Abstract
We used a 13-year time series of abundance estimates of breeding Northern Saw-whet Owls (Aegolius acadicus), and of small mammals from central Ontario, Canada, to assess the numerical response of the owls to small-mammal prey species. We found that the finite rate of increase of breeding owls was directly related to estimates of red-backed vole (Myodes gapperi) abundance. Thus, it appeared that the owls were nomadic, and made decisions about where to breed based on vole supply. The owls showed a much weaker response to deer mouse (Peromyscus maniculatus) abundance. Across all years, 55% of variation in owl rate of increase could be uniquely attributed to vole abundance, whereas only 3% could be attributed to mouse abundance. Consistent with the model of nomadism, there was only a weak relationship between the proportion of hatch-year owls caught at fall banding stations, and small-mammal abundance. Instead, it appeared that Northern Saw-whet Owls avoided years of widespread reproductive failure through the nomadic strategy of selecting breeding sites based on vole supply.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predator populations often show a numerical response to change in the abundance of prey species (Solomon 1949). This numerical response can be a product of birth, death, immigration, and emigration processes (Andersson and Erlinge 1977).
Predators that respond numerically to prey abundance may do so by showing either a delayed or direct response. A delayed response is lagged by some period of time, as a result of birth and death processes (Bulmer 1975). For example, the Great Horned Owl (Bubo virginianus) exhibits reduced recruitment and adult survival during snowshoe hare (Lepus americanus) population crashes, such that the owl population declines 1 year after the hares (Rohner 1996). An important characteristic of the Great Horned Owl related to their delayed numerical response is that they appear to maintain territories among years (Baumgartner 1939; Rohner 1996), a strategy that increases their familiarity with nesting and foraging opportunities on the territory. Thus, the owls maintain territories even during years with reduced food availability, favouring long-term benefits of site familiarity over shorter term risks of food shortage. Andersson (1980) has argued that such site tenacity is favoured in bird species that exhibit high adult survival and low clutch size.
In contrast, some avian predators appear to track prey resources rapidly, exhibiting a direct numerical response. Direct responses occur with no lag, and as such are mostly a result of immigration and emigration processes (Korpimäki 1994). This rapid-tracking of resources is often referred to as nomadism, and may be favoured in bird species with high juvenile survival, low adult survival, and large clutch size (Andersson 1980). For example, breeding density of the Tengmalm’s Owl (Aegolius funereus) in Finland is correlated with contemporaneous estimates of vole abundance, apparently due to vole supply-dependent migration (Korpimäki 1994).
The Northern Saw-whet Owl (Aegolius acadicus) is a common owl of North American boreal and eastern temperate forests that preys principally on small mammals, especially mice and voles (Rasmussen et al. 2008). It is commonly considered a short-distance migrant, although some birds remain on breeding territories year-round, particularly in the southern part of their range (e.g., Côté et al. 2007). There are several suggested migration routes for Northern Saw-whet Owls in eastern Canada (Rasmussen et al. 2008), and in autumn, there is a concentration of these migrating owls along the north shores of the Great Lakes.
In Ontario, breeding populations of Northern Saw-whet Owls have been monitored since 1995 by volunteers participating in the Ontario Nocturnal Owl Survey, a roadside survey of calling owls (Badzinski 2007). The relative abundance of owls is estimated through these surveys by quantifying the abundance of calls. It is assumed by the surveyors that changes in calls among years also reflect changes in owl abundance. These indices of owl abundance have fluctuated markedly among years, leading to the inference that the owls exhibit a numerical response to regional small-mammal populations. This inference, however, has never been tested. Although the similar Tengmalm’s Owl in Europe appears to be nomadic, small mammals in Canadian forests do not appear to exhibit cyclic dynamics as do those in Fennoscandia (Fryxell et al. 1998), and a cyclic prey base promotes nomadism (Andersson 1980). Northern Saw-whet Owls have two key characteristics of nomadism, however: high fecundity and relatively low adult survival (Marks and Doremus 2000). Moreover, in a study in Idaho, nest-box use was positively correlated with contemporaneous estimates of small-mammal abundance, and reuse of the area by banded adults was low (Marks and Doremus 2000). Consequently, we were interested in testing the hypothesis that Northern Saw-whet Owls in Canada are nomadic, such that annual spring abundance of breeding owls is a result of a direct numerical response to small-mammal abundance.
