Abstract
The carotenoid-based ornaments displayed by many birds often play key roles in social and sexual signalling, revealing information about individual quality. However, the proximate regulation of the honesty of sexual traits remains controversial. Understanding the mechanisms of coloured trait production and maintenance requires an accurate description of their chemical composition and of the physiological pathways involved in pigment production and deposition in the ornaments. Carotenoid-based colouration has been extensively studied in birds, but such information is often lacking for coloured integuments other than feathers, such as fleshy carotenoid-based ornaments. Here we report the carotenoid composition of the combs of the red grouse (Lagopus lagopus scoticus), a sexual trait that honestly reveals individual quality. In the present study, we also investigated blood carotenoid content, as well as associations between carotenoids, retinol and tocopherol (the active forms of vitamin A and E, respectively) within the ornament. We found that comb pigmentation was primarily the result of two red ketocarotenoids (astaxanthin and papilioerythrinone), which are synthesised from their dietary precursors (zeaxanthin and lutein) directly at the comb integument. These red ketocarotenoids are largely deposited esterified with fatty acids. Astaxanthin concentration in the comb was found to negatively correlate with retinol levels but positively correlate with tocopherol levels. Considering evidence from this and other studied species, we suggest that carotenoid esterification is a characteristic of coloured fleshy integuments, probably affecting pigment stability and colouration in living tissues, with subsequent effects on their signalling role and maintenance costs. We found little evidence that the honesty of this signal would result from a direct connection with vitamin A metabolism, as recently proposed. Rather, honest signalling via comb colouration appears more related to potential allocation trade-offs of some specific dietary precursors or to the capacity of individuals to manage the redox reactions interfering with carotenoid metabolism.
Zusammenfassung
Karotinoidprofil und Vitamine in den Hautlappen des Schottischen Moorschneehuhns ( Lagopus lagopus scoticus ): Folgen für die Ehrlichkeit eines sexuellen Signals Die Karotinoid-basierten Ornamente, die von vielen Vögeln zur Schau gestellt werden, spielen oft eine Schlüsselrolle in der sozialen und sexuellen Kommunikation und stellen Informationen über die Qualität eines Individuums bereit. Die proximate Regulierung der Ehrlichkeit sexueller Merkmale ist jedoch nach wie vor kontrovers. Um die Mechanismen der Produktion und Aufrechterhaltung farbiger Merkmale zu verstehen, ist eine genaue Beschreibung ihrer chemischen Zusammensetzung und der Stoffwechselwege, die an der Produktion der Pigmente und ihrer Einlagerung in den Ornamenten beteiligt sind, vonnöten. Karotinoid-basierte Färbung ist bei Vögeln intensiv untersucht worden, doch solche Informationen fehlen oft für farbige Integumente (mit Ausnahme von Federn), wie fleischige Karotinoid-basierte Ornamente. Hier berichten wir über die Karotinoidzusammensetzung der Hautlappen des Schottischen Moorschneehuhns (Lagopus lagopus scoticus), einem ehrlichen sexuellen Merkmal, das die individuelle Qualität anzeigt. Wir haben auch den Karotinoidgehalt im Blut untersucht, sowie die Zusammenhänge zwischen Karotinoiden, Retinol und Tocopherol (den aktiven Formen von Vitamin A bzw. E) innerhalb des Ornaments. Wir fanden heraus, dass die Pigmentierung der Hautlappen hauptsächlich auf zwei rote Ketokarotinoide (Astaxanthin und Papilioerythrinon) zurückgeht, die aus in der Nahrung vorkommenden Vorstufen (Zeaxanthin und Lutein) direkt im Hautlappen-Integument synthetisiert werden. Diese roten Ketokarotinoide werden hauptsächlich als Fettsäureester eingelagert. Die Astaxanthinkonzentration im Hautlappen korrelierte negativ mit dem Retinollevel, aber positiv mit dem Tocopherollevel. In Anbetracht von Befunden an dieser und anderen untersuchten Arten schlagen wir vor, dass die Veresterung von Karotinoiden eine Eigenschaft gefärbter fleischiger Integumente ist. Sie beeinflusst wahrscheinlich die Pigmentstabilität und die Färbung in lebenden Geweben, was wiederum deren Rolle als Signal und die energetischen Kosten ihrer Aufrechterhaltung beeinflusst. Wir haben kaum Hinweise darauf gefunden, dass die Ehrlichkeit dieses Signals aus einer direkten Verbindung mit dem Vitamin A-Stoffwechsel resultiert, wie kürzlich vorgeschlagen wurde. Vielmehr steht das ehrliche Signalisieren mittels der Hautlappenfärbung wohl damit in Zusammenhang, dass die in der Nahrung vorkommenden spezifischen Pigmentvorstufen verschiedenen Funktionen zugeteilt werden müssen, oder mit dem Vermögen von Individuen, die Redoxreaktionen zu regeln, die mit dem Karotinoidstoffwechsel interferieren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are large lipophilic molecules that often confer yellow, orange or red pigmentation to the teguments displayed by many taxa (Goodwin 1984). Vertebrates cannot synthesise carotenoid pigments de novo, and must obtain them from food, but they are able to transform ingested carotenoids through enzymatic reactions. As a result, the colour of carotenoid-based traits can be conferred by dietary or metabolised carotenoids, or by a mixture of both (McGraw 2006). These coloured traits often play key roles as social and sexual signals honestly revealing individual quality (Hill and McGraw 2006). This occurs in part because carotenoids available for trait pigmentation may be limited, but also because they have other functions in the organism or because their metabolism interferes with vital cellular processes, thus leading to physiological trade-offs (Lozano 1994; Pérez-Rodríguez 2009; von Schantz et al. 1999; Hill and Johnson 2012; Johnson and Hill 2013; Simons et al. 2012). Carotenoid metabolism and transformation are key processes ultimately determining the expression of carotenoid-based ornaments (McGraw 2006). It is therefore essential to study the biochemistry, physiology and metabolism of these pigments in order to understand the function and evolution of the signals that are based on them (McGraw 2006; Pérez-Rodríguez 2009; Hill and Johnson 2012; Johnson and Hill 2013).
