Abstract
In contrast to the majority of migratory songbirds in North America, which moult on or near their breeding grounds, the Bullock’s oriole (Icterus bullockii) is reported to stop during fall migration to moult en route to the wintering grounds. These birds seem to take advantage of food resources during the Mexican monsoon season in the Southwestern USA and Northwestern Mexico. We studied a population of Bullock’s orioles at the northern limit of their breeding range in Kamloops, British Columbia, Canada, using a combination of light-level geolocators and stable hydrogen isotope analysis. We found evidence that supports the existence of moult migration in this species, with geolocators indicating that all birds appeared to stay in the Mexican monsoon region for moult in an extended stopover period during fall migration. Feathers were isotopically enriched with deuterium compared to predicted breeding isotope values and were significantly more negative than winter-grown claws, confirming that moult occurred somewhere between the breeding and wintering grounds. Stable isotope data were consistent with complete prebasic stopover moult in adults and complete contour feather and variable tail feather moult in first-year orioles. Our results confirm that this northern population of Bullock’s orioles employs a moult migration strategy and highlight the usefulness of combining geolocator and stable isotope studies.
Zusammenfassung
Bestätigung des Mauserzugs beim Bullocktrupial ( Icterus bullockii ) mit Hilfe von Geolokatoren und stabiler Isotope
Im Gegensatz zu den meisten ziehenden Singvögeln Nordamerikas, die in oder in der Nähe ihres Brutgebiets mausern, wird über den Bullocktrupial (Icterus bullockii) berichtet, dass er auf dem Herbstzug ins Überwinterungsgebiet unterwegs einen Mauserstopp einlegt. Diese Vögel nutzen anscheinend Nahrungsressourcen im Südwesten der USA und im Nordwesten Mexikos während der mexikanischen Monsunsaison. Wir haben eine Population des Bullocktrupials am nördlichen Rand seines Brutgebiets in Kamloops in Britisch-Kolumbien, Kanada, mit Hilfe einer Kombination von Helldunkelgeolokatoren und stabiler Wasserstoffisotopenanalyse untersucht. Wir fanden Hinweise, welche die Existenz von Mauserzug bei dieser Art unterstützen; Geolokatoren zeigten an, dass alle Vögel zur Mauser offenbar in der mexikanischen Monsunregion blieben und dafür einen längeren Stopp auf dem Herbstzug einlegten. Die Federn waren im Vergleich zu den vorhergesagten Brutgebiet-Isotopenwerten mit Deuterium-Isotopen angereichert und signifikant negativer als im Winter gewachsene Krallen, was bestätigt, dass die Mauser irgendwo zwischen Brut- und Überwinterungsgebiet erfolgte. Die stabilen Isotopdaten standen mit einer kompletten Postnuptialmauser im Rastgebiet bei Adulttieren und einer kompletten Konturfeder- und variablen Schwanzfedermauser bei einjährigen Tieren im Einklang. Unsere Ergebnisse bestätigen, dass diese nördliche Population von Bullocktrupialen einen Mauserzug machen, und unterstreichen die Nützlichkeit einer Kombination von Geolokatorstudien und stabilen Isotopenanalysen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breeding, moulting, and migration are highly energy intensive events in the life of migratory birds (Lindstrom et al. 1993). As a result, they typically occur at different times during the annual cycle. When and where moulting occurs, however, and which feathers are replaced, varies considerably, particularly among Palearctic songbirds. Whereas most species moult in late summer on their breeding grounds, others migrate first, moulting after they have reached their wintering locations, and still others moult at stopover sites en route; some have only one annual moult, whereas others have two, typically replacing body feathers at one time and flight feathers elsewhere at another time (Pyle 1997; Svensson and Hendenström 1999; Rohwer et al. 2005; Barta et al. 2008; Newton 2011). Nearctic-Neotropical migrants, on the other hand, are less variable with respect to the timing of moult (Rohwer et al. 2005; Barta et al. 2008). In this group, most species undergo a prebasic moult in late summer, replacing all their body and flight feathers on or near their breeding grounds; some species also have a prealternate moult, which typically involves only body feathers, on the wintering grounds (Pyle 1997). However, an additional strategy exists whereby some species, particularly among those breeding in western North America, interrupt fall migration to fully or partially moult en route at a stopover site (Rohwer et al. 2005; Barta et al. 2008; Pyle et al. 2009; Newton 2011).