Migrating Northern Saw-whet Owls are caught and banded every fall at migration monitoring stations throughout the Great Lakes region of eastern Canada. It has long been posited that the number of hatch-year birds captured is related to small-mammal abundance in the breeding range (Coté et al. 2007; Weir et al. 1980) and that high numbers of hatch-year owls moving through a station result from abundant small mammals. If this were true, it would indicate a delayed numerical response, because production of fledglings by breeding adults would be lagged by some months. We hypothesised, however, that if Northern Saw-whet Owls are nomadic, then the abundance of migrating hatch-year birds should not depend on small-mammal abundance during the owls’ breeding season at one particular locality. Small-mammal populations in central Canada fluctuate synchronously over only small distances (<200 km; Bowman et al. 2008), and are asynchronous over longer distances. This suggests that nomadic predators should be able to track prey among asynchronous populations with fairly small breeding dispersals (i.e., the probability of breeding, P, is always >0; Andersson 1980). If Northern Saw-whet Owls are nomadic, and if the catchment area of birds migrating through a banding station spans >1 region of small-mammal synchrony, then the abundance of hatch-year birds at banding stations should be relatively stable among years and not depend strongly on small-mammal abundance at any one locality. If there is large-scale asynchrony among small-mammal populations then within any given year some localities within the banding station’s catchment area should have productive small-mammal populations. Therefore, nomadic owls should be able to avoid collapsed prey populations.
Our objective was to test for characteristics of nomadism in the numerical response of Northern Saw-whet Owls to small-mammal abundance in central Ontario, Canada. We compared spring abundance of breeding owls to summer abundance of small mammals, predicting that owls should exhibit a direct numerical response if they are nomadic. We also compared the proportion of hatch-year birds at a fall banding station to our estimate of summer small-mammal abundance, expecting that estimates of owl productivity should be independent of small-mammal abundance estimated from our single locality if owls are nomadic. We also predicted that our estimate of hatch-year owl abundance should be less variable than our estimate of small-mammal abundance. We assumed in these analyses that the owls banded at the migration monitoring station came from a broad geographic area that extended beyond the region where we sampled small mammals.
Methods
Study area
Our study took place in central Ontario, Canada. The largest extent of our study was defined by the central Ontario owl survey routes which extended from 47°N to the southern limit of the Canadian Shield (approximately 44°N). The area provided a mosaic of stand types typical of the Great Lakes–St. Lawrence forest region of Canada, a transition zone between the northern boreal forest and the southern temperate forest (Rowe 1972). The dominant forest types were tolerant hardwoods such as sugar maple (Acer saccharum), yellow birch (Betula alleghaniensis), and American beech (Fagus grandifolia) along with some softwood species such as hemlock (Tsuga canadensis), white spruce (Picea glauca), balsam fir (Abies balsamea), and white pine (Pinus strobus). The small-mammal surveys were conducted at 10 sites along the Highway 60 corridor of Algonquin Provincial Park (surveys centred approximately at 45.3°N, 78.4°W). Algonquin Park is a large park (7,725 km2) that occupies the geographic centre of our central Ontario study area. Thus, the small-mammal surveys represent samples of small mammal populations from within the owl study area.
Breeding owl surveys
Ontario Nocturnal Owl Survey data from central Ontario were used to estimate breeding abundance of the Northern Saw-whet Owl. Survey routes were situated along secondary roads, with pre-determined stations from which surveys were conducted. Routes were surveyed on a single evening in April, at least one half hour after sunset, and on clear calm nights with winds less than force 3 on the Beaufort scale, no precipitation and temperatures warmer than −15°C. Survey routes consisted of 10 stops spaced 2.0 km apart for a total route length of 18 km. The protocol at each stop consisted of 2 min of silent listening, followed by a series of Boreal Owl (Aegolius funereus) and Barred Owl (Strix varia) calls interspersed with silent listening. Mean (SD) number of routes completed per year from 1995 to 2007 was 77.2 (8.1). A generalised linear model with a Poisson error distribution and log-link function (PROC GENMOD; SAS Institute) was used to calculate annual indices. The model included year and route as class variables [number of owls = year (class), route (class)].