The carotenoid profiles of avian plumage have been described for many species (McGraw 2006). However, carotenoid pigmentation is also often present in metabolically active teguments such as beaks, skin, wattles and combs (McGraw 2006). Unlike the carotenoids in feathers, which are deposited in their free forms, carotenoids deposited in “living” teguments are largely esterified; i.e., the hydroxyl groups present on the carotenoid end rings are bound to one or two fatty acids, forming carotenoid monoesters or diesters, respectively (McGraw 2006; Garcia-de Blas et al. 2013). Carotenoid esterification may be functionally important, as it can affect perceived colouration, pigment stability and deposition into living tissues (Perez-Galvez and Minguez-Mosquera 2005; Pintea et al. 2005; Rao et al. 2007), with important implications for the colour lability and maintenance costs of these carotenoid-based traits. However, there is little information on carotenoid esterification in bird ornaments, as most studies use saponification to separate carotenoids from fatty acids during the analytical process, precluding the quantification of free and esterified forms of each pigment individually.
Carotenoids found in avian ornaments can be divided into xanthophylls, which contain carbon, hydrogen and oxygen, and carotenes, which contain only carbon and hydrogen. The former have been more frequently detected in both feathers and teguments (Stradi 1998; McGraw 2006). Xanthophylls can be further subdivided into hydroxycarotenoids (e.g., lutein, zeaxanthin) and ketocarotenoids (e.g., astaxanthin, canthaxanthin), depending on whether the oxygen-containing substituent is a hydroxyl or a ketone, respectively. Xanthophylls allocated to ornaments may be either directly obtained from the diet or as the product of enzymatic transformations from substrate xanthophylls (e.g., Prager et al. 2009; Prum et al. 2012). In many birds, red colours are produced by ketocarotenoids, which are the result of oxidation of yellow hydroxycarotenoids (McGraw 2006). These enzymatic transformations may involve some energetic and oxidative costs that should be added to those cited above, potentially reinforcing the reliability of the signal, and explaining the high frequency of ketocarotenoid-based ornaments among avian species (Hill 1996, 2000). Identifying the existence and nature of these metabolic costs is necessary for explaining the observed variability in colour expression among species and individuals. It was recently proposed that ornaments coloured by ketocarotenoids could be synthesised from dietary pro-vitamin A carotenoid precursors. Given the central role of vitamin A in homeostatic control, including redox status, ketocarotenoid synthesis may be closely connected to key cellular processes, providing a functional link between individual health and ornament expression (Hill and Johnson 2012). However, empirical evidence supporting this hypothesis is still scarce.
The anatomical site in which all of these carotenoid transformations take place remains controversial. The liver is a primary storage site for carotenoids and a key organ for lipid metabolism, which has led some authors to propose that biotransformation of hydroxycarotenoids into ornamental ketocarotenoids could take place in the liver (del Val et al. 2009; Hill and Johnson 2012). However, the conversion of dietary into ornamental carotenoids has also been reported to occur within the ornamental integuments for several species (McGraw 2004; McGraw et al. 2006; Garcia-de Blas et al. 2015). Identifying the organ(s) in which these metabolic conversions take place is essential for evaluating the potential costs associated with pigment transformation, transport and deposition in ornaments. Also, evaluating the link between ornamental carotenoids and other compounds such as antioxidants or vitamin A (see above) at the site of metabolism would provide functionally meaningful insight. Such information, however, is currently lacking for bird species.
