Abstract
Breeding birds often give alarm calls when a predator is near the nest. These calls have been proposed to serve as distraction displays for the predator, alerts for a mate conveying information about the presence of a threat, or a warning for nestlings about a potential risk. These functions, however, may not be mutually exclusive. In our study, we assessed if alarm calls uttered by breeding Southern House Wrens, Troglodytes musculus, are made to warn nestlings about risk. If so, we expected that nestlings would reduce overall activity in the nest and that the parents’ call rate would be related to the detectability of the young (e.g., vocalizations). We experimentally elicited parents’ alarm calls and compared nestling behavior before and after giving that stimulus. We found that Southern House Wren nestlings reduced their time spent vocalizing and remained inactive for longer when their parents called. Therefore, nestlings reduced their detectability by decreasing their activity inside the nest when their parents produced alarm calls. On the other hand, parental calling rates were not related to the nestling activity registered in any experimental stage. Therefore, we failed to find reliable results supporting the hypothesis that parent calling is uttered to silence nestlings. These results appear to indicate that alarm calling by breeding birds might fulfill other functions besides alerting nestlings. Future studies of this species are necessary to understand if parents are warning nestlings about a threat when they emit alarm calls.
Zusammenfassung
Brütende Vögel geben häufig Warnrufe ab, wenn sich in der Nähe des Nestes ein Räuber befindet. Es wird angenommen, dass diese Rufe zur Ablenkung für den Räuber, zur Warnung des Brutpartners oder als Warnung der Nestlinge vor einer Gefahrenquelle dienen. Diese Funktionen schließen sich dabei nicht unbedingt gegenseitig aus. In dieser Studie untersuchten wir, ob Warnrufe brütender Südlicher Hauszaunkönige für deren Nestlinge bestimmt waren. In diesem Fall erwarteten wir, dass Nestlinge ihre Aktivität im Nest verringern und dass die Frequenz der elterlichen Warnrufe mit der Erkennbarkeit der Jungen (z. B. durch Lautäußerungen) korreliert. Wir lösten experimentell Warnrufe der Elternvögel aus und verglichen das Verhalten der Nestlinge vor und nach dem Stimulus. Es zeigte sich, dass die Südlichen Zaunkönignestlinge weniger häufig Lautäußerungen von sich gaben und längere Zeit inaktiv waren, wenn die Eltern riefen. Dadurch verringerten die Nestlinge ihre Entdeckungswahrscheinlichkeit im Falle einer Gefahr. Andererseits war die Frequenz der Warnrufe nicht mit der Aktivität der Nestlinge verbunden. Die Ergebnisse unterstützen also nicht eindeutig die Hypothese, dass Eltern rufen, um ihre Jungen zum Schweigen zu bringen. Möglicherweise dienen Warnrufe von Brutvögeln anderen Funktionen außer der Alarmierung von Jungvögeln. Um festzustellen, ob Warnrufe der Eltern die Jungen vor Gefahr warnen sollen, wären weiterführende Studien notwendig.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many organisms, birds and mammals in particular, produce vocalizations when a predator is detected nearby. These calls are defined as alarm or warning calls (Hauser 1998; Caro 2005). Such signals could be uttered to inform of a risky situation to conspecifics (Greig-Smith 1980; Neudorf and Sealy 1992), or to predators as a distraction display, informing the receiver that it has been detected (perception advertisement) or that the prey is in a good condition to escape (condition advertisement) (Hasson 1991; Zahavi and Zahavi 1997; Laiolo et al. 2004; Caro 2005).
In altricial species, the production of alarm calls by breeding individuals during nesting is a common characteristic. There are three main hypotheses that have been proposed to explain the function of alarm calls in this context. First, alarm calls may be elicited to warn mates of an approaching predator, being a way of investing in reproduction where the survival of a mate is important for the sender’s fitness (Greig-Smith 1980; East 1981; Yasukawa 1989; Haftorn 1999; Gill and Sealy 2003; Krams et al. 2006). Second, alarm calls could serve as a parental defence of the nest attracting a predator’s attention away from the nest, thus reducing the risk of the nest being detected (Greig-Smith 1980; Högstedt 1983). Moreover, parents could be providing information about risk to conspecifics or heterospecifics resulting in a cooperative response of defence (Rohwer et al. 1976; Hurd 1996; Leavesley and Magrath 2005). Third, parents uttering alarm calls might be warning nestlings of the existence of risk near the nest, and the young may then reduce their detectability by decreasing their activity and vocalizations inside the nest (Knight and Temple 1988).
