Abstract
In many species of migratory birds, individuals of different populations, sexes and age classes migrate at different times and manage their energy reserves according to their specific migratory strategies. In this study, we analyzed the migratory patterns related to geographic provenance, sex and age in the Northern Wheatear Oenanthe oenanthe, a long-distance migratory passerine, during one spring season on the island of Ventotene (Italy), a Mediterranean stopover site. Individuals of different sex and age did not differ significantly in their average date of passage, but birds with longer and more pointed wings passed through later in the season than birds with shorter wings. Morphometric measurements combined with isotopic analysis revealed that late-arriving birds probably belonged to more distant breeding populations and this later date of passage probably mirrors the delayed arrival observed at their northern breeding grounds. Adult birds arrived in better condition than second-year birds, possibly as a result of better energy management. Birds passing through later in the season were also in better condition, which might be explained by their wing morphology favoring endurance flights and thus improving energetic efficiency when crossing the Mediterranean. Birds which migrate longer distances might also carry larger fuel loads to safely complete their journey. This intra-specific study shows that the birds’ organization of their migration schedule is population-specific in correspondence to the temporal requirements which depend on the different environmental conditions on the breeding grounds.
Zusammenfassung
Differentielles Zugverhalten und Körperkondition beim Steinschmätzer ( Oenanthe oenanthe ) an einen Rastplatz im Mittelmeer im Frühjahr
Bei vielen Zugvögeln ziehen verschiedene Populationen, Männchen und Weibchen und/oder verschiedene Altersgruppen zu verschiedenen Zeiten und gestalten ihre Energiereserven entsprechend ihrer spezifischen Zugstrategie. Wir betrachteten die Zugmuster des Steinschmätzer Oenanthe oenanthe, eines Langstreckenziehers, in Beziehung zur Lage des Brutgebietes, zu Geschlecht und Alter während einer Frühjahrssaison auf der italienischen Mittelmeerinsel Ventotene. Die Geschlechter und Altersgruppen unterschieden sich nicht in ihren mittleren Durchzugszeiten, doch zogen Vögel mit längeren und spitzeren Flügeln signifikant später durch als Vögel mit kürzeren Flügeln. Morphometrische Messungen in Kombination mit Isotopenanalysen zeigen, dass die spät durchziehenden Vögel wahrscheinlich zu weiter entfernten Brutpopulationen gehörten, korrespondierend zu deren späterer Ankunft in den nördlichen Brutgebieten. Altvögel kamen auf Ventotene in besserer Kondition an als Vögel in ihrem zweiten Kalenderjahr, möglicherweise eine Folge davon, dass Altvögel mit ihrer Energie besser haushalten. Auch waren später in der Saison durchziehende Vögel in besserer Kondition. Dies könnte eine Folge ihrer Flügelmorphologie sein, die lange Flüge begünstigt und so die Energiebilanz beim Flug über das Mittelmeer verbessert. Auch könnten die weiter ziehenden Vögel mehr Energiereserven für erfolgreichen Zug an sich benötigen. Diese intra-spezifische Studie zeigt, dass der Zugablauf populationsspezifisch ist, korrespondierend mit den zeitlichen Anforderungen, die von den unterschiedlichen Umweltbedingungen im Brutgebiet bestimmt sind.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In migratory birds, differences in timing and migratory routes often result from specific needs both at the individual and population level (Newton 2008). Birds need to adjust their migratory journeys in balance of two important criteria: arrival at their destination to initiate breeding at the optimal time, and high likelihood of completing their migration successfully. In long-distance migrants with broad breeding ranges and wintering areas, conditions may vary geographically and seasonally. This may require some adjustments of their migration strategies according to the birds’ place of origin and the distance to be covered. For example, birds should arrive at their breeding grounds in order to match the timing of optimal food availability for breeding (Lack 1968; van Noordwijk et al. 1995; Jonzén et al. 2007; Newton 2008). Furthermore, males and females or respective age classes may be exposed to different ecological and physiological constraints which may lead to different migration timing. For example, in protandrous bird species, males arrive earlier than females at the breeding grounds (Morbey and Ydenberg 2001; Coppack and Pulido 2009).
