Abstract
We studied the diet composition and behavioural responses to variable food conditions in Tengmalm’s Owls (Aegolius funereus). The abundance of main prey (voles and mice) of owls was higher in the Ore Mountains, Czech Republic, than in the Kauhava region, Finland. We monitored nests continuously by a camera system to estimate the feeding frequency and to identify prey items provided to nestlings. We recorded 990 prey deliveries at six nests in the Ore Mountains and 1,679 prey deliveries at nine nests in the Kauhava region. Mice (Apodemus) and voles (Microtus and Clethrionomys) were the main foods of owls in the Ore Mountains, whereas voles (Clethrionomys and Microtus) and shrews (Sorex) were the main foods in the Kauhava region. In consequence, on average smaller prey items were brought to nestlings at the Finnish site. However, both absolute and relative (per one nestling) feeding frequency was higher in the Kauhava region, and the biomass available to individual nestlings did not differ between the two areas. Moreover, the Finnish and Czech pairs produced about the same number of fledglings. Our results suggest that male owls are able to maintain the amount of food required for chicks by switching to alternative prey, and to increase their prey delivery rates under conditions of reduced abundance of main food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diet composition of generalist avian predators usually reflects the temporal and spatial variation in prey availability. They typically feed on the most abundant prey of the preferred size, and the diet composition is therefore strongly affected by the actual food supply (Jaksic and Braker 1983; Recher 1990; Marti et al. 1993; Korpimäki and Marti 1995; Valkama et al. 2005). Under conditions of the population decline of the dominant prey, raptors often switch to alternative prey items (Korpimäki 1986a, 1988, 1992; Korpimäki and Norrdahl 1991; Reif et al. 2001; Riegert and Fuchs 2004; Rutz and Bijlsma 2006) and/or change their foraging behaviour and microhabitats (Korpimäki 1986b; Jacobsen and Sonerud 1987; Hakkarainen and Korpimäki 1994; Valkama et al. 1995; Riegert et al. 2007), or enlarge the size of the feeding territories (Village 1981, 1987; Bonal and Aparicio 2008).
Alternative prey, however, could often be less accessible or have lower biomass (Korpimäki 1986a, 1986b, 1988; Steenhof and Kochert 1988; Korpimäki and Norrdahl 1991; Salamolard et al. 2000; Riegert and Fuchs 2004; Rutz and Bijlsma 2006). Many studies document that a drastic decline of main food and/or lack of alternative prey reduces the breeding success or even leads to nest desertion or failure of nesting attempts in birds of prey (Korpimäki and Sulkava 1987; Korpimäki 1987; Hörnfeldt et al. 1990; Wiehn and Korpimäki 1997; Salamolard et al. 2000; Tornberg et al. 2005; Salafsky et al. 2007). However, studies on changing of parental feeding effort as a response to temporal variation in food availability are relatively rare (Tolonen and Korpimäki 1994, 1996; Hakkarainen et al. 1997; Wiehn and Korpimäki 1997; Riegert et al. 2007). We suggest that this is mainly due to methodical difficulties in detailed food composition assessment and estimation of feeding effort.
Temgmalm’s Owl (Aegolius funereus) is a nocturnal avian predator which feeds mainly on voles in northern Europe (Korpimäki 1981, 1988) and voles and mice in central Europe (Korpimäki 1986c; Pokorný 2000; Pokorný et al. 2003). The abundance of small rodents varies strongly from year to year, particularly in northern Europe (Korpimäki et al. 2005), and the proportion of shrews and birds increases strongly in the diet of owls in years of low abundance of rodents (Korpimäki 1981, 1988; Koivunen et al. 1996). Males of the Tengmalm’s Owl provide food for females and also feed the young during the nestling period (Korpimäki 1981; Drdáková and Zárybnický 2004). There are marked differences in the types of parental care provided by each sex. Females incubate the eggs and brood the young until they are about 2 or 3 weeks old (Korpimäki 1981; Drdáková 2003). After the young leave the nest, females either take part in feeding them or pass all the work to the males and desert the brood (Korpimäki 1981, 1989; Solheim 1983). In European kestrels (Falco tinnunculus), females attempted to increase their prey delivery rate and parental effort under poor food conditions when male kestrels were not able to provide families with enough food (Jönsson et al. 1999).