Evidence supporting the third hypothesis comes mostly from studies in open-cup nesters, where nestlings are increasingly detectable as they grow. In these species, nestlings usually suppress begging calls and crouch into the nest after hearing parental alarm calls (Greig-Smith 1980; Knight and Temple 1986; Duckworth 1991; Kleindorfer et al. 1996; Halupka 1998; Yasukawa 1989; Gill and Sealy 2003; Davies et al. 2004; Platzen and Magrath 2004; Madden et al. 2005a; Anderson et al. 2010; but see Maurer et al. 2003). As nestlings get older, their appearance and vocalizations make them more vulnerable to detection by predators as these could use nestling vocalizations to locate and depredate the nest (Haskell 1994, 1999, 2002; Leech and Leonard 1997; Briskie et al. 1999; Dearborn 1999; McDonald et al. 2009). In accordance with this hypothesis, the rate of alarm calls registered in these species increases with the age of nestlings (Knight and Temple 1986; Kleindorfer et al. 1996; Gill and Sealy 2003; Davies et al. 2004). In contrast to nests of open-cup nesters, nest sites of cavity nesters are usually hidden, meaning that predators might use nestling acoustic cues (e.g., begging or vocalizations) to find nests. Furthermore, cavity nesters are louder and have more locatable calls than open-cup nesters, and have longer nestling periods, which place nestlings at greater risk of being detected (Redondo and Arias de Reyna 1988). So, these characteristics could provide a selection pressure for the evolution of alarm signals in cavity-nesting adults directed at their nestlings.
The majority of studies analyzing the possibility that alarm calls by parents may act as a signal to nestlings have focused on a single element of the interaction, usually nestling behavior, by manipulating the parents’ signal (Gill and Sealy 2003; Davies et al. 2004; Platzen and Magrath 2004; Anderson et al. 2010). However, nestlings could adjust their behavior in response to parental alarm calls even if they are not directed at them.
Our aim was to evaluate the function of parental alarm calls of Southern House Wrens, Troglodytes musculus, uttered during the nestling period. We achieved this using a field experiment to assess if adult alarm calls function to communicate to nestlings about the presence of a threat. Fasanella and Fernández (2009) found that, when threatened with a predator model, breeding individuals of the Southern House Wren increased the rate with which they gave alarm calls as their nestlings grew older. This finding appears to support the hypothesis that these calls might alert nestlings since, by the end of the nestling period, nests become more conspicuous because nestlings increase their vocalization rate and activity (Kleindorfer et al. 1996; Platzen and Magrath 2005). Therefore, we tested whether parental alarm calls act as a signal of immediate danger to nestlings which they use to change their behavior in order to reduce their risk of predation. If so, nestlings should respond by reducing overall activity and vocalization rates. Also, if there exists a parent–offspring communication system, we expected that parental calls should vary according to nestling vocalizations and activity, displaying higher rates when nestlings are more active.
Methods
Study site and species
The study took place in General Lavalle (36°20′S, 56°54 O), Buenos Aires, Argentina, over two breeding seasons, October–January, 2006–2007. The experiments were conducted in forests of Celtis tala, Scutia buxifolia and Schinus longifolius where nest-boxes are regularly used as nesting sites by Southern House Wrens (Llambías and Fernández 2009).
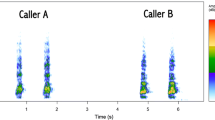
The Southern House Wren is a small passerine (12 g) distributed in America from eastern Oaxaca (México) to Tierra del Fuego (Argentina) (Skutch 1953). This species builds well-hidden nests in natural cavities in wooded areas and is well adapted to habitats modified for human use (Skutch 1953; Young 1994; Llambías and Fernández 2009). The breeding season extends from October to January in the south temperate region. Clutch size are typically 4–5 eggs (Llambías and Fernández 2009) which are incubated exclusively by female for 14–15 days and the nestlings are reared by both parents for 15–17 days before they fledge (Skutch 1953; Young 1994; Llambías and Fernández 2009). Adults usually give alarm calls when a predator approaches the nest. Two types of alarm calls have been recognized: Type I and Type II, which differ in frequency and duration (Fig. 1; see also Fasanella and Fernández 2009). Type I calls have a frequency that varies from 1 to 9 kHz (reaching a maximum of intensity at 6 kHz) and have a duration of between 400 and 600 ms. Type II alarm calls exhibit lower frequencies (1–6 kHz, reaching a peak of intensity at 3 kHz) and are shorter (~100 ms). Furthermore, it is known that Type I calls are used more often when a predator is perched nearby and is the one that increases in frequency with nestling age (Fasanella and Fernández 2009). During the treatment period, Type I calls were mostly uttered by females.