In general, migratory birds are faced with a trade-off between minimizing energy expenditure and reducing the total time needed for the journey (Hedenström and Alerstam 1997). Both early arrival and body condition at arrival have an impact on the reproductive fitness of birds (Potti and Montalvo 1991; Sandberg and Moore 1996; Currie et al. 2000; Smith and Moore 2005), although the relative importance of these factors may differ between sexes and age classes. Since the speed of migration depends to a greater extent on stopover duration than on actual flight speed (Hedenström and Alerstam 1997), we might expect birds seeking to reduce journey duration to shorten stopovers, thus carrying lower fuel stores, whereas birds aiming to arrive at their breeding grounds in good body condition might travel more slowly, but carrying higher fuel reserves. However, these strategies are not mutually exclusive and may vary throughout the season. Environmental variables such as adverse weather (e.g., low temperatures or wind drift) may also affect migratory strategies. Timing of migration and physiological correlates vary between populations, sexes, and age classes, and different species may adopt different strategies in order to match their specific requirements. Therefore, investigating the patterns of differential migration within a species is of paramount importance to understand the selective pressures acting at both the individual and population levels.
Differential migration can be observed en route at staging places (Newton 2008) provided that sexes, age classes, and populations can be distinguished from each other. Morphometric measurements are a useful tool for this purpose, since some morphological variables (e.g., body size or wing shape; Lockwood et al. 1998) vary in many species with the distance covered during migration (Chandler and Mulvihill 1990; Marchetti et al. 1995; Fiedler 2005). In addition, the analysis of stable isotopes in feathers has provided valuable information on migratory connectivity and offers a further tool for assigning individual birds to specific breeding populations (Rubenstein et al. 2002; Hobson 2005; Hobson and Wassenaar 2008). Over the past decade, stable hydrogen ratios of feathers have helped to identify migratory connectivity in a range of migratory species (Hobson and Wassenaar 2008), and the analysis of δ13C and δ15N has shown the different wintering grounds of two different subspecies of Willow Warbler (Phylloscopus trochilus) from Scandinavia (Chamberlain et al. 2000).
The Northern Wheatear (Oenanthe oenanthe) is a widespread passerine with a Holarctic breeding range; all populations winter in sub-Saharan Africa (Cramp 1988). It has been shown that populations and sexes differ in their timing of migration (Dierschke et al. 2005), and these findings have been confirmed by laboratory studies which have revealed the endogenous basis of these differences (Maggini and Bairlein 2010; 2012). Previous studies on the Wheatear were focused on northern stopover sites, where birds of different subspecies were easily assigned to their breeding population (Delingat et al. 2011). In this study, we wanted to investigate the migratory patterns of Northern Wheatears at a more southern stopover site placed just after the Mediterranean crossing, offering a unique opportunity to sample staging migrants belonging to a mix of geographical populations, all belonging to the nominate subspecies. In particular, we addressed the following questions:
-
1.
Are differences in the date of passage related to sex, age, and population?
-
2.
Do birds differ in body condition depending on passage date, sex, age, or population?
Methods
Study site
We trapped Northern Wheatears on the island of Ventotene, Italy (40°47′N, 13°25′E, some 50 km off the western Italian coast), during spring 2006. The island is only 1.54 km2 in area and is known for attracting large numbers of migrants during spring migration (Spina et al. 1993). Trapping effort was continuous between 1 March and 30 May, and personal observations did not reveal the presence of Wheatears outside this study period. Therefore, we assume to have covered the whole spring migratory season of this species on the island.
Capture protocol
Birds were mostly trapped within a standardized program using a total of 282 m of mist-nets (4 shelves, mesh 16 mm), placed in the macchia vegetation on the southern tip of the island. Additionally, birds were trapped during selected days in an open area on the northern end of the island using spring traps baited with a mealworm. After capture, birds were ringed and morphometric measurements were taken following Bairlein (1995). We measured the length of the primary feathers P2 to P9, the total length of the flattened and stretched wing (maximum chord), tail length, bill length to the skull, bill width, bill depth, and tarsus length. Sex and age were determined from plumage characters (Svensson 1992; Jenni and Winkler 1994). Fat and muscle scores were determined according to Bairlein (1995). Fat scores were given values from 0 to 8, and muscle scores ranged between 0 and 3. Birds were weighed with a precision of ±0.1 g. For isotopic analysis we sampled the second outermost tail feather (TF5) from every bird.