Owl migration monitoring
Migrating Northern Saw-whet Owls were caught and banded each fall at two banding stations of the Long Point Bird Observatory (LPBO), on the north shore of Lake Erie. The Old Cut station was located at the base of Long Point and the Tip station was situated at the end of the peninsula. Banding operations began on 1 October of each year and consisted of a minimum of 4 h of standardised trapping (beginning one half hour after sunset) using an audio lure to attract migrating owls. Owls were trapped on nights with favourable weather conditions and when sufficient personnel were present. To catch the owls, three 60-mm mistnets (2.6 m × 9.0 m) were set up in a triangular pattern with the audio lure at the centre. In addition to the standard owl net setup, the mistnets used for the daily banding operations were opened when conditions were favourable.
All owls caught in nets were promptly removed, banded, and standard morphological measurements were taken. Birds were aged as hatch year (HY) or older (AHY). A mean (SD) of 413 (90) owls was banded each year at LPBO during the years 1995, and 1997–2007.
Small-mammal surveys
Small-mammal trapping was undertaken on 10 traplines in Algonquin Provincial Park that have been surveyed every summer during late May to late August since 1952 (e.g., Fryxell et al. 1998). The trap lines were deployed in a variety of habitats typical of the Great Lakes–St. Lawrence forest region (Rowe 1972). Each trap line was approximately 90 m in length with a station every 10 m for a total of 10 stations per line. There were two small (7.5 cm × 7.5 cm × 25 cm) Sherman live traps per station resulting in 20 traps per line. Lines were trapped either once or twice per month, resulting in a mean of 4,589 trap nights per year across the 10 lines (range 4,200–4,800). The 13-year time series used for the present study (1995–2007) consisted of 59,660 trap nights.
The traps were baited in the evening between 1600 and 2000 hours and were checked the following morning starting at 0600 hours. The traps contained a ball of polyester batting and were baited with water-soaked sunflower seeds. Processing of captured mammals included identification to species, ear tagging with 1-g Monel tag (National Band and Tag, Newport, Kansas, USA), weighing, sexing, and determining the breeding condition. All mammals caught were released at the trap site after processing. All animal handling procedures were approved by the Animal Care Committee at the University of Guelph.
We considered the most likely prey species of the Northern Saw-whet Owl to be the deer mouse (Peromyscus maniculatus) and the red-backed vole (Myodes gapperi), given previous diet studies of the owl (Cannings 1993) and given that these are the most abundant forest-dwelling small mammals in the region (Fryxell et al. 1998). As such, we restricted analysis of the small-mammal dataset to these two species. Our index of abundance for each species was the mean annual captures per trap night.
Data analysis
We first visually inspected temporal trend data for vole, mouse, and breeding owl abundance (N t), and described annual changes in abundance. Because we were interested in testing the numerical response of owls to changing small-mammal abundance, we estimated the annual rate of increase of owls (N t/N t − 1), expecting that, for a nomadic species, breeding populations would increase in direct relation to small mammal abundance (N t) with no lag. Thus, we expected the rate of increase for nomadic species should be positively related to small-mammal abundance. We tested this prediction using ordinary least-squares regression. We compared bivariate models considering only abundance of voles or mice as the explanatory variable, and a combined multiple regression model considering both species.
We then inspected the migration data in relation to the small-mammal data. Here, we assumed that nomadic species can successfully reproduce every year (i.e., P always >0; Andersson 1980). This implies that the proportion of the post-fledging owl population that is hatch year should also always be >0, since nomadic birds will disperse to breeding sites with abundant small mammals. Consequently, we reasoned that there should be no relationship between the proportion of hatch-year Northern Saw-whet Owls banded during fall migration and the abundance of small mammals at a given locality. Rather than nomadism, a positive relationship between the proportion of migrating hatch-year birds and small-mammal abundance would indicate a delayed numerical response, related to increased productivity and chick survival. Thus, we predicted that if Northern Saw-whet Owls are indeed nomadic, they should not show such a delayed response. We tested our prediction with ordinary least-squares regression. We also predicted that if the owls are nomadic, then the proportion of hatch-year birds should be relatively stable among years, compared to our indices of breeding owl and small-mammal abundance. We compared coefficients of variation among these indices, expecting the CV for the hatch-year owl index to be lower.