We investigated diet composition and prey delivery rates of Tengmalm’s Owls in two study areas, which differed considerably in main food abundance, and in the taxonomic composition and mass of potential prey. The relatively recently developed methodological approach (see review in Reif and Tornberg 2006) of continuous nest recording by a camera system enabled us to obtain detailed data on both the parental feeding effort of the owls and on the identification of prey items brought to nestlings. We asked (1) whether males are able to increase their prey delivery rates (an estimate of feeding effort; see Tolonen and Korpimäki 1994) under conditions of poorer quality (smaller) prey, (2) if chicks are fed the same amount of food under different conditions of prey abundance, and (3) whether females are able to change their parental care and increase their provisioning of chicks under reduced food conditions.
Materials and methods
Our study was carried out during the breeding seasons (April to June) in 2004 in the Czech Republic (50°N, 13°E) and in 2005 in Finland (63°N, 23°E), respectively. The Czech site was situated in the Ore Mountains (730–960 m a.s.l.), covering 70 km2. The Finnish site was situated in the Kauhava region, western Finland (50–110 m a.s.l.), covering c. 1,300 km2.
The parental feeding behaviour was studied at six nests in the Ore Mountains (40% of the nest-box breeding population in 2004) and at nine nests in the Kauhava region (13% of the nest-box breeding population in 2005). Nests for observations were chosen randomly, but nest boxes near paths and roads were avoided due to visibility of technical equipment attached to the nest box. All nests in both study areas were successful, i.e. at least one young fledged from each nest. The nests were continuously monitored by a camera system for 24 h per day from hatching to fledging. Each nest was recorded over a mean period of 33.2 ± 5.4 days in Ore Mountains and 27.3 ± 4.6 days in the Kauhava region.
Technical equipment
The equipment we used to monitor the parental feeding effort and composition of prey delivered to the nest consisted of a camera (DECAM), a chip reader device, a movement data-logger, a movement infrared detector (KS96) and infrared lightning (IR diodes, SFH 485–2880 nm; Bezouška et al. 2005). The camera was installed inside the nest-box opposite the opening. It was triggered by the infrared detector sensitive to movements in the nest-box opening. The time of detection was recorded by the movement data-logger and one to three photos were taken for each event. During the night, the opening was illuminated by infrared diodes at the moment of taking photos by the camera. All adult owls and nestlings were marked by chip rings. A chip reader device fixed by the nest-box opening detected and archived all movements of chips in the nest opening. Using this equipment, we were able to record arrival and departure of parents to the nest box and feeding frequency and species composition of prey delivered to the nests.
Assessment of diet composition
We recorded 990 prey deliveries at six nests in the Ore Mountains and 1,679 prey deliveries at nine nests in Kauhava region. Total prey delivery rate (male and female together) was defined as the (1) number and (2) the mass of prey delivered to the nest during one night.
The diet composition was assessed combining the two methods. Pictures taken by the nest-box camera system enabled us to determine 87.4% (n = 865) of the delivered prey in the Ore Mountains and 75.6% (n = 1,269) in the Kauhava region at least to the family or genus level. We also recorded all cached prey during regular inspections (once per 3–5 days) of the nests (14.8 ± 2.3% from the total prey items in the Ore Mountains and 9.2 ± 1.5% in the Kauhava region). These prey items were determined to the species level. We used the species rate in cached prey to re-calculate the relative species composition of prey determined by the camera system.
Estimation of prey abundance and weight
Abundances of small mammals were assessed using snap-trap captures. The captures were carried out in both areas at the beginning of June. The traps were laid out in squares of 100 × 100 m (Ore Mountains, 3 squares) and 90 × 90 m (Kauhava region, 4 squares). Spacing of the traps was 10 m, i.e. totals of 121 traps/square were laid in the Ore Mountains and 100 traps/square in Kauhava region. The traps were in place for 3 days and checked once a day. All captured mammals (79 items in the Ore Mountains and 39 items in the Kauhava region) were determined to the species level. In the Ore Mountains, the average weight of each prey species was assessed based on the individuals captured by snap-traps laid in the squares (39 items) and along lines (315 items) during the same breeding season. In the Kauhava region, we used data from weights of each prey species from previous breeding seasons (789 items; Korpimäki 1988; Norrdahl and Korpimäki 2002).