Grouse are a subfamily of medium-sized birds that display brightly coloured supraorbital combs, a conspicuous sexual ornament that plays a major role in intra- and inter-sexual selection (Watson and Moss 2008). The yellow–red colour of grouse combs is often referred to as “carotenoid-based”. However, the carotenoid composition of combs has been accurately characterised only in the capercaillie (Tetrao urogallus), with astaxanthin identified as the most abundant carotenoid, accompanied by lower levels of zeaxanthin, lutein and an unidentified carotenoid similar to adonixanthin (Egeland et al. 1993). In red grouse (Lagopus lagopus scoticus), preliminary analyses also confirmed the carotenoid nature of comb colouration, but failed to accurately characterise their carotenoid profile (Mougeot et al. 2006). The signalling function of red grouse comb colouration, however, is better established: comb redness reflects individual body condition, parasite levels, circulating testosterone, immunocompetence and oxidative stress (Mougeot et al. 2006, 2007; Martinez-Padilla et al. 2007; Mougeot 2008; Martinez-Padilla et al. 2010, 2014; ; Pérez-Rodríguez et al. 2013). These previous works provided evidence for comb colouration to honestly advertise individual quality and for the existence of strong links between carotenoid metabolism and individual health status. However, assessing the mechanisms ensuring the honesty of this coloured trait requires a more precise knowledge of the carotenoid contents of combs and of the mechanistic control of comb colouration.
In this study, we used high-performance liquid chromatography to characterise the carotenoid composition of the supraorbital combs of male red grouse. The chromatographic techniques that we employed do not require sample saponification, allowing us to separately quantify the esterified and free forms of each carotenoid present in the comb. In order to determine the potential precursors, the metabolic pathway and the most probable anatomic site of ornamental carotenoid synthesis, we also analysed the carotenoid profile from plasma samples. Combs are living tissues whose colour can change within a few days, and so they require a continuous supply of metabolised carotenoids. Since carotenoids are delivered to peripheral tissues in the body through the bloodstream, if comb carotenoids are either present in the diet or synthesised at any internal organ, they must be present in the blood. Alternatively, if they are synthesised directly in the comb integument, they would not be detected in the bloodstream (McGraw 2004, 2009). Lastly, in order to explore the proximate links between ornamental pigmentation and redox homeostasis (von Schantz et al. 1999; Hill and Johnson 2012; Johnson and Hill 2013), we also analysed the associations between comb carotenoids and levels of retinol (the active form of vitamin A) and tocopherol (i.e., vitamin E). In this paper, we discuss the implications of these findings for the honesty of carotenoid-based signals, focusing in particular on the potential costs and trade-offs associated with carotenoid metabolism and expression in living ornamental traits.
Methods
Origin of the samples
In September 2007, we collected combs from eight adult male red grouse that were shot for sport on Catterick estate (North Yorkshire, England). Combs were kept refrigerated in individual sealed bags and then stored at −80 °C until analysis. Given the origin of the combs, we were not able to obtain blood samples from the same individuals. However, we collected blood from three living birds captured as part of another study (Martinez-Padilla et al. 2014). Comb- and blood-sampled birds were of the same sex (male) and age class (adults, i.e., >1 year), and were sampled on the same day and at the same study site, and all were in the same phase of the breeding cycle (establishing their breeding territories). Immediately upon bird capture, blood samples (1 mL) were drawn by extraction from the brachial vein using heparinized syringes. Plasma was separated by centrifugation of the blood for 10 min at 4000 g and stored at −80 °C until analysis.
Carotenoid and vitamin extraction and analysis
A detailed description of the methods used for carotenoid and vitamin extraction and identification has been described previously (García-de Blas et al. 2013, 2014, 2015). Briefly, approximately 70 mg of comb tissue (one comb per bird was analysed) were extracted with 5 mL of hexane:tert-butyl methyl ether (TBME) (1:1) in an electric homogeniser (VDI 12; VWR International LLC, Barcelona) for 30 s. The sample was kept on ice, avoiding exposure to light. The pooled extract was centrifuged (2026 g for 5 min), and the supernatant was evaporated to dryness under a nitrogen stream. The pooled organic extract was then redissolved in 200 µl of chromatographic phase A formed by MeOH:TBME:H2O (81:15:4) and acetone (1:1). For plasma, 50 μL of each sample was mixed with 200 μL of distilled water and 150 μL of ethanol and vortexed for 5 min. The mixture was then extracted twice with 1 mL of hexane using vortex mixing for 15 min each time. Hexane phases were recovered after centrifuging for 5 min at 2500 g (4 °C). These were combined and evaporated to dryness with a nitrogen flow. Residues were immediately redissolved in 100 μL of chromatographic phase A.
