sehr geringer Bodenbedeckung kaum eine starke Auswirkung auf die Prädationsraten haben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nest predation is one of the main causes of breeding failure in birds, and has been implicated in the population declines of a wide range of mainly ground-nesting birds [e.g. wading birds (Macdonald and Bolton 2008); forest songbirds (Schmidt 2003); game birds (Tapper et al. 1996)]. Increased predation rates are often attributed to landscape and habitat modification (e.g. Fuller and Gough 1999; Lahti 2001; Martin and Joron 2003). Landscape modification, particularly forest fragmentation, can lead to increases in generalist predators that feed in multiple habitats, and an increase in the proportion of forest edge may make some shrub-nesting species vulnerable to increased predator activity along woodland edges (Lahti 2001). Habitat modification effects are normally associated with the simplification of habitat structure associated with anthropogenic land management, resulting in less well-concealed nests that are more prone to predation (e.g. Eggers et al. 2005; and reviewed in Evans 2004).
Nest site-selection theory suggests that nest sites are selected to minimise nest-predation risks and that nest placement will be a reflection of local vegetation structure in relation to the pattern and availability of nest sites within a habitat. This may be based on characteristics of vegetation at a small scale, but in the context of variation in structure at larger scales (Martin 1993). Nest site selection can also vary in response to local predator abundance in situations where predation risk can be predicted and assessed prior to nesting (Forstmeier and Weiss 2004; Roos and Pärt 2004).
The placement of nests in many species, especially open- and ground-nesting species, is often a trade-off between concealment to avoid detection by predators and providing open views to detect approaching predators in order to either freeze, flee or defend the nest (Wiebe and Martin 1998). Both greater vegetation density and heterogeneity may increase nest crypsis and thus reduce nest-predation rates, with optimal nest site selection seeking small-scale dense vegetation in an area of patchy vegetation density (reviewed by Newton 1998; Willson et al. 2001). The selection for nest concealment is largely driven by predators that hunt using visual clues, which include most avian predators of nests. This is most effective at the egg stage; during the chick stage predators can also use chick begging calls and adult activity to locate nests. Where a large proportion of the predator community uses heat or scent to search, such as some mammals and reptiles, there is weaker selection for nest concealment (Braden 1999; Rangen et al. 1999).
A suggested cause of many observed forest bird population declines in the UK is a change in habitat structure arising from changes in management or grazing, through altering habitat quality and suitability for territory settlement (Fuller et al. 2005). However, the scale of observed forest habitat change does not consistently predict population trends (Mallord et al. 2016). One such declining forest bird is the Wood Warbler, a small Afro-Palaearctic migrant distributed in the non-breeding season in west and central Africa, and breeding across much of temperate Europe. Its breeding habitat comprises mature, closed canopy broadleaved or mixed woodland, where birds nest on the woodland floor and forage for invertebrates in the canopy (Mallord et al. 2012a; Huber et al. 2016; Pasinelli et al. 2016). The species is mainly single brooded, but if predated early in the nesting cycle, birds will re-nest in a new location. Populations of Wood Warblers have been declining across northwest Europe (− 33%), including the UK (− 56%) since the mid-1990s (Gregory et al. 2007; Harris et al. 2016). The species has declined across the UK, but declines in abundance and distribution are more apparent in the south and southwest of the UK breeding range (Amar et al. 2006; Balmer et al. 2013). In common with other ground-nesting species, nest predation is the main cause of breeding failure in Wood Warblers (Wesołowski and Maziarz 2009; Mallord et al. 2012b; Grendelmeier et al. 2015). A wide range of predators, including birds, mammals and reptiles, have been recorded taking chicks and eggs; on continental Europe predation is mostly by mammals, and in the UK mostly by avian, visual hunting predators such as Eurasian Jay Garrulus glandarius and Common Buzzard Buteo buteo (Mallord et al. 2012b; Grendelmeier et al. 2015).
In this paper we investigate, from three UK study areas, fine-scale nest site selection within Wood Warbler territories to determine whether habitat structure at nest sites influences predation rates. Factors relating to nest concealment have a stronger influence on nest survival compared with factors relating to weather, tree structure, topography and predation risk (Pasinelli et al. 2016). If vegetation structure has a strong influence on nest predation, this can have implications for managing habitats to encourage structures likely to reduce nest predation.
We therefore tested the hypothesis that Wood Warblers select nest sites with habitat structure that minimises the probability of nest predation.