Statistical analyses
We used conventional statistical methods with parametric tests where the data fit normal distribution. We used t tests to compare the differences between the two areas in number of fledglings, prey abundance, number and weight of prey items and total mass of prey delivered to nests and χ 2 tests to compare the taxonomic composition of the food supply and the diet of nestlings between the two areas. Trapping squares were used as a unit of replication in food supply comparisons, and nests were used as a unit of replication to test the differences in diet of nestlings and number of fledglings between the two areas. To correct the analysis for the age of nestlings, we recorded all nests during the period between 10 and 30 days of age of the oldest nestling in a particular nest. All data analyses were processed in the Statistica 6.0 software package (StatSoft 2003). Values are reported as means ± SD per nest or trapping site.
Results
We found differences between the two study sites both in small mammal abundance and species composition. The abundance of small mammals was significantly higher (t test: t = 3.5, P < 0.02, n 1 = 3, n 2 = 4) in the Ore Mountains (26.3 ind./ha) than in the Kauhava region (9.8 ind./ha). The taxonomic composition of the food supply also differed significantly between the two study localities (χ 2 = 132.4, P < 0.001, df = 4). In the Ore Mountains, Yellow-necked Mouse Apodemus flavicollis was the dominant prey species and accounted for 70.9% of all trapped small mammals, while Field Vole Microtus agrestis (12.7%), Bank Vole Clethrionomys glareolus (11.4%) and Common Shrew Sorex araneus (5.0%) were less frequently caught. In the Kauhava region, Bank Vole Clethrionomys glareolus (71.8%) and Common Shrew Sorex araneus (15.4%) were the most abundant species. Harvest mouse Micromys minutus (7.7%) and Field Vole Microtus agrestis (5.1%) were only rarely trapped. The average weight of small mammals caught in the traps differed between the two study sites (t test: t = 5.7, P = 0.002, n 1 = 3, n 2 = 4; Ore Mountains: mean = 23.3 ± 0.7 g, Kauhava region: mean = 17.3 ± 1.4 g).
We found significant differences in species composition of prey delivered to the owl nests at the two study sites (χ 2 = 92.1, P < 0.001, df = 5; Table 1). In the Ore Mountains, adults delivered mostly Apodemus mice (58.6%) and Microtus and Clethrionomys voles (32.6%), whereas adults in the Kauhava region fed their nestlings mostly on Microtus and Clethrionomys voles (61.5%) and Sorex shrews (27.6%). Adults also delivered significantly heavier prey (t test: t = 6.7, P < 0.0001, n 1 = 6, n 2 = 9) to their nestling in the Ore Mountains (24.0 ± 0.4 g) than in the Kauhava region (19.3 ± 1.6 g).
The average number of prey delivered by adults to nests per night was significantly lower (t test: t = 2.3, P = 0.04, n 1 = 6, n 2 = 9) in the Ore Mountains (7.5 ± 1.0) than in the Kauhava region (9.2 ± 1.5). However, we found no significant differences between the two sites in the total mass of prey delivered to the nests per night (t test: t = 0.1, P = 0.9, n 1 = 6, n 2 = 9): the average mass of prey delivered by the adults per night was 179.6 ± 26.7 g in the Ore Mountains and 176.9 ± 36.7 g in the Kauhava region.
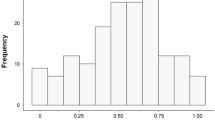
The two study sites did not show obvious differences in the number of fledglings per nest (t test: t = 0.8, P = 0.4, n 1 = 6, n 2 = 9): the average number of fledglings in the Ore Mountains was 4.7 ± 0.5, whereas 4.1 ± 1.5 chicks fledged in the Kauhava region. We found a significant difference between the two study sites in the number of prey delivered per one surviving nestling per night (t test: t = 2.9, P = 0.01, n 1 = 6, n 2 = 9; Fig. 1a): 1.6 ± 0.1 items in the Ore Mountains; 2.1 ± 0.4 items in the Kauhava region, but not in the weight of prey delivered per one surviving nestling per night (t test: t = 0.6, P = 0.5, n 1 = 6, n 2 = 9; Fig. 1b): 37.7 ± 4.1 g in the Ore Mountains and 39.7 ± 6.0 g in the Kauhava region.