For carotenoid and vitamin identification and quantification, we used an Agilent 1100 Series (Agilent Technologies, Santa Clara, CA, USA) system equipped with a diode array (DAD) and fluorescence (FLD) detectors simultaneously and a C30 column (YMC Carotenoid 5 mm, 250 × 4.6 mm i.d.; YMC Co., Ltd. Kyoto, Japan). The injection volume was 20 µL. Phase A was MeOH:TBME:H2O (81:15:4), and phase B was MeOH:TBME (10:90). Samples were initially eluted with 99 % of phase A and 1 % of phase B, changing by gradient to 44 % of phase A and 56 % of phase B in 39 min, and reaching final conditions of 100 % of phase B in 6 min. The system then returned to initial conditions in 5 min, and these were maintained for 5 min to stabilise the column. The flow was 1 mL/min. DAD detection and quantification was performed at 480 nm. In FLD, the excitation and emission wavelengths used for α-tocopherol were 295 and 325 nm, respectively. The identification of free carotenoids, carotenoid esters and vitamins was performed by comparison of retention times and UV–Vis spectra with commercially available carotenoid standards at a working concentration of 100 ng/mL in MeOH and by comparison with spectra available in the literature (Britton et al. 2004). Standards of all carotenoids (adonirubin, astaxanthin, lutein, canthaxanthin, β-carotene, β-cryptoxanthin, echinenone and zeaxanthin) were purchased from CaroteNature (Lupsingen, Switzerland), except for the papilioerythrinone standard, which was obtained from natural sources, as described in (Garcia-de Blas et al. 2014, 2015). The standard for free retinol and tocopherol were purchased from Sigma-Aldrich (Sigma-Aldrich Quimica SL, Madrid, Spain). From chromatograms, we calculated the concentrations of each compound in µmol per gram of comb tissue or nmol per milliliter of plasma sample.
Results
Carotenoid profile and vitamin concentration in combs and plasma
An illustrative chromatogram of the carotenoids found in red grouse combs is shown in Fig. 1a. Average concentrations of each compound detected in the chromatogram are reported in Table S1 (Electronic Supplementary Material, ESM), and a summary of that information, together with retinol and tocopherol concentrations in these tissues, is given in Table 1. Astaxanthin was the primary carotenoid present in combs (90 ± 2.9 % of total carotenoids), followed by papilioerythrinone (8.1 ± 2.7 %), and two unidentified carotenoids contributing less than 1 % to total carotenoids each. Although we were not able to identify these two minor compounds, a close inspection of their absorbance spectra revealed peaks at 468 and 466 nm in the mobile phase of the present study for unidentified free carotenoids 1 and 2, respectively (see Fig. S1 in ESM), suggesting that these two compounds are orange ketocarotenoids.
Chromatograms of a red grouse a comb and b plasma sample. Numbers and letters above each peak indicate the different carotenoids and carotenoid esters found in the samples. The concentration of each compound is summarised in Table 1. A detailed list of all of these compounds and their concentrations is given in Table S1 (Electronic Supplementary Material)
Most of the astaxanthin deposited in combs was present in its mono- or diesterified form (27 ± 6.40 and 53 ± 8.44 % of total astaxanthin, respectively). Most of the papilioerythrinone found in combs was monoesterified (74 ± 4.57 %), and no diesters of this carotenoid were detected. We were not able to identify peak 8 of the chromatogram (Fig. 1a). However, a detailed inspection of its absorbance spectrum indicates that it is probably the result from the co-elution of two different astaxanthin or papilioerythrinone esters.
Lutein and zeaxanthin were the two primary carotenoids detected in plasma (Fig. 1b; Table 1), and represented 75 ± 4.7 and 18 ± 2.5 %, respectively, of total carotenoids circulated by red grouse. Three minor carotenoids were also detected in plasma samples (Fig. 1b; Table 1). Although we were not able to identify them, a careful inspection of their absorbance spectra indicated that they were hydroxycarotenoids (their absorbance spectra and retention times are given in Fig. S2 of the ESM). Therefore, none of the carotenoids detected in the combs were found in blood plasma. No pro-vitamin A carotenoid (e.g., β-carotene, β-cryptoxanthin) was found in either the plasma or combs of red grouse.
Relationship between vitamins and carotenoids in combs
Associations between the levels of retinol, tocopherol and the primary carotenoids detected in red grouse combs are shown in Table 2. Overall, retinol showed no relationship with comb carotenoids except for free astaxanthin, where a negative correlation was detected. In contrast, tocopherol levels in combs were positively related to free and esterified astaxanthin levels (Table 2). Both retinol and tocopherol levels and papilioerythrinone and astaxanthin levels were unrelated (both p > 0.29).
Discussion
While the signalling function of comb colouration in red grouse has been studied in detail (Martinez-Padilla et al. 2007, 2010, 2014; Mougeot 2008; Mougeot et al. 2006, 2007; Pérez-Rodríguez et al. 2013), the present work reports the first detailed description of the chemical basis of comb colouration, thus allowing us to provide novel insight into the proximate mechanism linking ornament expression and individual quality.
We found that the red colour of the combs of red grouse was due to two primary red ketocarotenoids, astaxanthin and papilioerythrinone, which represented 98 % of carotenoids detected in the comb, in an approximate ratio of 1:10. Although astaxanthin has been detected in the skin of other birds (McGraw 2006), papilioerythrinone has only been recently described in living external tissues of another avian species, the red-legged partridge (Alectoris rufa) (Garcia-de Blas et al. 2014). Among grouse species, comb carotenoids have been accurately characterised only in the capercaillie, for which astaxanthin was the main carotenoid (88 % of total carotenoids), while papilioerythrinone was not detected (Egeland et al. 1993).