Methods
Study areas
This study was carried out in three study areas in the UK between 2009 and 2013: mid-Wales (2009–2011), Dartmoor in southwest England (2012–2013) and the New Forest in southern England (2011–2013). All study areas were within well-wooded landscapes. The mid-Wales study area (19 woods, largest 42.5 ha) is mostly marginal grazed upland with scattered remnants of native woodland and larger blocks of plantation on hill slopes. The Dartmoor study area consisted of 30 woods along wooded river valleys within a mainly grazed open upland landscape (954 km2) in which all suitable woodland was searched. Most of the Dartmoor woodlands were located along three river valleys with continuous woodland for 5–11 km. The New Forest study area is a largely continuous woodland block with some lowland heath areas (11 km2) in a landscape (566 km2) of low-intensity management, i.e. mainly extensive grazing and forestry, with 41% woodland cover. In Dartmoor and mid-Wales, the woods were largely broadleaved and dominated by oak Quercus petraea and Quercus robur, with birch Betula pendula, beech Fagus sylvatica, holly Ilex aquifolium and sycamore Acer pseudoplatanus in the canopy or sub-canopy. The New Forest woods were mixed broadleaved and conifer, with birch, beech, ash Fraxinus excelsior, larch Larix sp. and Douglas fir Pseudotsuga menziesii the predominant canopy trees. Woodland understorey was characterised by species such as holly, rowan Sorbus aucuparia, hazel Corylus avellana and blackthorn Prunus spinosa and ground cover by various mosses, grasses, ferns, bracken Pteridium aquilinum, bilberry Vaccinium myrtillus and bramble Rubus fruticosus. Woodlands in mid-Wales have traditionally been used to provide shelter for high densities of sheep grazing on adjacent land; sheep densities reached their highest in the late twentieth century and have since declined in response to changes in agricultural subsidies and encouragement of more environmentally sensitive management (Welsh Government 2015). Woodlands in Dartmoor have not had such high grazing impacts despite intensive livestock grazing on the adjacent open common land (Mercer 2009). The New Forest has a long history of protection of the deer population and common livestock grazing, which has resulted in woodlands being intensively grazed and browsed over many hundreds of years (Peterken and Tubbs 1965). However, the majority of the woodlands in the study area are enclosed and mainly grazed by deer with occasional incursions by ponies. These differences in management history may have resulted in regional differences in woodland structure.
Nest and predator monitoring
Wood Warbler territories were identified by the presence of singing males during weekly visits to each wood. Surveys were conducted from the time of arrival in mid- to late April until the end of the breeding season (mid-July) in each year. Territories were defined as locations where singing males were present in the same area for a minimum of 2 weeks, thus excluding transient birds. The extent of the territory was a minimum convex polygon encompassing all registrations. Within territories, females were located usually by their vocalisations, and watched for 30–60 min to confirm nesting, or until a nest was found. A total of 436 nests were found as follows: in Wales, 41 in 2009, 64 in 2010, and 63 in 2011; in the New Forest, 46 in 2011, 37 in 2012, and 35 in 2013; on Dartmoor, 95 in 2012, and 65 in 2013. Nests were visited every 3–4 days to monitor outcomes and to determine the date of failure as accurately as possible. First egg date was either determined at nests visited during egg-laying, or if found after incubation had started, was back-calculated after chicks had hatched based on chick development (Mallord et al. 2012b). Nests were found at all stages of the nesting cycle: 134 at nest-building, 55 during egg-laying, 164 during incubation and 83 during chick-rearing.
Nests were assumed to have been predated if, at inspection, all eggs, or chicks younger than 10 days old, had disappeared from the nest, and there were no signs from nearby adults to suggest that chicks had fledged. Date of nest failure was estimated as the midpoint between the last date with chicks recorded and the next visit when nest had failed, unless an accurate date was recorded on camera. Nests where partial predation resulted in a reduction in brood size but still fledged some young were classified as ‘non-predated’. In most cases it is not possible to distinguish between brood reduction through starvation and dead chick removal by parents. Nests abandoned by adults pre-laying or that were trampled by large mammals were not included in the final analysis. Nest predation probabilities were calculated as 1 − daily survival probabilitynesting period, where daily survival probability = (total nest monitoring days − failed nests) ÷ total nest monitoring days, and nesting period = 31 days [5.77 days egg-laying, 12.77 incubation, 12.59 nestling; data are mean values from Robinson (2017)].
Each year nest cameras were deployed at a sample of nests to determine predator identities, and the stage during the nesting cycle when predation occurred. Nest cameras were purpose-built [for details, see Bolton et al. (2007)] and deployed 0.5–1.5 m from nests as detailed in Mallord et al. (2012b). These cameras have high responsiveness, work in darkness as well as daylight, and typically capture several images of predation events. Cameras also confirmed successful fledging of non-predated nests. At the start of the breeding season cameras were deployed at nests randomly during the egg stage, and subsequently redeployed on nests at any stage of development to maximise the number of nests monitored by cameras and chances of recording predation. Predator identities were only determined by nest camera images, as previous work has shown that predator identities cannot be reliably inferred from nest remains (Larivière 1999; Mallord et al. 2012b). A total of 143 nests were monitored by camera: in Wales, nine in 2009, nine in 2010, and 15 in 2011; in the New Forest, none in 2011, 28 in 2011, and 17 in 2013; on Dartmoor, 40 in 2012, and 25 in 2013.