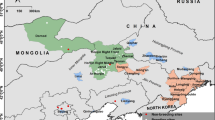
As many as 19 western North American migratory songbirds, including the Bullock’s oriole (Icterus bullockii), lazuli bunting (Passerina amoena), Lucy’s warbler (Vermivora luciae), black-headed grosbeak (Pheucticus melanocephalus), western kingbird (Tyrannus verticalis), warbling vireo (Vireo gilvus), ash throated flycatcher (Myiarchus cinerascens), and western tanager (Piranga ludoviciana), are reported to have a distinctive moult migration strategy (Leu and Thompson 2002; Rohwer et al. 2005; Pyle et al. 2009). These species, termed moult-migrants, appear to forego a moult on the breeding grounds; instead, they interrupt migration to stop and moult in what has been referred to as the Mexican monsoon region in the Southwestern USA and Northwestern Mexico (Leu and Thompson 2002; Rohwer et al. 2005; Pyle et al. 2009), timing this stopover to coincide with an increase in food abundance following late summer monsoon rains (Rohwer and Manning 1990; Rohwer et al. 2005). Stopover sites such as that in the Mexican monsoon region may be crucial, as they provide abundant resources that allow birds to build up vital fat stores, as well as grow high-quality plumage, which is important for both flight and communication (Hutto 1998; Leu and Thompson 2002). Moulting site fidelity within the Mexican monsoon region appears to be low, with local environmental factors playing an important role in the spatial distribution of birds during the moult period. A study by Pyle et al. (2009; see also Chambers et al. 2011) over 2 years demonstrated that during a wet year, moult migrants spread out into more xeric regions, whereas in a drier year, birds appeared to cluster around riparian areas. All species of the western North American moult migrants have been detected in the Mexican monsoon region, but there appears to be intraspecific and interannual variability in moult migration dynamics, making it unclear as to the general importance of this region and the generality of stopover moult behaviour at the species level (Pyle et al. 2009). Other researchers have drawn attention to the fact that, in contrast to the situation for New World migrants breeding in western North America, use of a stopover site for moulting seems to be rare among birds breeding in eastern North America (Rohwer et al. 2005). By comparison, there is little difference between eastern and western breeding Old World species with respect to whether they moult before or after fall migration (Rohwer et al. 2005; Barta et al. 2008).
Until recently, it has been extremely difficult to track small migratory birds throughout the year, though stable isotope analysis has proved quite useful for linking different phases of the annual cycle. Stable isotopes are incorporated into growing animal tissue from the food and water they ingest, which permits the delineation of prior location information and habitat use (Wassenaar 2008). Because tissues may be grown in different locations or during temporally discrete periods, these analyses have been used to infer spatial and dietary information for a number of species, revealing patterns of migratory connectivity (Hobson et al. 2004), habitat use (Marra et al. 1998; Bearhop et al. 2004), and important carry-over effects (Marra et al. 1998; Reudink et al. 2009). Stable hydrogen isotope ratios in animal tissues can provide a rough latitudinal approximation, as δ 2H values vary predictably across North America, becoming more negative moving from the southeast to the northwest (Hobson 2008). By comparing information from isoscape basemaps with stable hydrogen isotope analysis of animal tissues, we can determine the general area in which a bird moulted. For example, if a species typically moults on its breeding grounds, feathers from individuals captured at overwintering sites can allow us to determine roughly where the feather was initially grown, thus linking breeding and wintering populations (Wassenaar and Hobson 2000; Hobson 2008). This approach has been used to link breeding and wintering grounds in Bicknell’s thrush (Catharus bicknelli; Hobson et al. 2004) and many other species (Wassenaar and Hobson 2000; Kelly et al. 2002; Rubenstein and Hobson 2004; Pérez and Hobson 2007), as well as make connections between migratory stopover sites and breeding populations (Paxton et al. 2007; Gonzalez-Prieto et al. 2011). A recent study by Quinlan and Green (2011) found that overwintering yellow warblers (Dendroica petechia) could be assigned to breeding grounds using stable hydrogen isotope analysis, but that it was more difficult to estimate the locations of where feathers were grown on wintering grounds, based on weak gradients in precipitation isotopes.
Naturally occurring stable isotopes can have similar ratios across large areas and there may be considerable variation among individuals within a population, making spatial information gleaned from their use limited (Langin et al. 2007). Alternative, extrinsic marker approaches, such as global positioning system (GPS) tracking of individuals and the use of light-level geolocators, can provide incredibly detailed information about migratory routes, even in smaller birds (Bridge et al. 2011a). A geolocator is a small instrument, consisting of a light sensor and a data logger that records time of day and ambient light level; the timing of sunrise and sunset can then be used to determine the geographical location of the device (Bridge et al. 2011a). The accuracy of geolocator-based location estimates is moderate, especially due to high latitudinal error during or approaching the equinoxes, and stationary location estimates may have an associated error of tens of kilometers to over 100 km, although longitudinal accuracy generally exceeds latitudinal accuracy (Bridge et al. 2011a; Fudickar et al. 2011). Recently, geolocator use has exploded, and is providing many new insights into the ecology of migratory birds. Pioneering work with geolocators revealed faster than expected migration speeds in purple martins (Progne subis) and wood thrushes (Hylocichla mustelina; Stutchbury et al. 2009). Geolocators have also revealed longer than expected migration and multiple over-wintering sites in veeries (Catharus fuscescens; Heckscher et al. 2011) and bobolinks (Dolichonyx oryzivorus; Renfrew et al. 2013). Migratory connectivity has been estimated in gray catbirds (Dumetella carolinensis), showing different wintering sites for breeding populations in the eastern and mid-western USA (Ryder et al. 2011), and differences in migratory routes, stopover locations, and wintering sites have also been detected in coastal and inland subspecies of Swainson’s thrush (Catharus ustulatus; Delmore et al. 2012).
Combining the use of geolocators with stable isotope data can be particularly informative. A comparison of geolocator and feather hydrogen isotope data in ovenbirds (Seiurus aurocapilla) demonstrated that the two techniques can be complementary and also showed that the use of isotopes alone could lead to incorrect interpretations of migratory connectivity (Hallworth et al. 2013). The combination of stable hydrogen isotopes and geolocators has great potential for studying moult migration. For example, research on an eastern North American moult migrant, the painted bunting (Passerina ciris), revealed differing moult migration strategies among individuals, even within the same breeding population (Contina et al. 2013). A full annual cycle approach to studying moult migration is critical as stopover in the Mexican monsoon region represents a large and vital habitat for a number of species, and this region may be especially important for conservation efforts for moult migrants and other songbirds (Leu and Thompson 2002).