Comb carotenoids presented an overall degree of esterification of approximately 80 %, which is very similar to that described for similar avian traits such as the eye rings of red-legged partridges (84 %) (Garcia-de Blas et al. 2013) or the combs of capercaillies (83 %) (Egeland et al. 1993). However, these esterification levels are much higher than those reported in the wattles of domestic hens (29 %, Gallus gallus domesticus) or turkeys (10 %, Meleagris gallopavo) (Czeczuga 1979). The relative proportions of astaxanthin in free forms or forming mono- and diesters (20, 28 and 52 %, respectively) in red grouse combs were very similar to those reported in the closely related capercaillie. In contrast to astaxanthin, papilioerythrinone was only present in free or monoesterified forms, and never in its diesterified form, as was recently reported for red-legged partridge ornamental traits (Garcia-de Blas et al. 2014). Our results support the growing evidence that carotenoid esterification is the rule for living teguments in birds, as well as in fish and reptiles (Pike et al. 2011; San-Jose et al. 2012; Casagrande et al. 2011; Garcia-de Blas et al. 2013). This contrasts with feather carotenoids, which are deposited in free form or, in some cases, bound to proteins (Mendes-Pinto et al. 2012). Carotenoid esterification plays an important role favouring pigment solubility in cell membranes and in improving pigment stability, particularly against photodegradation (Pintea et al. 2005; Subagio et al. 1999; Perez-Galvez and Minguez-Mosquera 2005), which may be relevant for carotenoids deposited in exposed teguments. In addition, the degree of carotenoid esterification may influence ornament colour perception, enhancing colour intensity (Pérez-Rodríguez and Vinuela 2008; Garcia-de Blas et al. 2013). Previous studies have demonstrated a link between comb redness and carotenoid levels in red grouse (Martinez-Padilla et al. 2007; Mougeot et al. 2007), but whether the degree of carotenoid esterification (and the types and proportions of these esters) affects comb colour has yet to be properly evaluated.
In this study, we found that the carotenoid profile of red grouse combs was exclusively formed by ketocarotenoids. In contrast, plasma samples of male grouse captured on the same day and at the same site contained only hydroxycarotenoids, mostly lutein and zeaxanthin, as is typical in most species with plant diets. Comb redness is updated daily by a continuous deposition of carotenoids in the comb epidermis (Mougeot el al. 2006). Therefore, a direct dietary origin of comb carotenoids or a metabolic conversion of dietary hydroxycarotenoids into ornamental ketocarotenoids in an internal organ (i.e., liver; del Val et al. 2009) should be detected in the blood of living individuals. Our findings indicate, rather, that the carotenoids used for comb pigmentation are produced in situ, within the comb integument, similar to that found in the plumage and teguments of several passerine birds (McGraw 2004) and in the red-legged partridge (Garcia-de Blas et al. 2014). It should be noted, however, that our results are based on a reduced number of individuals and that blood and comb samples came from different birds. Although the sources of our samples were totally comparable (see “Methods”), and blood and comb samples showed qualitatively invariable patterns, this calls for some caution. Future studies based on larger sample sizes and on blood and tissue samples collected from the same individuals are needed to confirm our conclusions. To date, unfortunately, this type of information is available for very few bird species. It has been hypothesized that carotenoid metabolism is linked to vital physiological processes, and entails costs in terms of altered redox status (von Schantz et al. 1999; Hill and Johnson 2012; Johnson and Hill 2013). In such a case, the trade-offs regulating colour expression could be radically different if these transformations take place in a vital organ like the liver or in peripheral locations like skin or feather follicles.
Irrespective of the anatomical site of carotenoid biotransformation, the carotenoid profiles of combs and plasma suggest two primary metabolic routes leading to the conversion of dietary hydroxycarotenoids into ornamental ketocarotenoids. Papilioerythrinone would result from the oxidation, followed by the dehydrogenation, of each molecule of dietary lutein (Stradi et al. 2001; LaFountain et al. 2013). Astaxanthin can also be produced from lutein in fish (Hsu et al. 1972). However, the most likely route reported for birds consists of two consecutive oxidation reactions of each zeaxanthin molecule to produce astaxanthin (McGraw 2006; LaFountain et al. 2013). These transformation pathways would be expected to lead to the production of some intermediary compounds, such as papilioerythrin or fritschiellaxanthin in the case of papilioerythrinone production, or adonixanthin in the case of the zeaxanthin route. However, we found none of these compounds in the combs or plasma of red grouse. Moreover, the spectral properties and retention times of the minor unidentified peaks in plasma and combs were not compatible with these compounds. This scenario resembles that of the red-legged partridge (Garcia-de Blas et al. 2015), suggesting that these—still unknown—metabolic routes are very efficient (perhaps involving simultaneous transformations), preventing the detection of intermediate compounds (Hill and Johnson 2012).