Habitat measurement
The habitat requirements for territory settlement and nest location are well known for Wood Warbler, and habitat variables selected for analysis were those that could affect detection by predators of the nest itself or adults visiting the nest (Cody 1978; Delahaye and Vandervyvre 2008; Mallord et al. 2012a; Huber et al. 2016). Field layer variables are most likely to affect detection of the nest, whereas understorey and canopy vegetation are likely to affect the ability of predators to detect adult Wood Warbler activity. The habitat location variables, ground slope and distance to edge of wood are thought to affect predator activity.
At each nest location the structure of the surrounding vegetation was recorded using methods previously used in other studies of woodland bird-habitat associations (Mallord et al. 2012a; Charman et al. 2012; Amar et al. 2006). At each nest location, variables describing ground vegetation were recorded from 1- and 5-m radius plots. Variables describing understorey and canopy structure were recorded from 25-m-radius plots around the nest.
The same habitat variables, except ground vegetation within a 1-m radius, were recorded from non-nest locations in areas of the wood used by Wood Warblers in Dartmoor and Wales, and were used to test for nest site selection (see Mallord et al. 2012a for details). Non-nest locations were based on random points within the territory with the constraint that locations were at least 50 m apart. Points were selected to cover as much of the territory as possible. Due to variability in territory areas in some small territories it was not possible to fit another territory point, whereas in other cases more than one could be accommodated. Overall, a similar number of nest and non-nest locations were measured for this analysis (267 nests, 273 non-nests).
Ground layer vegetation was described by percentage cover in 5% increments estimated by eye for seven categories of vegetation or non-vegetated ground (bracken P. aquilinum, bramble Rubus fruticosus agg, bilberry Vaccinium myrtillus, herbs, grass, moss, leaf litter, rock, bare ground) and percentage of the vegetation in three height categories (< 10 cm, 10–30 cm, 30–50 cm). Percentage cover of vegetation in the understorey in 5% increments was estimated by eye for a 25-m-radius plot around the nest for three different height bands (0.5–2 m, 2–4 m, 4–10 m). Variables describing canopy cover, canopy height and horizontal visibility were collected at four points 12.5 m from nest locations in the four cardinal compass directions, with the mean of the four measurements for each nest used in the analyses. Horizontal visibility, a measure of density of understorey vegetation, was recorded as the proportion of the 12 bands, each 10 cm long on a vertical pole placed at the nest, visible from 12.5-m distance. Distance to nearest side branch was the distance measured with a tape measure from the nest to the nearest low branch that could be used as a perch by adults when approaching the nest. Canopy cover was estimated as the number of squares that showed more than 50% canopy cover above 10-m height, from a 4 × 4 grid of 2-cm squares held vertically 60 cm above the observer’s eye. Maximum height of the tallest tree was visually estimated and expressed to the nearest metre. Distance between nest locations and woodland edges was calculated within a geographic information system (GIS) (ArcMap 10; ESRI) using a woodland cover GIS data layer (Forestry Commission 2013, http://www.forestry.gov.uk/datadownload) which defined woodland boundaries. A description of habitat variables and the scale at which they were collected is given in Table 1.
In mid-Wales and Dartmoor, habitat measurements were taken after the conclusion of each associated nesting attempt, mainly in June and July. In the New Forest, all habitat measurements were taken at the end of the 2013 breeding season. Habitat features were unlikely to vary considerably during the few years studied as there was no major forest management or changes in grazing intensity. Highest nest-predation rates occurred during June and July after the main period of rapid seasonal vegetation change, therefore the vegetation post-breeding reflected the structure during the period of nest predation. The largest vegetation change between the active nest stage and measurement of vegetation was the late growth of bracken, which forms a tall layer (1–1.5 m) above herbs and grasses. Cover of these layers was recorded separately so that the cover of bracken was additional to the cover of shorter field vegetation. This resulted in 21 habitat variables of interest: 12 measures of field layer composition and structure, each recorded at 1-m and 5-m radius from the nest; seven measures of understorey and canopy structure recorded at 12.5-m or 25-m scales, and two location variables.