Results
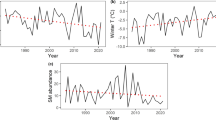
Northern Saw-whet Owls fluctuated in abundance between a low of 0.23 owls per route in 2000 and a high of 2.52 owls in 1999. Based on a visual inspection of trend graphs, it appeared that owl abundance in central Ontario closely tracked the abundance of red-backed voles from the Algonquin Park survey (Fig. 1). Voles fluctuated between a high of 0.099 captures per trap night in 1999 and a low of 0.018 captures in 2004. The greatest single-year increase by voles occurred in 1999, when voles exhibited a fourfold increase in abundance. The greatest decline was also fourfold, occurring in 2004. These changes were reflected by a 3.2-fold increase by owls in 1999, and a 2.6-fold decline in 2004. Mice exhibited greater fluctuations than did either voles or owls, increasing a maximum of 6.5-fold in 1999, and decreasing a maximum of 11-fold in 2002.
Abundance of red-backed vole (Myodes gapperi), deer mouse (Peromyscus maniculatus), and Northern Saw-whet Owl (Aegolius acadicus) in central Ontario, Canada, during 1995–2007. Vole and mouse indices are captures per trap night during summer, and the owl index is the number of owls calling per route surveyed in spring
There was a positive, linear relationship between the finite rate of increase of Northern Saw-whet Owls at time t and our index of vole abundance, also at time t (F = 28.37, df = 1,10, P < 0.0001, R 2 = 0.74) (Fig. 2). This model was a better fit than was the relationship between the finite rate of increase of owls at time t and mouse abundance at t (F = 2.72, df = 1,10, P = 0.130, R 2 = 0.21) (Fig. 3). There was not a strong correlation between mouse and vole abundance trends in the Algonquin study area (r = 0.37, n = 13, P = 0.210). A multiple regression with both voles and mice as independent variables explained only slightly more variation in owl rate of increase than did the vole only model (F = 14.66, df = 2,9, P = 0.001, R 2 = 0.77). From this regression, we partitioned the explained variation in owl rate of increase and found that 55% could be uniquely attributed to voles, 19% could be attributed to shared variation between mice and voles, and only 3% could be attributed to mice.
Northern Saw-whet Owl (Aegolius acadicus) rate of increase (N t/N t − 1) at time t, compared to red-backed vole (Myodes gapperi) abundance at t. Owl rate of increase is based on breeding abundance estimates in central Ontario, Canada. Vole abundance is the number of captures per trap night during summer surveys in central Ontario. Parameters for ordinary least-squares regression are shown
Northern Saw-whet Owl (Aegolius acadicus) rate of increase (N t/N t − 1) at time t, compared to deer mouse (Peromyscus maniculatus) abundance at t. Owl rate of increase is based on breeding abundance estimates in central Ontario, Canada. Mouse abundance is the number of captures per trap night during summer surveys in central Ontario. Parameters for ordinary least-squares regression are shown
The proportion of birds banded in autumn that were hatch year appeared to weakly track vole abundance (Fig. 4). Generally, however, this proportion remained between about 0.40 and 0.80, whereas vole abundance fluctuated much more widely. The coefficient of variation (CV) for the proportion of hatch-year birds was 25%, compared to a CV of 57% for summer small-mammal abundance, and 70% for breeding owl abundance. During 1999, when there was a fourfold increase in vole abundance, the proportion of hatch-year birds increased by a factor of 1.1. Similarly, in 2004, when voles exhibited a fourfold decline, hatch-year owls declined 1.7-fold. A linear regression confirmed this weak relationship between hatch-year birds caught in fall, and the abundance of red-backed voles the previous summer (F = 2.51, df = 1,9, P = 0.148, R 2 = 0.22) (Fig. 5).
Proportion of Northern Saw-whet Owls (Aegolius acadicus) banded in autumn at Long Point, Ontario that were hatch-year birds compared to red-backed vole (Myodes gapperi) abundance in central Ontario. Vole abundance is the number of captures per trap night during summer surveys. Parameters for ordinary least-squares regression are shown
Discussion
The annual population growth of Northern Saw-whet Owls, estimated from the number of birds calling in spring, appeared to be directly related to red-backed vole abundance with no time lag. There was a much weaker, direct response to deer mouse abundance. Our study is the first to demonstrate that Northern Saw-whet Owl abundance is directly related to red-backed vole abundance. Our results support the hypothesis that Northern Saw-whet Owls are nomadic and rapidly track resources, mostly through vole supply-dependent migration. These findings support a model whereby breeding season Northern Saw-whet Owl abundance at a given site is determined largely by prey abundance, and by the immigration and emigration response of the birds, rather than by mortality and natality processes. The owls appear to make a decision to settle into a breeding territory based, at least in part, on vole abundance.