Stable isotope analysis
Stable-hydrogen isotope ratios of feathers were analyzed at the stable isotope laboratory of the Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany, using an isotope ratio mass spectrometer (Delta V Advantage; Thermo Finnigan, Bremen, Germany) connected to an elemental analyser (HT Elementaranalysator; HEKAtech, Wegberg, Germany) (see Erzberger et al. 2011 for details). We used the international standards IAEA NBS 22 (mineral oil) and IAEA-CH-7 (polyethylene) to determine the stable isotope ratio of the reference gas used in the mass spectrometer. We refer to stable hydrogen isotope ratios in the δ notation, which expresses isotope ratios in relation to an international standard (Vienna Standard Mean Ocean Water for hydrogen) in the unit parts per mil (‰) deviations from the standard.
A small section of feather tissue was clipped from the tip of the feather (350 ± 7 μg) and loaded in silver capsules (IVA Analysetechnik, Meerbusch, Germany), which were allowed to equilibrate with ambient air in a microtiter tray over 1 week. Subsequently, trays were stored for at least 24 h at 50 °C in a drying oven. We used the comparative equilibration method (Wassenaar and Hobson 2003) to account for the amount of exchangeable hydrogen in feather keratin. We used three standards that covered the range of expected δ2H values in our samples. These standards were also used to determine the δ2H of non-exchangeable hydrogen (Wassenaar and Hobson 2000, 2003). The stable hydrogen isotope ratios of the non-exchangeable hydrogen (mean δDn ± 1SD) of the standards were: −133.6 ± 1.2 ‰, −109.1 ± 1.2 ‰, and −87.2 ± 1.0 ‰. In the sequential order of one autorun, keratin standards were placed at positions 1–6 (2 × 3 standards) and at any 9th–11th position (three standards each time). Analytical precision based on the repeated analyses of laboratory keratin standards was always better than 0.851 ‰ (1SD).
Data analysis
We performed a principal component analysis (PCA) to assess the relative importance of morphometric variables for further analysis. Eight variables were included in the PCA: wing length, tail length, tarsus length, bill length to skull, bill depth, bill width, wing pointedness (index C2 in Lockwood et al. 1998), and wing concaveness (index C3 in Lockwood et al. 1998). To assess variation in morphometrics with date, we ran linear models with the first (PCA1) and the second (PCA2) principal components (see “Morphometrics” in the “Results” section), respectively, as response variables and date as the explanatory variable. Age and sex were included in the models as covariates.
To investigate whether there is a link between stable isotope variation and date of arrival, we ran a linear model with δ2H as response variable and date of capture as the explanatory variable. Age, sex, PCA1, and PCA2 were included as covariates.
To investigate variation in body condition with date, we first calculated a body condition index (BCI). BCI was obtained from a PCA of fat score, muscle score, and body mass. In order to correct body mass by size and time of sampling, we used residuals of a linear model with body mass as response variable and PCA1 (as a measure of size) and time of day as covariates. To do this, we had to assume that changes in body mass are linear over the course of the day. This seems to be a good approximation when considering only one mass measurement per individual (Delingat et al. 2009). We then ran a linear model with BCI as response variable and sex, age, date, PCA1, and PCA2 as explanatory variables.
All response variables were tested for normality with Shapiro–Wilk tests. In all models, non-significant terms were stepwise eliminated until reaching a simplified model, according to the principle of parsimony. The simplified models were tested for heteroscedasticity and non-normality of errors by plotting residuals as described in Crawley (2007). All statistics were performed using the software R 2.8.0 (R Development Core Team 2008).
Results
We trapped 134 Northern Wheatears between 27 March and 13 May 2006. Males passed the island about 3.5 days earlier (median = 16 April) than females (median = 19–20 April). This difference, however, was not significant (Wilcoxon–Mann–Whitney test: m = 73, n = 60, W = 1,940, p = 0.258) and, therefore we pooled data (median for adults = 17 April). Second-year birds passed the island at about the same time (median = 16 April) as adults (Wilcoxon–Mann–Whitney test: m = 47, n = 86, W = 2129.5, p = 0.611).