Statistical analysis
Analyses were carried out within R (R Core team 2015) using generalised linear mixed models (GLMM) with binomial error structure and logit link fitted with Laplace approximation, using the function glmer in the package lme4 (Bates et al. 2015). The response variable was in the format of outcome (predated = 1, not predated = 0) from n trials, where the number of trials is the number of days that the nest was monitored minus outcome, as in the Mayfield logistic regression (Hazler 2004). Location, categorised as the grid reference of the 1 km2 the nest was located in, was included as a random effect to account for local effects such as individual predators potentially predating multiple nests. First egg date as a measure of the timing of breeding was included as a potential confounding variable as there is often a seasonal increase in nest predation (e.g. Mallord 2012b). The effects of first egg date (Likelihood ratio test, \(X_{1}^{2}\) = 9.39, p = 0.002) provided a significantly better fit than intercept and random factor alone. Year, another potential confounding variable, due to year to year variation in overall predation levels, was tested, but did not significantly increase model fit (Likelihood ratio test, \(X_{1}^{2}\) = 2.15, p = 0.14). A null model including the random effect of location (1 km2), and fixed effect of first egg date, was used to compare the influence of region and measures of habitat structure, on nest predation.
We first tested whether nest site locations differed significantly from habitat elsewhere in territories in terms of habitat structure, then tested whether habitat structure had an influence on probability of nest predation.
Comparison of vegetation between nest locations and other territory locations (non-nest locations) was examined using GLMMs with a binomial error structure (nest vs. non-nest), using a logit link and including location as a random factor. Vegetation variables were tested individually for linear or quadratic effects, and those variables with significant effects at P < 0.05 were included in the final model selection where models containing all combinations of variables were compared using the dredge function in the R package MuMIn (Barton 2014). Correlated variables were not omitted at this stage, to allow for the independent variance to contribute to models. The effects of any correlated variables is discussed in the interpretation of the model results (a full correlation matrix of variables is given in supplementary material Table S1). All models with a difference in Akaike information criterion adjusted for small sample size (ΔAICc) of < 2 from the best model are presented. As data for non-nest locations were only available for Dartmoor and Wales, this analysis was restricted to these sites and included field layer vegetation from 5-m-radius plots only.
To test whether daily nest-predation rates were significantly related to vegetation structure, habitat variables were added individually to the null model to test for linear and quadratic effects, with data from all three sites. Then final model selection compared all combinations of habitat variables that individually showed significant relationships at P < 0.05 as with nest site selection above. A summary of all models with ΔAICc < 2 is presented alongside the null model.
Results
Patterns of nest predation
In total 434 nests were monitored of which 221 failed to fledge any young. Of these failed nests, 182 were predated, 13 were abandoned at the egg stage and 24 abandoned at the chick stage. Of 144 nests monitored by cameras, 79 out of 95 predated nests had the predator identified from nest camera images, with 12 different predator species identified. The most common predators were Eurasian Jay (31.6%) and Common Buzzard (13.7%), which together accounted for 45.3% of nest predation (Table 2). Most predation (66.7%) was by predators that hunt using visual or aural cues. Predation occurred both during the day (84%) and at night (16%), although most unidentified predation occurred at night. A total of 418 monitored nests had a full set of nest data and showed highest predation rates in the New Forest (Table 3), with significantly higher nest predation compared to Wales and Dartmoor, but no significant difference between Dartmoor and Wales. A total of 379 nests had a full set of habitat variables and were used for the analysis of relationships between habitat and nest predation.
Relationships between predation rates and habitat structure
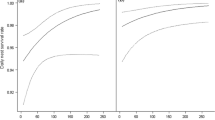
There was clear selection for nesting in a particular vegetation structure compared with the habitat in non-nesting areas of the wood used by singing males. Nine variables showed significant separation between nest and non-nesting locations, five describing field layer (cover of bracken, bramble, vegetation < 10 cm, vegetation 10–30 cm and vegetation 30–50 cm), one understorey (horizontal visibility), two canopy (canopy cover and height) and ground slope (Table S2, supplementary material). Although there was evidence for differences in vegetation characteristics between nest and non-nest locations, there was no single model that described this difference. Six models had an AICc difference of < 2 from the top model (Table 4). Five variables contributed to all six of the candidate models (cover of bramble and vegetation 30–50 cm high, horizontal visibility, canopy height and ground slope), canopy cover and cover of bracken contributed to 4 models and cover of vegetation < 10 cm and 10–30 cm high each contributed to one model. The variables that contributed to only a few models were all moderately correlated with other variables: Canopy cover was positively correlated with canopy height and horizontal visibility; vegetation 0.5–10 cm high was negatively correlated with bramble; vegetation 10–30 cm high was positively correlated with canopy height; bracken was negatively correlated with horizontal visibility (supplementary material Table S1a). There was little correlation between the variables that occurred in all models. Nests were more likely to be located on steeper slopes, where there was a greater cover of bramble, intermediate cover of tall field vegetation, intermediate horizontal visibility and extremes of canopy height (Fig. 1).