Among western North American songbirds, the Bullock’s oriole is ideally suited to investigate moult migration. Bullock’s orioles are relatively large (adults: 29–42 g) songbirds that breed as far north as the southern interior of British Columbia and overwinter as far south as Costa Rica. They have moderate to high rates of return to the breeding grounds (31–79 %; Butcher 1991) and high breeding site fidelity, averaging 111 m between nesting sites from 1 year to the next (Rising and Williams 1999), which makes them a practical species for geolocator deployment. Evidence thus far suggests that the birds depart breeding areas prior to moulting in order to undergo prebasic moult in the Mexican monsoon region before arriving in the wintering areas (Rising and Williams 1999; Pyle et al. 2009).
Using stable hydrogen isotope analysis of tail feathers, breast feathers, and claws from all age and sex classes, combined with data from geolocators, we examined the prevalence and pattern of moult migration in Bullock’s orioles nesting at the northern extent of their range. We predicted that the flight and breast feathers of after second year (ASY) orioles would show stable hydrogen isotope ratios consistent with the monsoon region, indicating that moult migration had taken place. We predicted that tail feathers for second year (SY) birds would show variation in moult location, as rectrices are initially grown in the nest, but first-cycle birds may replace a variable number of rectrices during the prejuvenile molt (Pyle 1997). Isotopically, breast feathers in SY birds are expected to be consistent with the occurrence of moult in the monsoon region, though it is possible that these feathers could be replaced on the wintering grounds. We also predicted that claw samples would show more positive (i.e., more enriched in deuterium relative to body and tail feathers) hydrogen isotope values than would feathers, because claws should be grown in a more southern location than feathers (i.e., on the wintering grounds in Mexico or Central America). In addition, we expected the geolocator data to reflect a similar pattern of moult stopover and the same general wintering region as indicated by the stable isotope data.
Methods
Field methods
Our study area was located in Kamloops, British Columbia, Canada (50.68 N 120.34 W) at the northern extent of the Bullock’s oriole range, and field work took place from May through July in 2012 and 2013. Using a combination of passive mist netting around oriole feeders and active targeting using mist nets, a decoy, and song playback, we caught a total of 45 birds over two field seasons: 31 in 2012 (18 ASY males, 4 ASY females, 2 SY males, and 7 SY females), and 20 in 2013 (10 ASY males, 7 ASY females, 2 SY males, and 1 SY female), including a total of 6 between-year recaptures (3 with geolocators). Descriptions provided by Rohwer and Manning (1990) and Pyle (1997) were used to determine the sex and age group of captured orioles. Each captured bird was banded with a Canadian Wildlife Service-issued aluminum band and a unique combination of colour bands for individual identification. From each bird, we acquired ten breast feathers, a tail feather, and a small, ~3-mm segment of the claw tip. Claw samples were collected within 2 weeks of the birds’ arrival on the breeding ground in an attempt to ensure that hydrogen isotope values reflected winter conditions, following Reudink et al. (2009) (but see Ethier et al. 2010). For SY birds, contour feathers and a variable number (0–12) of rectrices are replaced during the first prebasic moult, which appears to occur in the Mexican monsoon region (Rohwer and Manning 1990; Pyle 1997). Unfortunately, we were unable to visually distinguish rectrices grown in the nest from those grown during the first prebasic moult. A prealternate moult may also occur in some individuals, but has only been reported in head feathers of SY birds (Rohwer and Manning 1990; Pyle 1997). Definitive prebasic moult occurs from October–November during migration, but some individuals may also moult on the wintering grounds (Rohwer and Manning 1990; Rising and Williams 1999).
Stable hydrogen isotope analysis
We conducted our stable hydrogen isotope analysis at the Smithsonian Institution OUSS/MCI Stable Isotope Mass Spectrometry Facility in Suitland, Maryland, USA. We washed feather samples in a 2:1 chloroform–methanol solution, and then allowed them to dry and acclimate to the atmospheric conditions of the lab in a fume hood for over 72 h prior to sample preparation and analyses. Approximately 0.30–0.40 mg of feather vane, taken from the tip and avoiding the rachis, as well as claw samples were weighed and crushed in silver capsules. We then pyrolyzed these samples in a Thermo high temperature conversion elemental analyzer (TC/EA; Thermo Scientific) at 1350 °C and analyzed them using a Thermo Delta V Advantage isotope ratio mass spectrometer. Isotope ratios (2H/1H) are reported in δ notation in units per mil (‰) relative to Vienna standard mean ocean water (VSMOW). Four standards were run for every 10 samples, including the hydrogen standard International Atomic Energy Agency (IAEA-CH-7) and three additional standards [kudu horn standard (KHS), caribou hoof standard (CBS), keratin spectrum]. Measurements of the same feather were repeatable to within 3 ± 2 ‰ (mean ± standard deviation (SD), n = 10). Non-exchangeable δ 2 H values were corrected to keratin standards following Wassenaar and Hobson (2003). Predicted breeding ground (mean: −119 ± 5 ‰ SD) and moulting ground (mean: −64 ± 16 ‰ SD) feather isotope ratios were estimated using a growing season precipitation isoscape map (Meehan et al. 2004) with a 50-km buffer surrounding our breeding ground study site in Kamloops, British Columbia. The Mexican monsoon region was delineated as described by Comrie and Glenn (1998). Predicted wintering ground (−50 ± 9 ‰ SD) feather isotope values were estimated using a different growing season precipitation isotope map (Bowen and Revenaugh 2003), which had lower resolution, but which provided coverage of the entire Bullock’s oriole wintering region, unlike the Meehan et al. (2004) map. Tissue isotope fractionation was estimated using the precipitation δ 2H to feather-tissue δ 2H fractionation equation for non-ground foraging Neotropical migrants described by Hobson et al. (2012).