In both areas, the great majority of the prey items were delivered by males. Females took part in feeding of nestlings after they stopped to brood the young in the nest, but they brought prey to nestlings very sporadically. In the Ore Mountains, only 1.2% ± 1.8 of all prey items was delivered by females; in the Kauhava region, this proportion tended to be close to significantly higher (13.6% ± 14.2 prey items; t test: t = 1.918, P = 0.077, n 1 = 6, n 2 = 9).
Discussion
The abundance of small mammals was greater in the Ore Mountains than in the Kauhava region during the study years. Small mammals were the dominant prey of Tengmalm’s Owls in both areas, whereas birds were rarely taken. Mice (Apodemus) and voles (Microtus and Clethrionomys) were the staple food of Tengmalm’s Owls in the Ore Mountains. In contrast, owls in the Kauhava region fed their nestlings mainly on voles (Microtus and Clethrionomys) and shrews. Shrews and small birds represent the most important alternative prey for Tengmalm’s Owls in decreasing and low vole years (Korpimäki 1981, 1988). This kind of prey is suggested to be suboptimal for this species because it is typically smaller and less abundant and/or its hunting is more energetically costly (Korpimäki 1986a, b, 1988; Village 1987; Korpimäki and Norrdahl 1989, 1991). It has previously been shown that in poor vole years a majority of Tengmalm’s Owls skip breeding (Hakkarainen et al. 2002) and probably only individuals in good body condition are capable of breeding (Korpimäki 1984, 1994; Korpimäki and Rita 1996; Brommer et al. 2002). We asked in this study whether such individuals are able to buffer against scarcity of the main prey and to provide enough food for their nestlings by switching to alternative prey.
Tengmalm’s Owls in the Kauhava region provided smaller prey items to their nestlings than those in the Ore Mountains. However, both absolute and relative (per one nestling) feeding frequency was significantly higher in the Kauhava region than in the Ore Mountains, where abundance of the main food was higher. Accordingly, the biomass brought to individual nestlings did not show obvious differences between the two study sites. This suggests that male owls in the Kauhava region increased their hunting effort to maintain the amount of food required for optimal chick development (Underhill-Day 1993; Hakkarainen et al. 1997; Durant et al. 2004), or that the availability of prey was better there although the density of the main prey was lower. This might be because vegetative cover was lower in the Finnish than the Czech study site during the Tengmalm Owl breeding season.
The lack of suitable prey often results in lower breeding success in birds of prey (Korpimäki 1984; Korpimäki and Norrdahl 1991; Korpimäki and Hakkarainen 1991; Hörnfeldt et al. 1990; Wiehn and Korpimäki 1997; Salamolard et al. 2000; Tornberg et al. 2005; Salafsky et al. 2007). Although the two areas differed significantly in main prey abundance, the breeding success measured as the number of young fledging did not differ between the two areas. We therefore suggest that male owls were able to compensate for a reduction in food supply by increasing their hunting and feeding effort. Alternatively, differences in prey availability could also explain the similar offspring production, because we did not estimate the hunting and feeding effort of the males (Tolonen and Korpimäki 1994). Moreover, it is possible that, despite the males maintaining constant prey biomass per offspring, nestling growth may differ if fed with the staple prey or with alternative prey.