Plots showing relationships of habitat variables that showed a difference between nest and non-nest locations. Histograms show the distribution of nest location (top) and non-nest locations (bottom) with sample sizes given next to bars; modelled relationship is shown by the fitted line. The modelled line is based on the top candidate model in Table 4. Relationships are shown for all variables that occurred in all of the six candidate models within a difference in Akaike information criterion adjusted for small sample size (ΔAICc) of 2
There was also a relationship between nest-predation probability and vegetation structure (all models testing individual habitat variables are given in supplementary material Table S3). Twelve of the models that included habitat and site had an ΔAICc < 2 from the best model. One variable was included in all 12 models—nest-predation probability increased with decreasing cover of field layer vegetation of height 10–30 cm at the 5-m scale. Other variables retained in the top models were herbs (1 m, 8/12 models), understorey cover 0.5–2 m (6/12), moss (5 m, 6/12) and bramble (1 m, 4/12) (Table 5; Fig. 2). Predated nests had a lower cover of low understorey 0.5–2 m high (21.0 ± 1.4%) than non-predated nests (24.9 ± 1.5%), lower cover of medium height field layer (predated = 27.9 ± 1.7%, non-predated = 33.4 ± 1.5%), lower cover of bramble (predated = 7.4 ± 1.2%, non-predated = 11.3 ± 1.1%) and lower cover of herbs (predated = 4.7 ± 0.7%, non-predated = 7.0 ± 1.0%). Predated nests also had a higher cover of moss (24.4 ± 1.9%) than non-predated nests (19.9 ± 1.5%). The only moderate correlation between these variables was a positive correlation between understorey cover 0.5–2 m and cover of medium-height field layer vegetation (10–30 cm) (supplementary material Table S1b). The models which included habitat variables improved the model fit over site variation only, almost doubling the deviance explained, from 6.5 to 12.3%. However, this effect was still small and the greater amount of variability in nest predation was unexplained by the factors explored here.
Plots showing relationships between habitat variables and nest predation. Histograms show the distribution of predated nests (top) and non-predated nests (bottom) with sample sizes given next to bars; modelled relationship is shown by the fitted line. The modelled line is based on the top candidate model in Table 5. Figures are given for all variables that contributed to the candidate models within a ΔAICc of 2
The ground cover and understorey attributes associated with nest locations (greater bramble cover, intermediate cover of tall ground vegetation and intermediate density of low understorey) were similar to the attributes associated with lower nest predation (greater bramble cover, greater medium height ground vegetation and greater cover of low understorey). This suggests that the selected nesting sites are also locations that minimise nest-predation rates.
Discussion
This study shows a measurable relationship between the structure of vegetation surrounding Wood Warbler nests and daily nest-predation rates, and provides information on what optimal nesting habitat comprises in the UK. However, the magnitude of the relationship between predation probability and vegetation structure is small with most variation unrelated to the vegetation structure.
The vegetation structure around nest locations differed from that of non-nest areas within the same woods used by Wood Warblers, in terms of ground slope, tree canopy extent, understorey density and ground cover. This suggests that females select areas of woodland to nest in that have a specific structure, which subtly differs from where males sing. Nest locations had greater cover of bramble and taller field layer vegetation, consistent with greater nest concealment. However, selection for particular canopy and understorey, as well as ground layer structure, suggests nest location is not determined only for nest concealment. The selection for nesting on steeper slopes reflects the behaviour of adults when accessing nests. The nests are domed with the entrance facing downslope to allow adults to fly directly out of the nest and above the surrounding vegetation. Nests on flatter ground still tend to be located on small raised areas such as banks or rocks for the same reason. The circular vegetation plots recorded in our study do not take account of orientation of the nest entrance which affects the adults’ view, but may be valid from the perspective of the predator, which may observe adults visiting the nest from any angle.
The features of habitat structure identified for nest site selection are similar to those identified for male territories within a wood suggesting that nest site location is a further refinement of the same habitat features (Mallord et al. 2012a). However one notable difference is that Wood Warblers selected for areas with higher levels of bare ground or a short field layer for territory establishment. For nest sites they selected areas with intermediate levels of tall vegetation, but there was no relationship with shorter vegetation. Therefore nest concealment may be important, with nests located in areas of relatively taller vegetation in a sparsely vegetated area. This selection for nests concealed within vegetation on a forest floor that has a patchy low or intermediate vegetation cover has been found in other studies (Wesolowski 1985; Grendelmeier et al. 2015). The cover and nature of the ground vegetation is related to the structure and density of the tree canopy. Thus, the woodland structure associated with territory selection may be related to settlement in forest stands that provide suitable nesting conditions. Wood warblers select breeding territories with high tree cover, density or canopy closure (Delahaye et al. 2008; Huber et al. 2016; Passinelli et al. 2016), which normally results in low light levels below the canopy leading to low vertical diversity of vegetation (Huber et al. 2016), with low or intermediate levels of understorey cover and field vegetation (Delahaye et al. 2008; Mallord et al. 2012a; Pasinelli et al. 2016). This combination of specific canopy, understorey and ground structures is important for territory settlement, display flights and providing safe nest sites. In important Wood Warbler areas sufficient suitable closed canopy woodland could be maintained in the landscape by appropriate forest management.