An accurate description of the carotenoid composition of red grouse combs has further implications for our understanding of the mechanisms underlying the honesty of this signalling trait. Papilioerythrinone and, particularly, astaxanthin are produced by oxidative transformations that are likely irreversible. This is not compatible with the flexible mechanisms of reallocation proposed for other ketocarotenoids, such as 3-hydroxy-echinenone, which may potentially be reallocated to maintain the vitamin A pool, as proposed by the vitamin A redox hypothesis (Hill and Johnson 2012). In fact, neither lutein nor zeaxanthin, nor the potential intermediate compounds generated in the above-mentioned transformations, are pro-vitamin A carotenoids. In addition, the in situ production of papilioerythrinone and astaxanthin in small and specific skin regions precludes the direct implication of these two pigments in key physiological processes, as they are not susceptible to such allocation trade-offs since they do not circulate through the organism. Our results therefore suggest that the link between comb colouration and individual condition that has been reported by several studies (Pérez-Rodríguez et al. 2013) could arise from an allocation trade-off in dietary precursors (lutein and zeaxanthin) rather than from the use of comb ketocarotenoids directly, or from in situ oxidative costs associated with carotenoid metabolism (see below). Interestingly, comb colouration may fade rapidly (within days) as a result of worsened condition, suggesting that colour maintenance requires a continuous supply of carotenoids to the ornament to bolster signal expression. Such a carotenoid fuelling mechanism would explain both the cost of signal expression, which is key for its honesty, and its value as a dynamic and updated signal of health (Pérez-Rodríguez 2008).
Remarkably, the relative proportions of papilioerythrinone and astaxanthin (1:10) in the combs of red grouse do not match those of their respective precursors, lutein and zeaxanthin, in plasma (5:1). In other words, the main ketocarotenoid pigmenting red grouse combs is synthesised from the less abundant dietary hydroxycarotenoid substrate. Zeaxanthin is proportionally less abundant in food, and thus may be more limited, such that only good foragers could maximise the relative contribution of astaxanthin to comb colouration. Also, the synthesis of astaxanthin from zeaxanthin, which involves two oxidative steps, could be more sensitive to alterations in the redox state than the synthesis of papilioerythrinone from lutein, which involves one oxidation and one dehydrogenation step. If only high-quality birds are able to endure these respective biotransformation costs, the reliability of coloured traits as a signal of individual quality is thus reinforced, a hypothesis recently proposed for the red-legged partridge (Garcia-de Blas et al. 2014).
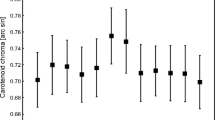
The sample sizes of this study are sufficient to obtain a reliable carotenoid profile of red grouse combs and to support our conclusions derived from it, but our correlative results (Table 1) must be considered with some caution. Nonetheless, vitamin levels in ornamental teguments, despite their central role in several hypotheses addressing the honesty of carotenoid-based signals, are rarely reported, and in this sense our results are valuable. We found that retinol was negatively related to free astaxanthin, whereas tocopherol levels in the comb were positively related to free astaxanthin, suggesting some interference between carotenoid synthesis and vitamin A metabolism. However, as noted above, neither astaxanthin nor its dietary precursor or intermediary compounds are pro-vitamin A carotenoids, which implies an indirect link rather than the shared pathway depicted by the vitamin A redox hypothesis (Hill and Johnson 2012). In contrast to retinol, tocopherol concentration in the comb was positively related to free and esterified astaxanthin levels. The oxidative transformation of dietary zeaxanthin into astaxanthin can act to increase oxidative stress at the site of metabolic transformation. Tocopherol is a powerful dietary antioxidant (Halliwell and Gutteridge 2007), and its association with astaxanthin levels in the comb may thus be mechanistically meaningful. The honesty of the signal may come from the higher capacity of healthy (i.e., not oxidised) individuals to allocate greater amounts of antioxidants at the site of ketocarotenoid synthesis, boosting colour production. Therefore, ketocarotenoid synthesis may reflect the efficiency of key redox cellular processes (Hill 2011, 2014). Interestingly, we found that the ratio of tocopherol to retinol ratio in red grouse blood was only 5:1, whereas it increased to 258:1 in combs, which is consistent with the above-mentioned strategic antioxidant allocation of antioxidants to buffer the potential oxidative costs of these metabolic transformations.