This model of nomadism in Northern Saw-whet Owls is generally supported by other studies. In addition to a direct numerical response to prey (Korpimäki 1994), nomadic predators should also show low site fidelity (Andersson 1980). Marks and Doremus (2000) found that during a 13-year nest-box study in Idaho, USA, there was a positive correlation between the number of Northern Saw-whet Owl nests and small-mammal abundance. Moreover, only 1 of 52 breeding adults banded in their study area was recaptured in a subsequent year, and 0 of 139 banded fledglings were subsequently recaptured in the area. Cannings (1993) also found low site fidelity by Northern Saw-whet Owls; 0 of 83 nestlings banded in his British Columbia study area during 1984–1991 were subsequently recaptured. Nomadism by predators is also promoted by high fecundity (Andersson 1980). The clutch size of Northern Saw-whet Owls is large for a raptor, usually 5–6 eggs (Cannings 1993).
Nomadism has been demonstrated in other owl species, and notably, most examples come from Europe where the owls’ food supply is cyclic. Long-eared and Short-eared Owls (Asio otus and A. flammeus, respectively), Tengmalm’s Owls, and Eurasian Kestrels (Falco tinnunulus) all show evidence of nomadism (Korpimaki 1994; Korpimäki and Norrdahl 1991). In Canada, Short-eared Owls have been found to exhibit a direct numerical response to meadow vole (Microtus pennsylvanicus) abundance, such that Poulin et al. (2001) concluded they were nomadic.
Overall, breeding owl abundance varied more widely among years than did the proportion of hatch-year owls banded. This is consistent with immigration and emigration making a larger contribution to the numerical response than natality. There was a weak relationship between the proportion of hatch-year owls and vole abundance, possibly indicating a small, delayed numerical response. The strength of this relationship was limited by the lack of variation in the proportion of hatch-year birds. We interpret these data as being largely consistent with nomadism in Northern Saw-whet Owls. We expected that fecundity would not vary greatly from year to year if adult birds disperse from breeding sites to track prey resources. In this way, breeding owls would be protected from years of prey collapse leading to reproductive failure of the sort that affects predators with a pronounced delayed numerical response (e.g., Bowman et al. 2006; O’Donoghue et al. 1997; Rohner 1996). A difficulty in interpreting these migration data is that we do not know the origins of the Northern Saw-whet Owls that are passing through Long Point Bird Observatory. If owls passing through this station all originate from the central Ontario region (which is unlikely), than we would expect a positive relationship between the owl productivity estimates at the banding station and our small-mammal index. As the extent of the catchment area increases, the relationship between these two metrics should decrease.
Nomadism in predators may be promoted by prey species that are cyclic, and by long intervals occurring between years of abundant prey (Andersson 1980). Red-backed voles are not cyclic in central Ontario (Fryxell et al. 1998), although they may be in other parts of their range (e.g., Elias et al. 2006). They also do not typically exhibit long intervals between years of peak abundance. Instead, red-backed voles in the area tend to undergo wide, irregular fluctuations in abundance that are spatially synchronous over only a small extent (<200 km) (Bowman et al. 2008; Fryxell et al. 1998). Thus, over a large spatial extent, vole populations may often be out of phase with one another, with dramatically different levels of abundance. This approximate scenario was addressed by Andersson (1980), who concluded that when food production is temporally random, nomadism will be favoured for species with large clutch size when the probability of reproducing in a given year (P) is greater than the ratio of 1/t, where t is the interval between productive years at a given site. This suggests that, since the voles are synchronous over a fairly small area, the relatively short-distance search required by owls in spring to find a breeding site increases the chance that P > 1/t, promoting nomadism.