In this study we did not directly study nest site selection at the time of settlement, but inferred preferences from the differences in vegetation recorded after the breeding season. Given the strength of separation between territory and nest locations, it is likely that the features used for nest site selection are related to later season vegetation recorded here. It seems likely from other studies of nest site concealment for forest passerines that there could be selection for nest concealment (e.g. Braden 1999; Toumenpuro 1991). The fact that selection is taking place at the 5-m scale suggests that vegetation structure on the forest floor in the vicinity of nests may affect whether predators can locate them in addition to any immediate nest concealment effect, which we did not detect in our study. For Wood Warbler, a stronger relationship between vegetation structure at a scale wider than the immediate nest site has been found elsewhere (Grendelmeier et al. 2015). The combination of vegetation affecting nest site selection at both the immediate nest location and the surrounding area has also been observed in studies of other species (Martin and Roper 1988).
The probability of nest predation was found to have a detectable relationship with nest location in terms of vegetation. The variables involved are consistent with concealment of the nest acting to reduce predation rates. A greater cover of medium-height vegetation, largely consisting of bramble, herbs and grasses, could make it more difficult for predators to locate the nest. Conversely, moss cover is associated with lower cover of taller ground vegetation and greater canopy openness, which may lead to nests being located more easily. The fact that nest predation was related to field layer variables at both the 1- and 5-m scales suggests that the surrounding vegetation is important, as is nest concealment. A greater cover of taller vegetation may make it more difficult for predators to locate potential nest sites from above. Unfortunately, data describing vegetation at the nest itself were only consistently recorded from one site, which meant we were not able to investigate the potential impact of immediate nest concealment.
Despite finding a relationship between nest-predation rates and vegetation structure, the size of the effect was small with a large amount of variation in nest-predation rates remaining unexplained. Another potential source of variation in nest predation could be variability in predator abundance, diversity or activity. Predators were not monitored and so predator population sizes are unknown and could have varied between sites, although all predators identified are common and widespread. In continental studies it has been hypothesised that individual Wood Warblers can assess rodent populations as a proxy of predation pressure, leading to spatial variation in settling patterns detected as population changes at the local scale (Wesolowski et al. 2009b; Pasinelli et al. 2016). While nest-predation rates in the UK vary between years, there is no indication of large-scale annual variation in Wood Warbler density similar to that found in continental Europe, with observed variation in breeding density between years a seemingly local effect at the woodland scale (Mallord et al. 2012b; Harris et al. 2016). Also, forest rodent populations appear to be synchronised across the UK (Flowerdew et al. 2004) and so are unlikely to lead to the redistribution of Wood Warbler populations on a broad scale within the UK. The avian predators prevalent in this study do not show the same widespread redistribution or fluctuation in numbers between years as smaller mammalian predators. The main nest predators are widespread with similar densities in all three study areas (Balmer et al. 2013).
In the UK, interest in Wood Warbler nest predation is linked to understanding possible causes of recent declines in their populations (Fuller et al. 2005). Changes in predation rates and productivity over time can give insight into whether predation is having a major effect on a population trend. Within Wales there was no difference in nest-predation rates between the 1980s and the present study (Mallord et al. 2012b). Also, UK-wide trends in numbers of young fledged per breeding attempt have not shown any overall trend during the period 1966–2013. However, there has been an increase in nest-failure rates during the chick stage counterbalanced by decreases in failure rates during the egg stage, which may relate to changes in predation pressure (Robinson et al. 2016). The evidence from these temporal trends in nest productivity is inconclusive regards the role of predation as a driver of population change.
Of the three sites we studied, the New Forest had particularly high nest-predation rates, which may even be high enough to cause local population declines in the absence of immigration into the population. This area has the heaviest grazing pressure and lowest cover of vegetation in the field layer. However, this study area also had higher diversity of predators. Thus, there may be greater predation pressure in this area, but we were unable to separate the potential interactions between predation pressure and the role of vegetation affecting predation rates.
In situations where there is an interaction between vegetation structure and predation rates affecting local populations, conservation management to reduce nest predation has involved both managing predator populations and/or habitat structure (e.g. White et al. 2008; Fletcher et al. 2010). Based on our results for Wood Warbler, there is limited potential for managing ground vegetation, e.g. by varying grazing to improve the cover of nesting habitat in woods with low ground cover and high predation rates, in order to improve nesting success locally.
Conclusion
There was support for the hypothesis that Wood Warblers select habitat that reduces nest predation. There was a separation between territory and nest locations based on measures of canopy, understorey and field layer structure. Nest predation rates were also affected by field layer vegetation consistent with the features selected for nest location. Nest predation was lower where there was greater cover of medium-height vegetation, including bramble and herbs and lower cover of moss. However, the size of this effect was small and explained only a small amount of variation in nest-predation rates. Widespread conservation management involving modification of field layer vegetation is therefore unlikely to have an effect on population declines of the Wood Warbler. However, any improvement in breeding productivity could have a buffering effect and ameliorate population declines caused by other pressures, particularly in situations where there are high levels of bare ground or sparse vegetation cover in the ground layer.