Geolocators
We deployed 1.2-g British Antarctic Survey (BAS) MK10 geolocators on 17 ASY male Bullock’s orioles caught during the 2012 field season (mid-May to early July), and were able to recover three of them in 2013. We attached the geolocators to birds via a leg loop harness made of Stretch Magic™. Geolocators measure light intensity levels at 1-min intervals and allow us to estimate the locations of birds at noon and midnight on given dates, based on changes in sunrise and sunset times. Of these three geolocators, two functioned for an entire annual cycle, while the other lost power during fall migration. We calibrated individual geolocators using recorded locations of the orioles while the birds were still on the breeding ground for a minimum of 11 days and a maximum of 14 days and using individual solar angles of −2.5° (bird 22), −3.1° (bird 24), and −2.4° (bird 41). Using the BASTrak suite of geolocator software, we downloaded, decompressed, and examined each individual light response curve in TransEdit2. We eliminated dates within 20 days of the spring and fall equinoxes from the analyses, as latitudinal location estimates become extremely poor during these periods. We also eliminated dates in which an obvious shading event was detected by examining the sunrise and sunset transition, or in which a significant amount of noise occurred close to sunrise or sunset (76/383 [bird 22] + 18/416 [bird 24] + 3/72 [bird 41] = 97 of 871 days = 11 % of the total number of days). Because there is very little known about the behaviour of Bullock’s orioles away from the breeding grounds, we used only the midnight location estimates during stationary phases off the breeding grounds, as the birds should be staying in one spot (i.e., not flying) at this time (Rising and Williams 1999). As location was known during the breeding season, and the birds may be migrating at night, we used both midnight and noon positions for these phases. We estimated error (range: 1–309 km, latitude: 88 ± 67 SD, 90 ± 63 km SD, 87 ± 69 SD km, longitude: 69 ± 59 SD, 54 ± 42 SD, 81 ± 71 SD km for birds 22, 24, and 41) in measurement while the birds were known to be on their breeding grounds in 2012. Migratory departure dates were determined by movements greater than 100 km away from the stationary location followed by subsequent movements further away from the stationary location. Stopover sites were determined by a stay of ≥14 days at one location. Using ESRI’s ArcGIS, we ran kernel density estimates (50, 75, and 90 % of maximum density) with an output cell size of 1 km and a search radius of 309 km. These kernel density estimates were converted to polygons and mapped.
Statistical analysis
To examine differences among tissues, we used mixed model analyses with δ 2H as a response variable, year as a fixed effect, and band number as a random effect when individuals captured in both years (n = 6) were included in the sample. Where year was not significant, we eliminated it as a fixed effect and re-ran the models. We also used paired t tests to compare the δ 2H values of different tissues for each individual, considering sex and age class separately. To compare the δ 2H values of male and female orioles, we separated age classes and built mixed models using sex and year as main effects and individual as a random effect. When individuals were not duplicated in the sample, we used Student’s t tests. When we examined differences in δ 2H between ASY and SY orioles, we separated individuals by sex and built mixed models using δ 2H as the response variable, age and year as main effects and individual as a random effect. We used Pearson correlation to examine relationships between the δ 2H values of different tissues within individuals. Data points were classified as outliers if they fell outside the 1.5 × inter quartile range (n = 2 ASY male claw samples, 2 ASY female breast feathers, and 1 ASY female tail feather) and were excluded from analyses. Results of all analyses including outlier samples are shown in the Supplementary Material.
Results
Comparisons among tissues
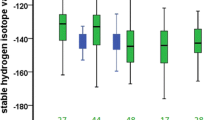
When we examined differences among the tissues of ASY males (tail feathers, breast feathers, claws), we found that δ 2H values differed significantly among all tissue types (mixed model: n = 6 claw, 27 tail, 28 breast, F 2,36.29 = 16.49, p < 0.0001; Fig. 1). Two of the eight claw samples were excluded as outliers. These outliers had δ 2H values (96.6 and 99.6 ‰) that were consistent with a location south of the breeding grounds, but far north of the wintering grounds. This was potentially due to birds either arriving in more northern latitudes earlier, or taking a longer time to migrate in spring, thus allowing the claws to begin incorporating the isotope value of the higher latitude areas en route. The analyses reported below exclude these two outlier claw samples. (Results from each of these same tests with the outliers included are reported in the Supplementary Material, Appendix 1.)