Generally, nomadic species are thought to be specialist predators, following preferred prey to a new territory, rather than maintaining a territory in the absence of that prey and switching to an alternative food (Korpimäki and Norrdahl 1991). This specialist tendency is undoubtedly related to the apparent habitat preferences of Northern Saw-whet Owls, which are found in greatest abundance during breeding in coniferous forests (Cannings 1993). Red-backed voles are also most abundant in coniferous forests (Bowman et al. 2001a, b). Deer mice, however, are more generalist in habitat use than red-backed voles, occurring in a wide range of habitats (Baker 1968). We predict that Northern Saw-whet Owls in the study area should specialise on red-backed voles.
In a recent paper, Bowman et al. (2008) argued that a lack of interspecific synchrony among small mammals in the same Ontario region indicated that nomadic predation was not spatially synchronising prey population dynamics. Our findings concerning the owl’s numerical response suggest, however, that the interspecific synchrony assumption may have been inappropriate. If Northern Saw-whet Owls are specialising on voles rather than mice, it is conceivable that owls could contribute to the observed short-distance synchrony of voles and not to the spatial pattern of mouse population growth. It appears that we must still consider nomadic predation a potential contributor to small-mammal population synchrony in the region, at least for red-backed voles.
The prospect that migrating Northern Saw-whet Owls evaluate vole supply prior to establishing a breeding territory implies a mechanism for comparing relative vole abundance among sites. In Fennoscandia, it appears that Eurasian Kestrels and Rough-legged Buzzards (Buteo lagopus) evaluate the prevalence of vole scent marks, which are visible under ulraviolet light (Koivula and Viitala 1999; Viitala et al. 1995). This particular mechanism does not appear to be used by nocturnal Tengmalm’s Owls, however (Koivula et al. 1997), suggesting that it may not be used by Northern Saw-whet Owls either. Some other mechanism appears necessary for Northern Saw-whet Owls to make an accurate assessment of vole supply in breeding territories. At present, we are uncertain what the mechanism might be, and this points toward an interesting avenue of future research.
To summarize, we observed a direct numerical response by breeding Northern Saw-whet Owls to red-backed vole abundance in central Ontario, Canada. We observed a weaker response by Northern Saw-whet Owls to deer mouse abundance. These findings suggest that Northern Saw-whet Owls are nomadic predators, specialising in the region on red-backed voles.
Zusammenfassung
Numerische Antwort von brütenden Sägekäuzen Aegolius acadicus lässt Nomadentum vermuten
Wir haben anhand einer 13-jährigen Zeitreihe bestehend aus Abundanzschätzungen von Sägekäuzen und von Kleinsäugern in Central Ontario, Kanada, die Veränderung in der Anzahl von Sägekäuzen als Antwort auf Beutetierabundanzen bestimmt. Die begrenzte Zunahme brütender Sägekäuze hing direkt mit den Abundanzschätzungen von Rötelmäusen (Myodes gapperi) zusammen. Es scheint also als wären die Käuze nomadisch, und machten Entscheidungen über den Brutort in Abhängigkeit vom Rötelmausangebot. Die Käuze zeigten jedoch eine viel schwächere Antwort auf die Abundanz von Hirschmäusen (Peromyscus maniculatus). Über alle Jahre hinweg konnte 55% der Variation in der Zunahme von Käuzen eindeutig den Wühlmausabundanzen zu geordnet werden, und wir fanden nur einen schwachen Zusammenhang zwischen dem Anteil einjähriger Käuze, die im Herbst an Berinungsstationen gefangen wurden, und Kleinsäugerabundanzen. Stattdessen scheinen Sägekäuze Jahre, in denen der Bruterfolg weitläufig sehr gering war, zu meiden und durch die nomadische Strategie Brutgebiete nach dem Wühlmausangebot auszuwählen.