Although nestlings typically beg when parents visit the nest (see below), they also vocalize with a soft brief chip call during the period between parental visits (Serra and Fernández, unpublished data).
Experimental procedure
We conducted a field experiment to study the nestlings’ behavior in response to alarm calls elicited by the parents in presence of a threat nearby the nest. Nestlings were tested at 9–11 days old since studies of altricial young indicate a late onset of the alarm call response (Platzen and Magrath 2005), and because of our knowledge that the rate at which Type I House Wren alarm calls rises as nestlings grow older (Fasanella and Fernández 2009). The experiment was performed at 15 nests with 4–6 nestlings, during the morning (0600–1200 hours). At least one adult of each nest was individually colour ringed. We divided the experiment into three stages: habituation, control and treatment. Nestling activity was videotaped with a Sony Handycam Hi8 (model CCD TR940; Sony, Tokyo, Japan) during all stages. Video cameras were fitted into replacement nest-box lids which showed a complete view of the brood and were covered with a camouflage cover. In the first stage, adults were habituated to the presence of the camera during 30 min. In the second stage, nestling activity was recorded without the human presence for 10 min (control stage). Finally, in the third stage, nestling activity was videotaped during 10 min in the presence of a human observer standing 1–1.5 m from the nest-box (treatment).
We later played the movies back and scored nestling behavior using Etholog 2.2 (Ottoni 2000). We chose one nestling at the beginning of the control stage and performed a focal continuous sampling record of its behavior. Selection of this focal animal was arbitrary, subject to the visibility on the videotape. We defined four categorical behaviors to study the activity of nestlings: (1) begging, which consisted in a succession of events in the following order: stretching of the neck, wide opening of the bill and production of a sound (we only considered the begging events that were given in absence of parents); (2) vocalization (when nestlings rested and gave a chip sound as described before); (3) activity (when nestlings exhibited movements inside the nest other than those included in 1 and 2); and (4) inactivity (when nestlings remained silent and resting) (Lichtenstein 1997; Bachman and Chappell 1998; Maurer et al. 2003). In spite of defining the first two behaviors as different, we infer they both communicate hunger to parents. Parental behavior was also analyzed during the treatment period by recording the number of Type I calls emitted by the breeding adults of each nest.
Statistical analysis
We analyzed the behavior of nestlings and adults from 15 nests. Data were analyzed using non-parametric tests because they did not fulfill the assumptions required by parametric tests. The comparison of nestlings’ behavior between the control and treatment stages was analyzed using Wilcoxon matched-pairs tests. The relationship between parents and nestlings behavior was tested using Spearman rank correlations. As we performed multiple tests on the same database, we corrected the critical significance level using Bonferroni correction setting the α–level to 0.0125 for the analysis of nestling behavior and to 0.025 for the analysis of parental behavior. All analyses were performed using the statistical package GenStat DE3 (VSN International).
Results
Brood size did not affect nestling behavior in either of the stages studied (Spearman rank correlation tests, n = 15, P > 0.05 for begging, vocalization, activity, and inactivity during control and treatment sessions). Moreover, parental alarm calls were not significantly correlated with the number of nestlings in the brood (Spearman rank correlation, n = 15, r s = 0.15, P = 0.59).
When parents uttered alarm calls during the treatment, nestlings spent less time eliciting vocalizations (Wilcoxon matched-pairs tests, n = 15, T = 9, P = 0.01 for vocalization; Fig. 2a) and more time inactive (Wilcoxon matched-pairs test, n = 15, T = 12, P = 0.006; Fig. 2b). The time nestlings spent displacing and begging did not change between control and treatment stages (Wilcoxon matched-pairs test, n = 15, T = 47, P = 0.73 for movement, meancontrol ± SE = 26.61 ± 7.69 s, meantrial ± SE = 26.42 ± 5.98; n = 15, T = 6.0, P = 0.68 for begging, meancontrol ± SE = 7.68 ± 6.08 s, meantrial ± SE = 1.30 ± 0.75 s).