In conclusion, we have provided detailed information regarding the chemical composition of a well-studied carotenoid-based sexual ornament: the red colouration of red grouse combs. This has allowed us to shed new light on the expression and proximate mechanisms regulating the honesty of this sexual signal. The two primary carotenoid pigments responsible for comb redness were ketocarotenoids synthesised in situ, within the comb integument, from dietary precursors. The site of carotenoid transformation (i.e., integument or feather follicle vs. liver) has been determined for few bird species, and warrants further research in order to better understand the variability among species and to draw general patterns or hypotheses. We also found that ketocarotenoids were largely deposited in combs as mono- and diesters. There exists significant variability within and among species in the degree of carotenoid esterification, but we are far from understanding its biological significance. Experimental studies analysing its role in pigment deposition, stability and colour properties are needed. We found little support for the Vitamin A redox hypothesis (Hill and Johnson 2012) in this species, as metabolic pathways leading to red ornamental ketocarotenoids did not involve the participation of pro-vitamin A carotenoids. We hypothesise that the proximate regulation of the honesty of carotenoid-based comb colour could come from 1) the constraints imposed by limitations in the availability of specific dietary precursors of the primary comb carotenoid, and 2) a lower capacity of oxidised individuals to allocate sufficient antioxidant defences to combs to bolster ketocarotenoid synthesis. Future studies should experimentally address how systemic redox state relates to antioxidant allocation at the site of pigment transformation, and whether this ultimately results in variations in ornament colouration.
References
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids handbook. Birkhaüser, Basel
Casagrande S, Dijkstra C, Tagliavini J, Goerlich VC, Groothuis TG (2011) Differential effects of testosterone, dihydrotestosterone and estradiol on carotenoid deposition in an avian sexually selected signal. J Comp Physiol A 197:1–13
Czeczuga B (1979) Carotenoids in the skin of certain species of birds. Comp Biochem Physiol B 62:107–109
del Val E, Senar JC, Garrido-Fernandez J, Jaren M, Borras A, Cabrera J, Negro JJ (2009) The liver but not the skin is the site for conversion of a red carotenoid in a passerine bird. Naturwissenschaften 96:797–801
Egeland ES, Parker H, Liaaen-Jensen S (1993) Carotenoids in combs of capercaillie (Tetrao urogallus) fed defined diets. Poult Sci 72:747–751
Garcia-de Blas E, Mateo R, Vinuela J, Pérez-Rodríguez L, Alonso-Alvarez C (2013) Free and esterified carotenoids in ornaments of an avian species: the relationship to color expression and sources of variability. Physiol Biochem Zool 86:483–498
Garcia-de Blas E, Mateo R, Guzman Bernardo FJ, Rodriguez Martin-Doimeadios RC, Alonso-Alvarez C (2014) Astaxanthin and papilioerythrinone in the skin of birds: a chromatic convergence of two metabolic routes with different precursors? Naturwissenschaften 177:259–271
Garcia-de Blas E, Mateo R, Alonso-Alvarez C (2015) Accumulation of dietary carotenoids, retinoids and tocopherol in the internal tissues of a bird: a hypothesis for the cost of producing colored ornaments. Oecologia 177:259–271
Goodwin TW (1984) The Biochemistry of Carotenoids, Vol. 2 Animals. 2nd edn, London
Hill GE (1996) Redness as a measure of the production cost of ornamental coloration. Ethol Ecol Evol 8:157–175
Hill GE (2000) Energetic constraints on expression of carotenoid-based plumage coloration. J Avian Biol 31:559–566
Hill GE (2011) Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol Lett 14:625–634
Hill GE (2014) Cellular Respiration: the nexus of stress, condition, and ornamentation. Integr Comp Biol 54:645–657
Hill GE, Johnson JD (2012) The vitamin A-redox hypothesis: a biochemical basis for honest signaling via carotenoid pigmentation. Am Nat 180:E127–E150
Hill GE, McGraw KJ (2006) Bird Coloration, Vol. 2. Function and evolution. Harvard University Press, Cambridge
Hsu WJ, Rodríguez DB, Chichester CO (1972) The biosynthesis of astaxanthin. VI. the conversion of [14c]lutein and [14c] β-carotene in goldfish International. J Biochem 3:333–338
Johnson JD, Hill GE (2013) Is carotenoid ornamentation linked to the inner mitochondria membrane potential? A hypothesis for the maintenance of signal honesty. Biochimie 95:436–444
LaFountain AM, Frank HA, Prum RO (2013) Carotenoids from the crimson and maroon plumages of Old World orioles (Oriolidae). Arch Biochem Biophys 539:126–132
Lozano GA (1994) Carotenoids, parasites, and sexual selection. Oikos 70:309–311
Martinez-Padilla J, Mougeot F, Pérez-Rodríguez L, Bortolotti GR (2007) Nematode parasites reduce carotenoid-based signalling in male red grouse. Biol Lett 3:161–164
Martinez-Padilla J, Mougeot F, Webster LM, Pérez-Rodríguez L, Piertney SB (2010) Testing the interactive effects of testosterone and parasites on carotenoid-based ornamentation in a wild bird. J Evol Biol 23:902–913