Change history
05 May 2018
In the original publication of the article, there is a misalignment of the last two columns in table 2. The correct Table 2 is provided below.
References
Amar A, Hewson C, Thewlis R, Smith KW, Fuller RJ, Lindsell J, Conway G, Butler S, MacDonald M (2006) What’s happening to our woodland birds? Long-term changes in the populations of woodland birds. RSPB research report 19/BTO research report 169. RSPB/BTO, Sandy/Thetford
Balmer D, Gillings S, Caffrey BJ, Swann RL, Downie IS, Fuller RJ (2013) Bird atlas 2007–11: the breeding and wintering birds of Britain and Ireland. BTO, Thetford
Barton K (2014) MuMIn: multi-model inference. R package version 1.10.5. http://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bolton M, Butcher N, Sharpe F, Stevens DK, Fisher G (2007) Remote monitoring of nests using digital camera technology. J Field Ornithol 78:213–220
Braden GT (1999) Does nest placement affect the fate or productivity of California Gnatcatcher nests? Auk 116:984–993
Charman EC, Smith KW, Dodd S, Gruar DJ, Dillon IA (2012) Pre-breeding foraging and nest site habitat selection by Lesser Spotted Woodpeckers Dendrocopos minor in mature woodland blocks in England. Ornis Fenn 89:182–196
Cody ML (1978) Habitat selection and interspecific territoriality among the sylviid warblers of England and Sweden. Ecol Monogr 48:351–396
Delahaye L, Vandevyvre X (2008) Le Pouillot siffleur (Phylloscopus sibilatrix) est-il une espece indicatrice de la qualite des forets feuillues ardennaises? Aves 45:3–14
Eggers S, Griesser M, Andersson T, Ekman J (2005) Nest predation and habitat change interact to influence Siberian Jay numbers. Oikos 111:150–158
Evans KL (2004) The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 146:1–13
Fletcher K, Aebischer NJ, Baines D, Foster R, Hoodless AN (2010) Changes in breeding success and abundance of ground-nesting moorland birds in relation to the experimental deployment of legal predator control. J Appl Ecol 47(2):263
Flowerdew JR, Shore RF, Poulton SMC, Sparks TH (2004) Live trapping to monitor small mammals in Britain. Mamm Rev 34:131–150
Forstmeier W, Weiss I (2004) Adaptive plasticity in nest-site selection in response to changing predation risk. Oikos 104:487–499
Fuller RJ, Gough SJ (1999) Changes in sheep numbers in Britain: implications for bird populations. Biol Cons 91:73–89
Fuller RJ, Noble D, Smith KW, Vanhinsbergh D (2005) Recent declines in populations of woodland birds in Britain: a review of possible causes. Br Birds 98:116–143
Gregory RD, Vorisek P, Van Strien A, Gmelig-Meyling AW, Jiguet F, Fornasari L, Burfield IJ (2007) Population trends of widespread woodland birds in Europe. Ibis 149(s2):78–97
Grendelmeier A, Arlettaz R, Gerber M, Pasinelli G (2015) Reproductive performance of a declining forest passerine in relation to environmental and social factors: implications for species conservation. PLoS One 10:e0130954
Harris SJ, Massimino D, Newson SE, Eaton MA, Marchant JH, Balmer DE, Noble DG, Gillings S, Procter D, Pearce-Higgins JW (2016) The Breeding Bird Survey 2015. BTO research report 687. British Trust for Ornithology, Thetford
Hazler KR (2004) Mayfield logistic regression: a practical approach for analysis of nest survival. Auk 121:707–716
Huber N, Kienast F, Ginzler C, Pasinelli G (2016) Using remote-sensing data to assess habitat selection of a declining passerine at two spatial scales. Landsc Ecol 31:1919–1937
Lahti DC (2001) The “edge effect on nest predation” hypothesis after twenty years. Biol Cons 99:365–374
Larivière S (1999) Reasons why predators cannot be inferred from nest remains. Condor 101:718–721
Macdonald MA, Bolton M (2008) Predation on wader nests in Europe. Ibis 150:54–73. https://doi.org/10.1111/j.1474-919X.2008.00869.x
Mallord JW, Charman EC, Cristinacce A, Orsman CJ (2012a) Habitat associations of Wood Warblers Phylloscopus sibilatrix breeding in Welsh oakwoods. Bird Study 59:403–415
Mallord JW, Orsman CJ, Cristinacce A, Butcher N, Stowe TJ, Charman EC (2012b) Mortality of Wood Warbler Phylloscopus sibilatrix nests in Welsh oakwoods: predation rates and the identification of nest predators using miniature nest cameras. Bird Study 59:286–295