Time spent emitting vocalizations (a), or remaining inactive (b) by nestlings of Southern House Wrens when they were exposed to the presence of a threat (an observer standing close to the nest) that elicited the alarm calling by parents (trial; open boxes) and during the immediately previous period without disturbance (control; hatched boxes). ***P < 0.01
During the treatment stage, parents emitted an average of 11.92 calls/min (SD = 12.24; range: 0.2–32.6 calls/min). In all cases, only one parent performed the calls. We found a significant reduction of time spent begging during the treatment along with an increase of alarm calls uttered by the parents (Spearman rank correlation, n = 15, Spearman rank correlation, n = 15, r s = −0.35, P = 0.05). Also, the period of time that nestlings remained inactive during the treatment increased but not significantly with parental calls (Spearman rank correlation, n = 15, r s = −0.26, P = 0.09). These relationships appear to reveal the response of nestlings to parental calls. The increase in the number of alarm calls given by parents implies an increase in the time nestlings remained inactive and a reduction in the time engaged in begging.
We couldn’t find any other relationship between alarm calls uttered by parents and nestlings’ behavior analyzed during the control or treatment stages (Spearman rank correlation tests, n = 15, P > 0.05).
Discussion
We found that alarm calls uttered by breeding Southern House Wrens during the rearing period resulted in a reduction of nestlings’ vocalization calls and in an increase of the time spent inactive. This result makes evident that offspring modify their behavior because they are able to recognize and decode the information contained in the alarm call signal given by their parents. These results coincide with others found in different species of birds (Gill and Sealy 2003; Davies et al. 2004; Platzen and Magrath 2004, 2005; Madden et al. 2005b; Anderson et al. 2010) and suggest an anti-predator response to parental acoustic stimulus. In the Southern House Wren, as well as very probably for other cavity-nesting species, nestling perception of a potential risk near the nest has to rely on the information provided mainly by acoustic cues. Therefore, alarm calls given by breeding parents appear to be a plausible mechanism for nestlings to evaluate risk near the nest and so modify their behavior.
Many studies have found that conspicuous begging and vocalizations of nestlings, and a high activity inside the nest, increase the risk of predation of the nest contents (Haskell 1994, 1999; Leech and Leonard 1997; Maurer et al. 2003). As a consequence, the reduction of nestling vocalizations and activity implies an adaptive response in order to reduce such risk in passerine species (Gill and Sealy 2003; Platzen and Magrath 2004, 2005).
Although there is growing evidence that offspring are taking advantage of the signals given by their parents to assess and recognize the existing risk, it does not necessarily mean that parents are signaling to them (McGregor 1993; McGregor et al. 1999; Valone and Templeton 2002). Breeding birds could give alarm calls to alert mates about the risk near the nest (Madden et al. 2005a) or to distract predators away from the nest, in order to prevent revealing its location (Greig-Smith 1980; Hasson 1991). In these cases, nestlings could still “parasitize” the signal to adjust their behavior and so reduce their own risk of predation.
In our study, we failed to find evidence supporting the hypothesis that parents are calling in order to alert nestlings about the threat. If parents try to communicate the presence of a threat, we should expect that the parental response would be related to nestling behavior. Calls uttered by adult Southern House Wrens were not related to any behavior displayed by nestlings during the period prior to the exposure to the treatment. In particular, higher nestling vocalizations detected during this period did not trigger a stronger response by the adults during the exposure to the threat. Therefore, it is possible that alarm calls are not being directed at the nestlings and fulfill another function. In a previous study, it was observed that Southern House Wren alarm calls are also given during the incubation period and during the early stages of nestling rearing even though at a low rate (Fasanella and Fernández 2009). It has also been found that the calling rate varies according to different risk-associated artificial models offered (Fasanella and Fernández 2009). Such variation might be indicating that alarm calls may also serve as a distraction or mobbing display directed towards the predator (Curio 1987). Given the acoustical characteristics of Type I alarm calls (highly repetitive calls at high frequencies; Fasanella and Fernández 2009; Fernández et al., unpublished data) and behavior displays during calling, Type I calls might be functioning rather as mobbing calls. Therefore, alarm calling could function as a defense mechanism against predators, and the increase in parental calling with nestling age could be due to a higher brood value (Curio 1987; Montgomerie and Weatherhead 1988; Redondo and Carranza 1989; Onnebrink and Curio 1991) or to a higher vulnerability at this stage (Weatherhead 1979; Andersson et al. 1980; Burger et al. 1989). Therefore, Southern House Wren nestlings might be parasitizing parental calls as a cue to assess the presence of a risk.
Further studies seeking the existence of additional functions of alarm calls uttered by breeding adults and the response of nestlings to the variation in the calling rate are necessary to completely understand the ecological value of parental alarm calling during the nestling period in this species. Particularly, an experiment manipulating nestling signals and measuring the parental response might be necessary to assess if it constitutes an effective communication system between parents and nestlings.