Morphometrics
The two first principal components resulting from the PCA were related to overall body size (PCA1) and to wing shape and bill size (PCA2).
PCA1 resulted from the formula:
PCA2 resulted from the formula:
In both formulas, the standardized values of the morphometric variables (subtracting the mean from every value and dividing the difference by the standard deviation) were used. High values of PCA1 indicate larger birds, whereas high values of PCA2 indicate birds with long, pointed wings and smaller bills. PCA1 explained 26.4 % of the total variance in the data (eigenvalue: 2.111), whereas PCA2 explained 19.1 % of the variance (eigenvalue: 1.530).
Shapiro–Wilk normality tests were non-significant for both PCA1 (W = 0.9949; p = 0.920) and PCA2 (W = 0.9879; p = 0.287), thus allowing the use of parametric statistics. PCA1 was significantly correlated with sex, males being larger than females (Table 1a) and date. Smaller birds passed through later in the season (Table 1a; Fig. 1). PCA2 was significantly related to sex (Table 1b) and date. Birds with longer and more pointed wings (representing populations flying longer distances; Delingat et al. 2011), and also smaller bills, passed later in the season than birds from populations flying shorter distances (Table 1b; Fig. 2). The interaction term of sex and date was close to significance (p = 0.057; Table 1b), and removing it from the model caused an increase in Akaike Information Criterion (AIC), so we did not drop this term from the final model. The increasing slope in PCA2 with date was steeper in males than in females (Fig. 2).
Stable isotopes
Preliminary data exploration of deuterium values revealed the presence of two outliers in the dataset (δ2H = −38.0 and +11.4 ‰, respectively). We removed these values from further analysis since feathers with such high δ2H values are likely to have been grown in the African wintering grounds (see, e.g., Bowen et al. 2005), and therefore do not give any indication of the breeding provenance of these two birds. Thus, we performed analysis of deuterium values of a total of 63 birds, which ranged from a minimum of δ2H = −152.9 ‰ to a maximum of δ2H = −54.2 ‰. After deletion of the two outliers, the Shapiro–Wilk normality test was not significant (W = 0.9773, p = 0.277), thus allowing the use of parametric statistics. δ2H was significantly correlated with date of passage and PCA2 (Table 2). Birds with long and pointed wings had lower δ2H values and passed the stopover site later in the season (Fig. 3).
Body condition
BCI (the first principal component of a PCA using the standardized values of size-corrected body mass, fat, and muscle scores) was obtained from the formula:
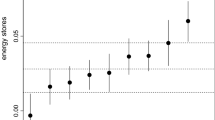
BCI accounted for 62.4 % of the variance and had an eigenvalue of 1.871. Preliminary data exploration of BCI data revealed one outlier (BCI = −5.754). We decided to remove this data point from further analysis because the individual was clearly exhausted (fat and muscle scores = 0, body mass = 16.6 g) and therefore we did not consider it as representative. After deletion of the outlier, the Shapiro–Wilk normality test was not significant (W = 0.9864; p = 0.221), which allowed us to use parametric statistics for further analysis. BCI was significantly correlated to age and date (Table 3). Second-year birds were in slightly poorer condition than adults (Fig. 4) and birds passing later in the season were in better condition (Fig. 5).
Body condition of adult (>second year) versus second-year wheatears on Ventotene in spring 2006, as indicated by a body condition index (BCI) calculated using a PCA with fat and muscle scores, and size-corrected body mass as factors. The horizontal lines show the median BCI value for both age classes, the bottom and top of the boxes show the 25 and 75th percentiles, respectively. Whiskers show 1.5 times the interquartile range of the data. Stars indicate the significance level, **p < 0.01
Discussion
During spring 2006, we observed no significant differences between sexes and age classes in the date of passage of migrating Northern Wheatears on Ventotene. This contrasts with observations of other species (Newton 2008) or of Wheatears at other sites (Dierschke et al. 2005). Protandry is known to occur at the breeding grounds (Currie et al. 2000; Pärt 2001) and to have an endogenous basis in the Wheatear (Maggini and Bairlein 2012). Analysis of data from Ventotene over many years, however, revealed a mean difference in passage dates between males and females of only 1 day (Spina et al. 1994). In our study, the difference in median passage between the two sexes was 3.5 days, albeit the difference was not significant. Possibly, the sex-related or age-related passage times were obscured by the presence of several distinct populations which may differ in their timing of migration, thus leading to a larger variation of passage times and, consequently, to differences that are not statistically detectable.