Mallord JW, Smith KW, Bellamy PE, Charman EC, Gregory RD (2016) Are changes in breeding habitat responsible for recent population changes of long-distance migrant birds? Bird Study 63:250–261. https://doi.org/10.1080/00063657.2016.1182467
Martin TE (1993) Nest predation and nest sites. Bioscience 43:523–553
Martin JL, Joron M (2003) Nest predation in forest birds: influence of predator type and predator’s habitat quality. Oikos 102:641–653
Martin TE, Roper JJ (1988) Nest predation and nest-site selection of a western population of the Hermit Thrush. Condor 90:51–57
Mercer I (2009) Dartmoor. A statement of its time. New Naturalist 111
Newton I (1998) Population limitation in birds. Academic Press, London
Pasinelli G, Grendelmeier A, Gerber M, Arlettaz R (2016) Rodent-avoidance, topography and forest structure shape territory selection of a forest bird. BMC Ecol 16:1–18
Peterken G, Tubbs C (1965) Woodland regeneration in the new forest, Hampshire, since 1650. J Appl Ecol 2:159–170
R Core Team (2015) R3.2.1: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Rangen SA, Clark RG, Hobson KA (1999) Influence of nest-site vegetation and predator community on the success of artificial songbird nests. Can J Zool 77:1676–1681
Robinson RA (2017) BirdFacts: profiles of birds occurring in Britain and Ireland (BTO research report 407). BTO, Thetford. http://www.bto.org/birdfacts. Accessed on 23 Jan 2017
Robinson RA, Leech DI, Massimino D, Woodward I, Hammond MJ, Harris SJ, Noble DG, Walker RH, Eglington SM, Marchant JH, Sullivan MJP, Baillie SR (2016) BirdTrends 2016: trends in numbers, breeding success and survival for UK breeding birds. Research report 691. BTO, Thetford. http://www.bto.org/birdtrends
Roos S, Pärt T (2004) Nest predators affect spatial dynamics of breeding Red-backed Shrikes (Lanius collurio). J Anim Ecol 73:117–127. https://doi.org/10.1111/j.1365-2656.2004.00786.x
Schmidt KA (2003) Nest predation and population declines in Illinois songbirds: a case for mesopredator effects. Conserv Biol 17:1141–1150. https://doi.org/10.1046/j.1523-1739.2003.02316.x
Tapper S, Potts G, Brockless M (1996) The effect of an experimental reduction in predation pressure on the breeding success and population density of Grey Partridges Perdix perdix. J Appl Ecol 33:965–978. https://doi.org/10.2307/2404678
Tuomenpuro J (1991) Effect of nest site on nest survival in the Dunnock Prunella modularis. Ornis Fenn 68:49–56
Welsh Government (2015) June 2015 survey of agriculture and horticulture: results for Wales. http://gov.wales/docs/statistics/2015/151119-survey-agriculture-horticulture-june-2015-en.pdf
Wesolowski T (1985) The breeding ecology of the wood warbler phylloscopus sibilatrix in primaeval forest. Ornis Scand 16(1):49
Wesolowski T, Maziarz M (2009) Changes in breeding phenology and performance of Wood Warblers Phylloscopus sibilatrix in a primeval forest: a thirty-year perspective. Acta Ornithol 44:69–80
Wesolowski T, Rowinski P, Maziarz M (2009) Wood Warbler Phylloscopus sibilatrix: a nomadic insectivore in search of safe breeding grounds? Bird Study 56:26–33
White PJ, Stoate C, Szczur J, Norris K (2008) Investigating the effects of predator removal and habitat management on nest success and breeding population size of a farmland passerine: a case study. Ibis 150(s1):178–190
Wiebe KL, Martin K (1998) Costs and benefits of nest cover for Ptarmigan: changes within and between years. Anim Behav 56:1137–1144
Willson MF, Morrison JL, Sieving KE, de Santo TL, Santisteban L, Diaz I (2001) Patterns of predation risk and survival of bird nests in a Chilean agricultural landscape. Conserv Biol 15:447–456
Acknowledgements
This work was funded by Natural England and RSPB under the Action for Birds in England partnership. We would like to thank the many landowners for access to study sites. Derek Gruar and Anna Riach contributed to field data collection in 2009, and Alice Tribe in 2012. All aspects of this study complied with UK law.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellamy, P.E., Burgess, M.D., Mallord, J.W. et al. Nest predation and the influence of habitat structure on nest predation of Wood Warbler Phylloscopus sibilatrix, a ground-nesting forest passerine. J Ornithol 159, 493–506 (2018). https://doi.org/10.1007/s10336-017-1527-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-017-1527-7