References
Anderson M, Brunton DH, Hauber ME (2010) Species specificity of grey warbler begging solicitation and alarm calls revealed by nestling responses to playbacks. Anim Behav 79:401–409
Andersson MC, Wiklund G, Rundgren H (1980) Parental defence of offspring: a model and an example. Anim Behav 28:536–542
Bachman GC, Chappell MA (1998) The energetic cost of begging in nestling house wrens. Anim Behav 55:1607–1618
Briskie JV, Martin PR, Martin TE (1999) Nest predation and the evolution of nestling begging calls. Proc R Soc Lond B 266:2153–2159
Burger J, Gochfeld M, Saliva JE, Gochfeld D, Gochfeld D, Morales H (1989) Antipredator behaviour in nesting zenaida doves (Zenaida aurita): parental investment or offspring vulnerability? Behaviour 65:129–143
Caro T (2005) Anti-predator defences in birds and mammals. University of Chicago Press, Chicago
Curio E (1987) Brood defence in the great tit: the influence of age, number and quality of young. Ardea 75:33–42
Davies NB, Madden JR, Butchard SHM (2004) Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc R Soc Lond B 271:2297–2304
Dearborn DC (1999) Brown-headed cowbird nestling vocalizations and risk of nest predation. Auk 116:448–457
Duckworth JW (1991) Responses of breeding reed warblers Acrocephalus scirpaceus to mounts of sparrowhawk Accipiter nisus, cuckoo Cuculus canorus and jay Garrulus glandarius. Ibis 133:68–74
East M (1981) Alarm calling and parental investment in the robin Erithacus rubecula. Ibis 123:223–230
Fasanella M, Fernández GJ (2009) Alarm calls of the southern house wren, Troglodytes musculus: variation with nesting stage and predator model. J Ornithol 150:853–863
Gill SA, Sealy SG (2003) Test of two functions of alarm calls given by yellow warblers during nest defense. Can J Zool 81:1685–1690
Greig-Smith PW (1980) Parental investment in nest defence by stonechats (Saxicola torquata). Anim Behav 28:604–619
Haftorn S (1999) Contexts and possible functions of alarm calling in the willow tit, Parus montanus; the principle of “better safe than sorry”. Behavior 137:437–439
Halupka K (1998) Vocal begging by nestlings and vulnerability to nest predation in meadow pipits Anthus pratensis: to what extent do predation costs of begging exist? Ibis 140:144–149
Haskell DG (1994) Experimental evidence that nestling begging behavior incurs a cost due to nest predation. Proc R Soc Lond B 257:161–164
Haskell DG (1999) The effect of predation on begging-call evolution in nestling wood warblers. Anim Behav 57:893–901
Haskell DG (2002) Begging behaviour and nest predation. In: Wright J, Leonard ML (eds) The evolution of begging: competition, cooperation and communication. Springer, The Netherlands, pp 163–172
Hasson O (1991) Pursuit-deterrent signals: communication between prey and predator. Trends Ecol Evol 6:325–329
Hauser MD (1998) The evolution of communication. MIT Press, Cambridge
Högstedt G (1983) Adaptation unto death: function of fear screams. Am Nat 121:562–570
Hurd CR (1996) Interspecific attraction to the mobbing calls of blackcapped chickadees (Parus atricapillus). Behav Ecol Sociobiol 38:287–292
Kleindorfer S, Hoi H, Fessl B (1996) Alarm calls and chick reactions in the moustached warbler, Acrocephalus melanopogon. Anim Behav 51:1199–1206
Knight RL, Temple SA (1986) Nest defence in the American goldfinch. Anim Behav 34:887–897
Knight RL, Temple SA (1988) Nest-defense behaviour in the red-winged blackbird. Condor 90:193–200
Krams I, Krama T, Igaune K (2006) Alarm calls of wintering great tits Parus major: warning of mate, reciprocal altruism or a message to the predator? J Avian Biol 37:131–136
Laiolo P, Tella JL, Carrete M, Serrano D, López G (2004) Distress calls may honestly signal bird quality to predators. Proc R Soc Lond B 271:513–515
Leavesley AJ, Magrath RD (2005) Communicating about danger: urgency alarm calling in a bird. Anim Behav 70:365–373
Leech SM, Leonard ML (1997) Begging and the risk of predation in nestling birds. Behav Ecol 8:644–646
Lichtenstein G (1997) Begging behaviour and host exploitation in three species of parasitic cowbirds. PhD thesis, Cambridge University