We detected no year effects in any model comparing different tissues within ASY males; thus, all results are shown with the effect of year were removed from the model. In ASY males, claws exhibited the highest (i.e., indicating most southern) δ 2H values (n = 6, mean = −58 ± 4 ‰ SD), followed by breast (n = 28, mean = −71 ± 12 ‰ SD) and tail (n = 27, mean = −80 ± 12 ‰ SD; Fig. 1). Pairwise comparisons revealed that breast feathers and tail feathers differed significantly [breast and tail (n breast = 28, n tail = 27, F 1,29.97 = 22.45, p < 0.0001)], as did tail feathers and claws (n claw = 6, n tail = 27, F 1,18.12 = 13.33, p < 0.002), although there was no difference between breast feathers and claws (n breast = 28, n claw = 6, F 1,8.74 = 3.51, p = 0.09; Fig. 1). Paired t tests for ASY male orioles revealed similar trends within individuals: breast and tail feathers differed significantly (n = 27, t = −4.68, p < 0.0001), whereas there was no difference between breast feathers and claws (n = 6, t = 1.41, p = 0.22). Tail feathers were significantly more negative (potentially from further north) than claws (n = 6, t = 3.15, p = 0.03; Fig. 1). In addition, we found a significant correlation between the δ 2H values of breast and tail feathers for ASY males (n = 27, r = 0.63, p = 0.0004). However, there was no relationship between the δ 2H values of claws and tail (n = 6, r = −0.24, p = 0.65) or claws and breast (n = 6, r = 0.11, p = 0.85). ASY male breast (mean = −70 ± 12 ‰ SD) and tail (mean = −80 ± 12 ‰ SD) feathers are similar to, though more negative than, to the predicted value for feathers from the moulting grounds (mean = −64 ± 16 ‰ SD). Similarly, claw isotope values (mean = −59 ± 7 ‰ SD) were close to, but slightly more negative than, the values predicted (mean = −50 ± 9 ‰ SD) for tissues grown on the wintering grounds.
Because claw samples needed to be collected within 2 weeks of arrival, we were unable to collect an adequate number of such samples from females or SY males. When we compared the tail and breast feathers [replaced by hatch year (HY) birds during the previous fall migration] of individuals using paired t tests, the δ 2H values of breast feathers were more positive than those of tail feathers for SY males, although the difference was not significant (mean breast = −70 ± 19 ‰ SD; mean tail = −116 ± 33 ‰ SD; n = 4, t = −2.29, p = 0.11; Fig. 2c). We found no differences between the tail and breast feathers of individual females in both the ASY (paired t test: n = 8, mean breast = −77 ± 12 ‰ SD, mean tail = −83 ± 15 ‰ SD; n = 8, t = −1.05, p = 0.33; Fig. 2b) and SY (paired t test: n = 5, mean breast = −61 ± 11 ‰ SD, mean tail = −70 ± 13 ‰ SD; n = 5, t = −1.58, p = 0.19; Fig. 2d) age classes. A mixed effects model comparing the δ 2H values of breast and tail feathers within ASY females also showed that the two feather types were not significantly different (n = 9 breast, 9 tail, F 1,5.29 = 0.28, p = 0.62).
Comparing δ2H across age and sex classes
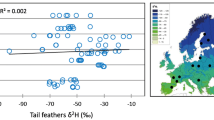
We examined the δ 2H values of breast and tail feathers, comparing ASY and SY birds for both sexes. For male orioles, we found no significant difference between the breast feathers of SY and ASY birds (mixed model: n total = 32, n ASY = 28, n SY = 4, F 1,26.69 = 0.03, p = 0.87), but did find a significant difference between the age classes with respect to tail feathers (mixed model: n total = 30, n ASY = 27, n SY = 4, F 1,24.92 = 16.84, p = 0.0004; Fig. 3b). There were no significant differences in δ 2H values between ASY and SY females for either breast (n total = 17, n ASY = 9, n SY = 8, F 1,0.92 = 16.30, p = 0.17; Fig. 3b) or tail (n total = 14, n ASY = 9, n SY = 5, t = 1.69, p = 0.12; Fig. 3b) feathers.
Within the ASY age class, males had significantly more positive δ 2H values than females for breast feathers (mixed model: n total = 37, n female = 9, n male = 28, F 1,28.19 = 5.90, p = 0.02; Fig. 3a), but not tail feathers (mixed model: n total = 36, n female = 9, n male = 27, F 1,25.97 = 0.37, p = 0.55; Fig. 3b). Additionally, t tests revealed no difference between the sexes of SY birds with respect to the δ 2H values of breast feathers (n total = 12, n female = 8, n male = 4, t = −1.06, p = 0.31; Fig. 3a). However, there was a significant difference for tail feathers: SY males exhibited more positive tail feather values than did SY females (mixed model: n total = 9, n female = 5, n male = 4, t = −2.91, p = 0.02; Fig. 3b).