Martinez-Padilla J, Pérez-Rodríguez L, Mougeot F, Ludwig S, Redpath SM (2014) Intra-sexual competition alters the relationship between testosterone and ornament expression in a wild territorial bird. Horm Behav 65:435–444
McGraw KJ (2004) Colorful songbirds metabolize carotenoids at the integument. J Avian Biol 35:471–476
McGraw KJ (2006) Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ (eds) Bird Coloration vol. 1: mechanisms and measurements, vol. 1: mechanisms and measurements. Harvard University Press, Cambridge
McGraw KJ (2009) Identifying anatomical sites of carotenoid metabolism in birds. Naturwissenschaften 96:987–988
McGraw KJ, Nolan PM, Crino OL (2006) Carotenoid accumulation strategies for becoming a colourful House Finch: analyses of plasma and liver pigments in wild moulting birds. Funct Ecol 20:678–688
Mendes-Pinto MM, LaFountain AM, Stoddard MC, Prum RO, Frank HA, Robert B (2012) Variation in carotenoid-protein interaction in bird feathers produces novel plumage coloration. J R Soc Int 9:3338–3350
Mougeot F (2008) Ornamental comb colour predicts T-cell-mediated immunity in male red grouse Lagopus lagopus scoticus. Naturwissenschaften 95:125–132
Mougeot F, Martínez-Padilla J, Pérez-Rodríguez L, Bortolotti GR (2006) Carotenoid-based colouration and ultraviolet reflectance of the sexual ornaments of grouse. Behav Ecol Sociobiol 61:741–751
Mougeot F, Pérez-Rodríguez L, Martínez-Padilla J, Leckie F, Redpath SM (2007) Parasites, testosterone and honest carotenoid-based signalling of health. Funct Ecol 21:886–898
Perez-Galvez A, Minguez-Mosquera MI (2005) Esterification of xanthophylls and its effect on chemical behavior and bioavailability of carotenoids in the human. Nutr Res 25:631–640
Pérez-Rodríguez L (2009) Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. BioEssays 31:1116–1126
Pérez-Rodríguez L (2008) Carotenoid-based ornamentation as a dynamic but consistent individual trait. Behav Ecol Sociobiol 62:995–1005
Pérez-Rodríguez L, Vinuela J (2008) Carotenoid-based bill and eye ring coloration as honest signals of condition: an experimental test in the red-legged partridge (Alectoris rufa). Naturwissenschaften 95:821–830
Pérez-Rodríguez L, Martinez-Padilla J, Mougeot F (2013) Carotenoid-based ornaments as signals of health status in birds: evidences from two galliform species, the red-legged partridge (Alectoris rufa) and the red grouse (Lagopus lagopus scoticus). In: Yamaguchi M (ed) Carotenoids: food sources, production and health benefits. Nova Science Publishers, New York
Pike TW, Bjerkeng B, Blount JD, Lindström J, Metcalfe NB (2011) How integument colour reflects its carotenoid content: a stickleback’s perspective. Funct Ecol 25:297–304
Pintea A, Diehl HA, Momeu C, Aberle L, Socaciu C (2005) Incorporation of carotenoid esters into liposomes. Biophys Chem 118:7–14
Prager M, Johansson EIA, Andersson S (2009) Differential ability of carotenoid C4-oxygenation in yellow and red bishop species (Euplectes spp.). Comp Biochem Physiol B 154:373–380
Prum RO, LaFountain AM, Berro J, Stoddard MC, Frank HA (2012) Molecular diversity, metabolic transformation, and evolution of carotenoid feather pigments in cotingas (Aves: Cotingidae). J Comp Physiol B 182:1095–1116
Rao AR, Sarada R, Ravishankar GA (2007) Stabilization of astaxanthin in edible oils and its use as an antioxidant. J Sci Food Agric 87:957–965
San-Jose LM, Granado-Lorencio F, Fitze PS (2012) Vitamin E, vitamin A, and carotenoids in male common lizzard tissues. Herpetologica 68:88–99
Simons MJ, Cohen AA, Verhulst S (2012) What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds—a meta-analysis. PLoS ONE 7:e43088
Stradi R (1998) The colour of flight: carotenoids in bird plumage. Solei Gruppo Editoriale Informatico, Milan
Stradi R, Pini E, Celentano G (2001) Carotenoids in bird plumage: the complement of red pigments in the plumage of wild and captive bullfinch (Pyrrhula pyrrhula). Comp Biochem Physiol B 128:529–535
Subagio A, Wakaki H, Morita N (1999) Stability of lutein and its myristate esters. Biosci Biotechnol Biochem 63:1784–1786
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc B-Biol Sci 266:1–12
Watson A, Moss R (2008) Grouse. Collins, London
Acknowledgments
This study was financed in part by project CGL2011-26318 (Ministerio de Ciencia e Innovación). LP-R was supported by a postdoctoral contract from the Spanish Ministerio de Economía y Competitividad (MINECO), through the Severo Ochoa Programme for Centres of Excellence in Research, Development and Innovation (SEV-2012-0262).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Fusani.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pérez-Rodríguez, L., de Blas, E.G., Martínez-Padilla, J. et al. Carotenoid profile and vitamins in the combs of the red grouse (Lagopus lagopus scoticus): implications for the honesty of a sexual signal. J Ornithol 157, 145–153 (2016). https://doi.org/10.1007/s10336-015-1261-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1261-y