The morphology of staging Northern Wheatears varied across spring migration on the island of Ventotene. Birds passing the island later were smaller and had proportionately longer, more pointed wings than earlier in the season. The fact that late birds were smaller than early birds could be explained by a higher proportion of migrating females later in the season (although this was not significant in our analysis). Wing length and pointedness have been demonstrated to be related to the overall distance covered on migration between and within species (Chandler and Mulvihill 1990; Marchetti et al. 1995; Fiedler 2005; Förschler and Bairlein 2011). In this study, we were able to give further support to this assumption by analyzing the stable hydrogen ratios in sampled feathers. Birds with longer and more pointed wings had the lowest values of δ2H, indicating their provenance from breeding grounds further away. When considering the potential breeding ranges of wheatears passing through Ventotene in spring (which most probably lie to the NE, Spina and Volponi 2008), decreasing values of δ2H are found towards the north or towards the east, i.e. in northern Scandinavia or western Siberia (Bowen et al. 2005; West et al. 2009). Thus, our data clearly indicate that birds migrating over longer distances pass through Ventotene later in the season, reflecting later arrival dates at the breeding grounds. Central European birds already occupy their breeding territories in April, whereas northern Scandinavian and Siberian birds are not on territory before the end of May (Suter 1988). The birds observed on Ventotene later in the season most probably originate from these areas. Differences in phenology of migrating birds belonging to different populations are known from a wide range of species (Lack 1968; van Noordwijk et al. 1995; Jonzén et al. 2007; Newton 2008; Conklin et al. 2010), and this study provides additional data supporting the idea that this is a general pattern.
Almost all Wheatears examined on Ventotene were in relatively good body condition. As only 1 bird out of 134 captures was found to be clearly exhausted, we are confident that our analysis was representative. Second-year birds were in slightly poorer condition than adults. However, the difference was relatively small (Fig. 4) and can be explained by age-related migratory history or experience. Birds migrating for the first time might be less efficient in adjusting their energy accumulation and consumption in response to environmental conditions (e.g., Pilastro and Magnani 1997; Woodrey and Moore 1997). Furthermore, birds arrived in better body condition when staging on Ventotene later in the season. This can be explained in several ways: (1) birds passing through later in the season may have allocated more time in refuelling at stopover sites than earlier passage migrants; (2) birds passing through later may have accumulated larger energy stores prior to migration; or (3) all birds may have embarked on the Mediterranean crossing with the same amount of reserves, but birds passing later may have been more efficient in using fuel stores during sea crossing thanks to their longer and more pointed wings. Previous studies of both captive and wild Wheatears have shown that the normal migratory strategy used by this species is daily stopover with refueling (Delingat et al. 2006) and that increased fuel loads are accumulated only prior to the crossing of ecological barriers (Delingat et al. 2008; Maggini and Bairlein 2010). These studies do not report correlations between fuel load and overall distance to be covered for Northern Wheatears (Maggini and Bairlein 2010). Thus, we believe that the Wheatears arriving on Ventotene accumulated just enough reserves on the coast of North Africa in order to cross the Mediterranean, irrespective of the distance to their breeding range further to the north or east. Wheatears are opportunists in terms of food choice (Suter 1988) and, therefore, they can expect to find refueling opportunities almost anywhere along their migration route. The same pattern can be observed in other species migrating through continental Europe (Bairlein 2003). Therefore, it seems likely that the differences in body condition observed in Wheatears on Ventotene are a consequence of their different wing morphology, although we cannot completely rule out the possibility that birds facing a longer flight are also carrying more energy reserves.
The results of this study indicate that different populations within a bird species adjust their migration schedule to reach their breeding grounds at an appropriate time. The data suggest that more northern and/or eastern populations of Northern Wheatears migrate later than conspecifics breeding in more southern areas. The observed pattern at the stopover sites may shed new light on what we observe in Northern Wheatears at their breeding grounds, in particular since environmental or climatic changes along migratory routes could affect some individuals or populations more than others.