Llambías PE, Fernández GJ (2009) Effects of nestboxes on the breeding biology of southern house wrens Troglodytes aedon bonariae in the southern temperate zone. Ibis 151:113–121
Madden JR, Kilner RM, Davies NB (2005a) Nestling responses to adult food and alarm calls: 1. Species-specific responses in two cowbird hosts. Anim Behav 70:619–627
Madden JR, Kilner RM, Davies NB (2005b) Nestling responses to adult food alarm calls: 2. Cowbirds and red-winged blackbirds reared by eastern phoebe hosts. Anim Behav 70:629–637
Maurer G, Magrath RD, Leonard ML, Horn A, Donnelly C (2003) Begging to differ: scrubwren nestlings beg to alarm calls and vocalize when parents are absent. Anim Behav 65:1045–1055
McDonald PG, Wilson DR, Evans CS (2009) Nestling begging increases predation risk, regardless of spectral characteristics or avian mobbing. Behav Ecol 20:821–829
McGregor PK (1993) Signalling in territorial systems: a context for individual identification, ranging and eavesdropping. Philos Trans R Soc Lond 340:237–244
McGregor PK, Otter K, Peake TM (1999) Communication networks: receiver and signaller perspectives. In: Espmark Y, Amundsen T, Rosenquist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Academic, Trondheim, pp 405–416
Montgomerie RD, Weatherhead PJ (1988) Risks and rewards of nest defense by parent birds. Q Rev Biol 63:167–187
Neudorf DL, Sealy SG (1992) Reactions of four passerine species to threats of predation and cowbird parasitism: enemy recognition or generalized responses? Behaviour 123:84–105
Onnebrink H, Curio E (1991) Brood defense and age of young: a test of the vulnerability hypothesis. Behav Ecol Sociobiol 29:61–68
Ottoni EB (2000) EthoLog 2.2. A tool for the transcription and timing of behaviour observation sessions. Behav Res Meth Instrum Comput 32:446–449
Platzen D, Magrath RD (2004) Parental alarm calls suppress nestling vocalization. Proc R Soc Lond B 271:1271–1276
Platzen D, Magrath RD (2005) Adaptive differences in response to two types of parental alarm call in altricial nestlings. Proc R Soc Lond B 272:1101–1106
Redondo T, Arias de Reyna L (1988) Locatability of begging calls in nestling altricial birds. Anim Behav 36:653–661
Redondo T, Carranza J (1989) Offspring reproductive value and nest defense in the magpie (Pica pica). Behav Ecol Sociobiol 25:369–378
Rohwer S, Frettwell SD, Tuckfield RC (1976) Distress screams as a measure of kinship in birds. Am Midl Nat 96:418–430
Skutch AF (1953) Life history of the southern house wren. Condor 55:121–149
Valone TJ, Templeton JJ (2002) Public information for the assessment of quality: a widespread social phenomenon. Philos Trans R Soc Lond B 357:1549–1557
Weatherhead PJ (1979) Do savannah sparrows commit the concorde fallacy? Behav Ecol Sociobiol 5:373–381
Yasukawa K (1989) The costs and benefits of a vocal signal: the nest-associated ‘chit’ of the female red-winged blackbird, Agelaius phoeniceus. Anim Behav 38:866–874
Young BE (1994) Geographic and seasonal patterns of clutch-size variation in house wrens. Auk 111:545–555
Zahavi A, Zahavi A (1997) The handicap principle. Oxford University Press, Oxford
Acknowledgments
We thank Paulo E. Llambías for the help given in the field, the Whisky-Michelli family and Luis Martinez for allowing us to work on their ranches at Buenos Aires, and Mario Beade for logistical support. We also thank Jose E. Crespo, Craig Barnett, Idrikis Krams and one anonymous reviewer for their helpful comments on an earlier version of this manuscript. This work was supported by grants to G.J.F. provided by the University of Buenos Aires (Grant X007 and X434) and CONICET Grants (PIP5223). All methods used in the present study meet the ethical requirements for science research and comply with the current laws of our country.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Friedl.
Rights and permissions
About this article
Cite this article
Serra, C., Fernández, G.J. Reduction of nestlings’ vocalizations in response to parental alarm calls in the Southern House Wren, Troglodytes musculus . J Ornithol 152, 331–336 (2011). https://doi.org/10.1007/s10336-010-0595-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-010-0595-8