References
Andersson M (1980) Nomadism and site tenacity as alternative reproductive tactics in birds. J Anim Ecol 49:175–184
Andersson M, Erlinge S (1977) Influence of predation on rodent populations. Oikos 29:591–597
Badzinski DS (2007) Ontario nocturnal owl survey: 2007 final report. Bird Studies Canada
Baker RH (1968) Habitats and distribution. In: King JA (ed) Biology of Peromyscus (Rodentia). Spec Publ No. 2, Am Soc Mammal, pp 98–126
Baumgartner FM (1939) Territory and population in the great horned owl. Auk 56:274–282
Bowman J, Forbes GJ, Dilworth TG (2001a) The spatial component of variation in small-mammal abundance measured at three scales. Can J Zool 79:137–144
Bowman J, Forbes GJ, Dilworth TG (2001b) Landscape context and small-mammal abundance in a managed forest. For Ecol Manag 140:249–255
Bowman J, Donovan D, Rosatte RC (2006) Numerical response of fishers to synchronous prey dynamics. J Mammal 87:480–484. doi:https://doi.org/10.1644/05-MAMM-A-202R2.1
Bowman J, Phoenix RD, Sugar A, Dawson FN, Holborn G (2008) Spatial and temporal dynamics of small mammals at a regional scale in Canadian boreal forest. J Mammal 89:381–387. doi:https://doi.org/10.1644/07-MAMM-A-147R1.1
Bulmer MG (1975) Phase relations in the 10-year cycle. J Anim Ecol 44:609–621
Cannings RJ (1993) Northern saw-whet owl (Aegolius acadicus). In: Poole A, Gill F (eds) The birds of North America, No. 42. The Birds of North America, Philadelphia
Côté M, Ibarzabal J, St-Laurent M-H, Ferron J, Gagnon R (2007) Age-dependent response of migrant and resident Aegolius owl species to small rodent population fluctuations in the eastern Canadian boreal forest. J Raptor Res 41:16–25. doi:https://doi.org/10.3356/0892-1016(2007)41[16:AROMAR]2.0.CO;2
Elias SP, Witham JW, Hunter ML Jr (2006) A cyclic red-backed vole (Clethrionomys gapperi) population and seedfall over 22 years in Maine. J Mammal 87:440–445. doi:https://doi.org/10.1644/05-MAMM-A-170R1.1
Fryxell JM, Falls JB, Falls EA, Brooks RJ (1998) Long-term dynamics of small-mammal populations in Ontario. Ecology 79:213–225
Koivula M, Viitala J (1999) Rough-legged buzzards use vole scent marks to assess hunting areas. J Avian Biol 30:329–332
Koivula M, Korpimäki E, Viitala J (1997) Do Tengmalm’s owls see vole scent marks visible in ultraviolet light? Anim Behav 54:873–877
Korpimäki E (1994) Rapid or delayed tracking of multi-annual vole cycles by avian predators? J Anim Ecol 63:619–628
Korpimäki E, Norrdahl K (1991) Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology 72:814–826
Marks JS, Doremus JH (2000) Are northern saw-whet owls nomadic? J Raptor Res 34:299–304
O’Donoghue M, Boutin S, Krebs CJ, Hofer EJ (1997) Numerical responses of coyotes and lynx to the snowshoe hare cycle. Oikos 80:150–162
Poulin RG, Wellicome TI, Todd LD (2001) Synchronous and delayed numerical responses of a predatory bird community to a vole outbreak on the Canadian Prairies. J Raptor Res 35:288–295
Rasmussen JL, Sealy SG, Cannings RJ (2008) Northern saw-whet owl (Aegolius acadicus). In: Poole, A (ed) The birds of North America online, No. 42. Cornell Laboratory of Ornithology
Rohner C (1996) The numerical response of great horned owls to the snowshoe hare cycle: consequences of non-territorial “floaters” on demography. J Anim Ecol 65:359–370
Rowe JS (1972) Forest regions of Canada. Canadian Forest Service, Department of the Environment, Ottawa
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–35
Viitala J, Korpimäki E, Palokangas P, Koivula M (1995) Attraction of kestrels to vole scent marks visible in ultraviolet light. Nature 373:425–427
Weir RD, Cooke F, Edwards MH, Stewart RB (1980) Fall migration of saw-whet owls at Prince Edward Point, Ontario. Wilson Bull 92:475–488
Acknowledgments
Funding for this work was provided by the Ontario Ministry of Natural Resources, Bird Studies Canada, Long Point Bird Observatory, the University of Guelph, and the Natural Sciences and Engineering Research Council of Canada. We thank the many technicians and the volunteer owl surveyors and banders who to helped collect the data. Jeff Marks and Matt Reudink provided helpful comments on the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Bowman, J., Badzinski, D.S. & Brooks, R.J. The numerical response of breeding Northern Saw-whet Owls Aegolius acadicus suggests nomadism. J Ornithol 151, 499–506 (2010). https://doi.org/10.1007/s10336-009-0482-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-009-0482-3