References
Bairlein F (1995) Manual of field methods. Revised edition. Wilhelmshaven, Germany
Bairlein F (2003) The study of bird migrations—some future perspectives. Bird Study 50:243–253
Bowen GJ, Wassenaar LI, Hobson KA (2005) Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 143:337–348
Chamberlain CP, Bensch S, Feng X, Ǻkesson S, Andersson T (2000) Stable isotopes examined across a migratory divide in Scandinavian willow warblers (Phylloscopus trochilus trochilus and Phylloscopus trochilus acredula) reflect their African winter quarters. Proc R Soc Lond B 267:43–48
Chandler CR, Mulvihill RS (1990) Wing-shape variation and differential timing of migration in dark-eyed juncos. Condor 92:54–61
Conklin JR, Battley PF, Potter MA, Fox JW (2010) Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat Commun 1:67
Coppack T, Pulido F (2009) Proximate control and adaptive potential of protandrous migration in birds. Int Comp Biol 49:493–506
Cramp S (1988) The birds of the western Palearctic, vol 5. Oxford University Press, Oxford
Crawley MJ (2007) The R book. Wiley, Chichester
Currie D, Thompson DBA, Burke T (2000) Patterns of territory settlement and consequences for breeding success in the northern wheatear Oenanthe oenanthe. Ibis 142:389–398
Delingat J, Dierschke V, Schmaljohann H, Mendel B, Bairlein F (2006) Daily stopovers as optimal migration strategy in a long-distance migrating passerine: the northern wheatear Oenanthe oenanthe. Ardea 94:593–605
Delingat J, Bairlein F, Hedenström A (2008) Obligatory barrier crossing and adaptive fuel management in migratory birds: the case of the Atlantic crossing in northern wheatears (Oenanthe oenanthe). Behav Ecol Sociobiol 62:1069–1078
Delingat J, Dierschke V, Schmaljohann H, Bairlein F (2009) Diurnal patterns of body mass change during stopover in a migrating songbird, the northern wheatear Oenanthe oenanthe. J Avian Biol 40:625–634
Delingat J, Hobson KA, Dierschke V, Schmaljohann H, Bairlein F (2011) Morphometrics and stable isotope differentiate populations of northern wheatears (Oenanthe oenanthe). J Ornithol 152:383–395
Dierschke V, Mendel B, Schmaljohann H (2005) Differential timing of spring migration in northern wheatears Oenanthe oenanthe: hurried males or weak females? Behav Ecol Sociobiol 57:470–480
Erzberger A, Popa-Lisseanu A, Lehmann GUC, Voigt CC (2011) Potential and limits in detecting altitudinal movements of bats using stable hydrogen isotope ratios of fur keratin. Acta Chiropterol 13:431–438
Fiedler W (2005) Ecomorphology of the external flight apparatus of blackcaps (Sylvia atricapilla) with different migration behavior. Ann NY Acad Sci 1046:253–263
Förschler MI, Bairlein F (2011) Morphological shifts of the external flight apparatus across the range of a passerine (northern wheatear) with diverging migratory behaviour. PLoS ONE 6:e18732
Hedenström A, Alerstam T (1997) Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J Theor Biol 189:227–234
Hobson KA (2005) Stable isotopes and the determination of avian migratory connectivity and seasonal interactions. Auk 122:1037–1048
Hobson KA, Wassenaar LI (eds) (2008) Tracking animal movements with stable isotopes. Elsevier, San Diego
Jenni L, Winkler R (1994) Moult and ageing of European passerines. Academic, London
Jonzén N, Hedenström A, Lundberg P (2007) Climate change and the optimal arrival of migratory birds. Proc R Soc Lond B 274:269–274
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lockwood R, Swaddle JP, Rayner JMV (1998) Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptations to migration. J Avian Biol 29:273–292
Maggini I, Bairlein F (2010) Endogenous rhythms of seasonal migratory body mass changes and nocturnal restlessness in different populations of northern wheatears Oenanthe oenanthe. J Biol Rhythms 25:268–276
Maggini I, Bairlein F (2012) Innate sex differences in the timing of spring migration in a songbird. PLoS ONE 7:e31271