Geolocators
We tracked the full annual cycle of two ASY males and part of the fall migration of another (Fig. 4). All three birds that returned with geolocators left the breeding grounds in mid- to late July of 2012 (birds 24 and 41 on July 18th and bird 22 on July 26th). Birds 22 and 24 arrived in the monsoon region on August 2nd and 5th, and left in October 18th and 21st, respectively, proceeding to their apparent wintering grounds in central Mexico. Spring migration towards the breeding grounds began on April 23rd (bird 22) and April 26th (bird 24) of 2013, with the two birds arriving on the breeding grounds on May 17th (bird 22) and 19th (bird 24). The remaining individual (bird 41) appeared to stopover in the Nevada-Idaho-Oregon region for 2 weeks before continuing south towards the moulting area. As a result, the rate of bird 41’s fall migration was much slower than that of the other two birds; bird 41 was just leaving this two-week stopover 10–13 days after the other two had reached the Mexican monsoon region. This last geolocator appears to have lost power while the bird was moving south through Arizona. Birds 24 and 22 did not have any long stopovers aside from the stopover in the Mexican monsoon region. During and around the equinoxes when latitudinal estimates are weakest, these birds were primarily stationary or moving longitudinally. The return rate of birds carrying geolocators was at least 35 % (6/17, though only three were recaptured), which exceeded the return rate of 25 % for banded birds without geolocators (7/28). In addition, each of the three recaptured birds had a body mass in 2013 that was greater than or equal to its mass in 2012.
Breast feather hydrogen isotope values for the two birds equipped with recovered geolocators that functioned year round were −75 ‰ (bird 22) and −75 ‰ (bird 24) in 2012 and −76 ‰ (bird 22) and −88 ‰ (bird 24) in 2013. For tail feathers, hydrogen isotope values were −82 ‰ (bird 22) and −90 ‰ (bird 24) in 2012 and −70 ‰ (bird 22) and −77 ‰ (bird 24) in 2013. Both birds were captured too late (>2 weeks after arrival) to collect a claw sample.
Discussion
Our results from both stable isotope and geolocator data support previous reports indicating that Bullock’s orioles stop in the Mexican monsoon region to moult en route to the wintering grounds. Bullock’s orioles breeding at the northern limit of the species’ range migrate to the Mexican monsoon region for a stopover moult before heading to the wintering grounds, as has been suggested previously based on captures of moulting orioles in the Mexican monsoon region (Rohwer and Manning 1990; Pyle 1997; Rising and Williams 1999; Pyle et al. 2009; Rohwer et al. 2009). The results obtained from two geolocators clearly show that the birds made a long stopover (approximately 73 and 80 days) in the Mexican monsoon region before they moved on to the wintering sites. This stopover was long enough for a complete moult to take place (de la Hera et al. 2009). While two of the geolocators showed similar fall migration patterns, the third geolocator revealed that the bird approached the edge of the expected moult region 2 weeks later than the others due to a stopover during southward migration. This two-week stopover was too short for a complete moult, but may have provided resources necessary to continue migration. It is also possible that this bird initiated moulting at this stopover, then suspended it before moving on to the monsoon region, as is common in some passerines and shorebirds (Pyle 1997, 2008). Migratory pathways also differed among individuals, with birds appearing to utilize both the Pacific and Central Flyways. Future geolocator studies with a larger number of Bullock’s orioles from this and other populations will be critical for understanding connections between migratory pathways, moult locations, and over-wintering areas.
Our isotopic data further support the idea that moult migration is prevalent and potentially obligatory within our population, with at least partial moulting in the monsoon region detected across all ages and sexes. In addition, substantial variation in feather isotopic values likely indicates that there is a broad range of moult locations within the Mexican monsoon region for birds of the same breeding population, suggesting low connectivity between breeding and moult locations. The significant isotopic differences between claws, which grow continuously and should reflect wintering areas, and feathers, suggests that the location of moult differs from the location of overwintering for ASY males, providing further evidence of moult migration rather than an over-winter moult. ASY and SY male birds did not show significant differences in δ 2H of breast feathers, but did in tail feathers, with SY tail feather values being much more negative than those of the ASY males, indicating growth at more northern latitudes. The δ 2H values for two of the four SY male tail feathers fell within the range of expected breeding range values, suggesting that these feathers were grown on the breeding grounds when the birds were nestlings. One consideration is that the young birds may have indeed moulted during stopover, but their body tissues had not yet equilibrated to the new isoscape, as has been suggested for painted buntings (Bridge et al. 2011b). The other two SY males had tail feather values similar to ASY birds, suggesting moult in the monsoon region. This result is consistent with the idea that juvenile birds re-moult a variable number of rectrices (0–12 feathers; Pyle 1997). Future studies able to discriminate juvenile from adult feathers may be useful for gaining information on both natal and moult migration location, especially among individuals that retain both generations of feathers. Among females, SY and ASY birds did not differ in either tail or breast feather δ 2H values. SY breast feathers (of both males and females) and SY female tail feathers were consistent with moult migration in the Mexican monsoon region. This area, thus, appears to be an important stopover moult location for all age and sex classes of Bullock’s orioles.
Perhaps surprisingly, we found that for ASY males, tail feathers had more negative δ 2H values than did breast feathers. While this result could indicate differences in the location of feather growth, strong within-individual correlation between the tail and breast feathers of individual birds suggests that the feathers were grown in the same location, and differences between tissues were likely due to isotopic fractionation. When compared to their body feathers, flight feathers of Wilson’s warblers (Wilsonia pusilla) had more negative (9.6 ‰ difference) δ 2H values (Kelly et al. 2002). In addition, merlins (Falco columbarius), red-tailed hawks (Buteo jamaicensis), and sharp-shinned hawks (Accipiter striatus) have all been shown to have more negative δ 2H values in flight feathers than in covert feathers, which has been suggested might be due to differences in the growth rates of these two types of feathers (Smith et al. 2008). We also observed isotopic differences between claw and feather samples; however, no within-individual correlations between breast feathers and claws or claws and tail feathers were detected and we suggest that it is unlikely the claws were grown in the same location as the feathers.
Our study is the first to our knowledge to examine moult migration in western North American songbirds by tracking individuals throughout the annual cycle. Earlier studies of Bullock’s orioles demonstrated that moult migration was occurring, but had not been able to assign breeding origin of birds, discern how widespread the phenomenon was within breeding populations, or provide any details of possible intraspecific differences in moult strategies (Rising and Williams 1999; Pyle et al. 2009). To our knowledge, there has been only one study (Contina et al. 2013) that used both stable isotope analysis and geolocators to examine moult migration. Whereas Contina et al. (2013) found differences in moult and migration strategies within a breeding population, our study suggested a consistent pattern of moult migration in the Mexican monsoon region, yet demonstrated variation in both moulting areas and over-wintering sites. As a number of western North American songbirds seem to employ this same moult migration strategy (Rohwer et al. 2005; Pyle et al. 2009), investigating patterns of moult and migration in these other species using an approach that combines the use of stable isotope analysis and geolocators would give us a broader understanding of the evolution of this interesting life history.
References
Barta Z, McNamara JM, Houston AI, Weber TP, Hedenström A, Feŕό O (2008) Optimal moult strategies in migratory birds. Philos Trans R Soc B 363:211–229
Bearhop S, Hilton GM, Votier SC, Waldron S (2004) Stable isotope ratios indicate that body condition in migrating passerines is influenced by winter habitat. Proc R Soc B 271:215–218
Bowen GJ, Revenaugh J (2003) Interpolating the isotopic composition of modern meteoric precipitation. Water Resour Res 39:1299
Bridge ES, Thorup K, Bowlin MS, Chilson PB, Diehl RH, Fléron RW, Hartl P, Kays R, Kelly JF, Robinson WD (2011a) Technology on the move: recent and forthcoming innovations for tracking migratory birds. Bioscience 61:689–698
Bridge ES, Fudickar AM, Kelly JF, Contina A, Rohwer S (2011b) Causes of bimodal stable isotope signatures in the feathers of a molt-migrant songbird. Can J Zool 89:951–959
Butcher GS (1991) Mate choice in female northern orioles with a consideration of the role of the black male coloration in female choice. Condor 93:82–88
Chambers M, David G, Ray C, Leitner B, Pyle P (2011) Habitats and conservation of molt-migrant birds in Southeastern Arizona. Southwest Nat 56:204–211
Comrie AC, Glenn EC (1998) Principal components-based regionalization of precipitation regimes across the southwest United States and northern Mexico, with an application to monsoon precipitation variability. Clim Res 10:201–215
Contina A, Bridge ES, Seavy NE, Duckles JM, Kelly JF (2013) Using geologgers to investigate bimodal isotope patterns in painted buntings (Passerina ciris). Auk 130:265–272
de la Hera I, Díaz JA, Pérez-Tris J, Luis Tellería J (2009) A comparative study of migratory behaviour and body mass as determinants of moult duration in passerines. J Avian Biol 40:461–465
Delmore KE, Fox JW, Irwin DE (2012) Dramatic intraspecific differences in migratory routes, stopover sites and wintering areas, revealed using light-level geolocators. Proc R Soc B 279:4582–4589
Ethier DM, Kyle CJ, Kyser TK, Nocera JJ (2010) Variability in the growth patterns of the cornified claw sheath among vertebrates: implications for using biogeochemistry to study animal movement. Can J Zool 88:1043–1051
Fudickar AM, Wikelski M, Partecke J (2011) Tracking migratory songbirds: accuracy of light-level loggers (geolocators) in forest habitats. Methods Ecol Evol 3:47–52
Gonzalez-Prieto AM, Hobson KA, Bayly NJ, Gomez C (2011) Geographic origins and timing of fall migration of the veery in northern Columbia. Condor 113:860–868
Hallworth MT, Studds CE, Sillett TS, Marra PP (2013) Do archival light-level geolocators and stable hydrogen isotopes provide comparable estimates of breeding-ground origin? Auk 130:273–282
Heckscher CM, Taylor SM, Fox JW, Afanasyev V (2011) Veery (Catharus fuscescens) wintering locations, migratory connectivity, and a revision of its winter range using geolocator technology. Auk 128:531–542
Hobson KA (2008) Isotopic methods to track animal movements. In: Hobson KA, Wassenaar LI (eds) Tracking animal migration with stable isotopes. Elsevier, London, pp 45–78
Hobson KA, Aubry Y, Wassenaar LI (2004) Migratory connectivity in Bicknell’s thrush: locating missing populations with hydrogen isotopes. Condor 106:905–909
Hobson KA, Van Wilgenburg SL, Wassenaar LI, Larson K (2012) Linking hydrogen (δ2H) isotopes in feathers and precipitation: sources of variance and consequences for assignment to isoscapes. PLoS One 7:e35137
Hutto RL (1998) On the importance of stopover sites to migrating birds. Auk 115:823–825
Kelly JF, Atudorei V, Sharp ZD, Finch DM (2002) Insights into Wilson’s warbler migration from analyses of hydrogen stable-isotope ratios. Oecologia 130:216–221
Langin KM, Reudink MW, Marra PP, Norris DR, Kyser TK, Ratcliffe LM (2007) Hydrogen isotopic variation in migratory bird tissues of known origin: implications for geographic assignment. Oecologia 152:449–457