Marchetti K, Price T, Richman A (1995) Correlates of wing morphology with foraging behaviour and migration distance in the genus Phylloscopus. J Avian Biol 26:177–181
Morbey YE, Ydenberg RC (2001) Protandrous arrival timing to breeding areas: a review. Ecol Lett 4:663–673
Newton I (2008) The migration ecology of birds. Academic, London
Pärt T (2001) The effect of territory quality on age-dependent reproductive performance in the northern wheatear, Oenanthe oenanthe. Anim Behav 62:379–388
Pilastro A, Magnani A (1997) Weather conditions and fat accumulation dynamics in pre-migratory roosting barn swallows Hirundo rustica. J Avian Biol 28:338–344
Potti J, Montalvo S (1991) Male arrival and female mate choice in pied flycatchers Ficedula hypoleuca in central Spain. Ornis Scand 22:45–54
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Rubenstein DR, Chamberlain CP, Holmes RT, Ayres MP, Waldbauer JR, Graves GR, Tuross NC (2002) Linking breeding and wintering ranges of a migratory songbird using stable isotopes. Science 295:1062–1065
Sandberg R, Moore FR (1996) Fat stores and arrival on the breeding grounds: reproductive consequences for passerine migrants. Oikos 77:577–581
Smith RJ, Moore FR (2005) Arrival timing and seasonal reproductive performance in a long-distance migratory landbird. Behav Ecol Sociobiol 57:231–239
Spina F, Volponi S (2008) Atlante della migrazione degli uccelli in Italia. 2. Passeriformi. Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Istituto Superiore per la Ricerca e la Protezione Ambientale (ISPRA). Tipografia SCR-Roma
Spina F, Massi A, Montemaggiori A, Baccetti N (1993) Spring migration across central Mediterranean: general results from the “Progetto Piccole Isole”. Vogelwarte 37(Suppl.):1–94
Spina F, Massi A, Montemaggiori A (1994) Back from Africa: who’s running ahead? Differential migration of sex and age classes in Palearctic-African spring migrants. Ostrich 65:137–150
Suter W (1988) Oenanthe oenanthe – Steinschmätzer. In: Glutz von Blotzheim UN, Bauer KM (eds) Handbuch der Vögel Mitteleuropas. Band 11/I. Passeriformes (2. Teil). Aula, Wiesbaden, pp 529–592
Svensson L (1992) Identification guide to European passerines, 4th edn. L. Svensson, Stockholm
van Noordwijk AJ, McCleery RM, Perrins CM (1995) Selection for the time of great tit breeding in relation to caterpillar growth and temperature. J Anim Ecol 64:451–458
Wassenaar LI, Hobson KA (2000) Improved method for determining the stable-hydrogen isotopic composition (δD) of complex organic materials of environmental interest. Environ Sci Technol 34:2354–2360
Wassenaar LI, Hobson KA (2003) Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isot Environ Health Stud 39:211–217
West JB, Bowen GJ, Dawson TE, Tu KP (2009) Isoscapes. Understanding movement, pattern, and process on earth through isotope mapping. Springer, New York
Woodrey MS, Moore FR (1997) Age-related differences in the stopover of fall landbird migrants on the coast of Alabama. Auk 114:695–707
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft DFG (Ba 815/16) and by the Progetto SIM, financed by the Riserva Naturale Statale Isole di Ventotene e Santo Stefano. We are grateful to the Ventotene ringing team for help in the field. Karin Sörgel performed the isotopic analysis. We thank Nigel Richards and two anonymous reviewers for comments on an earlier version of the manuscript. The authors declare that they have no conflict of interest. Results from the ‘‘Progetto Piccole Isole’’ ISPRA: paper n. 52.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Hedenström.
Rights and permissions
About this article
Cite this article
Maggini, I., Spina, F., Voigt, C.C. et al. Differential migration and body condition in Northern Wheatears (Oenanthe oenanthe) at a Mediterranean spring stopover site. J Ornithol 154, 321–328 (2013). https://doi.org/10.1007/s10336-012-0896-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0896-1