Leu M, Thompson CW (2002) The potential importance of migratory stopover sites as flight feather molt staging areas: a review for neotropical migrants. Biol Conserv 106:45–56
Lindstrom A, Visser GH, Daan S (1993) The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol Zool 66:490–510
Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282:1884–1886
Meehan TD, Giermakowski JT, Cryan PM (2004) GIS-based model of stable hydrogen isotope ratios in North American growing-season precipitation for use in animal movement studies. Isot. Environ. Healt. S. 40:291–300
Newton I (2011) Migration within the annual cycle: species, sex and age differences. –. J Ornithol 152:169–185
Paxton KL, Van Riper C III, Theimer TC, Paxton EH (2007) Spatial and temporal migration patterns of Wilson’s Warbler (Wilsonia pusilla) in the Southwest as revealed by stable isotopes. Auk 124:162–175
Pérez GE, Hobson KA (2007) Feather deuterium measurments reveal origins of migratory western Loggerhead Shrikes (Lanius ludovicianus excubitorides) wintering in Mexico. Divers Distrib 13:166–171
Pyle P (1997) Identification guide to North American birds. Part 1: Columbidae to Ploceidae. Slate Creek Press, Point Reyes Station
Pyle P (2008) Identification guide to North American birds. Part II Anatidae to Alcidae. Slate Creek Press, Point Reyes Station
Pyle P, Leitner WA, Lozano-Angulo L, Avilez-Teran F, Swanson H, Limón EG, Chambers MK (2009) Temporal, spatial, and annual variation in the occurrence of molt-migrant passerines in the Mexican monsoon region. Condor 111:583–590
Quinlan SP, Green DJ (2011) Variation in deuterium (δD) values of yellow warbler Dendroica petechia feathers grown on breeding and wintering grounds. J Ornithol 152:93–101
Renfrew RB, Kim D, Perlut N, Fox J, Marra PP (2013) Phenological matching across hemispheres in a long-distance migratory bird. Divers Distrib 19:1008–1019
Reudink MW, Marra PP, Kyser TK, Boag PT, Langin KM, Ratcliffe LM (2009) Non-breeding season events influence sexual selection in a long-distance migratory bird. Proc R Soc B 276:1619–1626
Rising JD, Williams PL (1999) Bullock’s oriole (Icterus bullockii). In: Poole A, Gills F (eds) The birds of North America online. Cornell Lab of Ornithology, Ithaca
Rohwer S, Manning J (1990) Differences in timing and number of molts for Baltimore and Bullock’s orioles: implications to hybrid fitness and theories of delayed plumage maturation. Condor 92:125–140
Rohwer S, Butler LK, Froehlich DR, Greenberg R, Marra PP (2005) Ecology and demography of east–west differences in molt scheduling of Neotropical migrant passerines. In: Greenberg R, Marra PP (eds) Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press, Baltimore, pp 87–105
Rohwer VG, Rohwer S, Ortiz-Ramirez MF (2009) Molt biology of resident and migrant birds of the monsoon region of west Mexico. Ornitologia Neotropical. 20:565–584
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol 19:256–263
Ryder TB, Fox JW, Marra PP (2011) Estimating migratory connectivity of gray catbirds (Dumetella carolinensis) using geolocator and mark-recapture data. Auk. 128:448–453
Smith AD, Donohue K, Dufty AM Jr (2008) Intrafeather and intraindividual variation in the stable-hydrogen isotope (δD) content of raptor feathers. Condor. 110:500–506
Stutchbury BJ, Tarof SA, Done T, Gow E, Kramer PM, Tautin J, Fox JW, Afanasyev V (2009) Tracking long-distance songbird migration by using geolocators. Science 323:896–896
Svensson E, Hendenström A (1999) A phylogenetic analysis of the evolution of moult strategies in Western Palearctic warblers (Aves: Sylviidae). Biol. Jour. Linn. Soc. 67:263–276
Wassenaar LI (2008) An introduction to light stable isotopes for use in terrestrial animal migration studies. In: Hobson KA, Wassenaar LI (eds) Tracking animal migration with stable isotopes. Elsevier, London, pp 21–44
Wassenaar LI, Hobson KA (2000) Stable-carbon and hydrogen isotope ratios reveal breeding origins of red-winged blackbirds. Ecol Appl 10:911–916
Wassenaar LI, Hobson KA (2003) Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isot Environ Health Stud 39:211–217
Acknowledgments
We would like to thank D. Carlyle-Moses, and D. Green for insightful comments and suggestions on this manuscript. We would also like to thank S. Joly, O. Greaves, and J. Crawford for field assistance on this project and C. France for assistance with stable isotope analysis. Thank you also to the Dreger family, the owners of the Knutsford Campground and T. McLeod at Tranquille on the Lake for access to study sites and the Kamloops Naturalists Club for information on oriole locations. Funding was provided by a Natural Sciences and Engineering Research Council Discovery Grant to M. W. R. and a Natural Sciences and Engineering Research Council Canada Graduate Scholarship to A. G. P.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Guglielmo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pillar, A.G., Marra, P.P., Flood, N.J. et al. Moult migration in Bullock’s orioles (Icterus bullockii) confirmed by geolocators and stable isotope analysis. J Ornithol 157, 265–275 (2016). https://doi.org/10.1007/s10336-015-1275-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1275-5