Previous studies on food provisioning behaviour of Tengmalm’s Owls showed that males provide food for females and exclusively feed the young during the early nestling period (Korpimäki 1981; Drdáková and Zárybnický 2004). This has been confirmed by our detailed study of feeding behaviour using continuous camera recording. Females, however, stop brooding the young and leave the nest-hole when the chicks are about 3 weeks old (Klaus et al. 1975; Korpimäki 1981; Drdáková 2003) and could then take part in food provisioning of the nestlings. We hypothesised that females should increase their prey delivery rate under conditions of poor food supply as has been shown in other bird of prey species (Jönsson et al. 1999; Durant et al. 2004). Indeed, females of Tengmalm’s Owls showed a higher prey delivery rate in the Kauhava region, where the food abundance was lower. However, their relative contribution to feeding of nestlings was only marginal compared to the males in both areas. A reduction in prey abundance in the Kauhava region therefore mainly resulted in an increased prey delivery rate by males to maintain a sufficient amount of food required by the chicks. However, as it has been shown that the annual survival rate of adult males is low in the years of low vole abundance (Hakkarainen et al. 2002), we suggest that high prey delivery rate in poor vole years could be one of the causes of increased mortality of male owls in addition to the low food supply in winter.
Zusammenfassung
Ändern Rauhfußkäuze den elterlichen Fütterungsaufwand, wenn die Hauptbeute unterschiedlich verfügbar ist?
Wir haben die Nahrungszusammensetzung sowie die Verhaltensantworten auf variable Nahrungsbedingungen bei Rauhfußkäuzen untersucht. Die Abundanzen der Hauptbeutetiere der Eulen (Wühlmäuse und Mäuse) waren höher in den Ore-Bergen (Tschechische Republik) als in der Kauhava-Region (Finnland). Wir haben die Nester fortwährend mit Hilfe eines Kamerasystems überwacht, um die Fütterfrequenz abzuschätzen und den Nestlingen angebotene Beutestücke zu identifizieren. Wir haben 990 Beutelieferungen an sechs Nestern in den Ore-Bergen und 1679 Lieferungen an neun Nestern in der Kauhava-Region aufgezeichnet. Mäuse (Apodemus) und Wühlmäuse (Microtus und Clethrionomys) waren die Hauptnahrung der Eulen in den Ore-Bergen, während Wühlmäuse (Microtus und Clethrionomys) und Spitzmäuse (Sorex) die Hauptbeute in der Kauhava-Region darstellten. Folglich wurden den Nestlingen am finnischen Standort durchschnittlich kleinere Beutestücke gebracht. Die Fütterfrequenz, sowohl die absolute als auch die relative (pro einem Nestling), war jedoch höher in der Kauhava-Region, und die für einzelne Nestlinge verfügbare Biomasse unterschied sich nicht zwischen den beiden Gebieten. Darüber hinaus produzierten finnische und tschechische Paare etwa dieselbe Anzahl flügger Jungvögel. Unsere Ergebnisse deuten darauf hin, dass Eulenmännchen in der Lage sind, die von den Küken benötigte Nahrungsmenge einzuhalten, indem sie auf alternative Beutetiere umstellen, und ihre Fütterfrequenzen zu erhöhen, wenn die Abundanz der Hauptbeute reduziert ist.
References
Bezouška V, Děd P, Drdáková M (2005) The automatic system for monitoring of owls′ nests. ITCE 2005 Conference abstracts, CZU TF Praha, pp 173–182. CD-ROM. ISBN 80-213-1358-7
Bonal R, Aparicio JM (2008) Evidence of prey depletion around lesser kestrel Falco naumanni colonies and its short term negative consequences. J Avian Biol 39:189–197. doi:10.1111/j.2008.0908-8857.04125.x
Brommer JE, Pietiäinen H, Kolunen H (2002) Reproduction and survival in a variable environment: Ural owls (Strix uralensis) and the three-year vole cycle. Auk 119:544–550. doi:10.1642/0004-8038(2002)119[0544:RASIAV]2.0.CO;2
Drdáková M (2003) Breeding biology of the Tengmalm’s Owl in air-pollution damaged areas of the Ore Mountains. Sylvia 39:35–51 (in Czech with English summary)
Drdáková M, Zárybnický J (2004) Does activity of Tengmalm’s Owl (Aegolius funereus) change during breeding period? Sluka 1:23–36 (in Czech with English summary)
Durant JM, Gendner JP, Handrich Y (2004) Should I brood or should I hunt: a female barn owl’s dilemma. Can J Zool 82:1011–1016. doi:10.1139/z04-078
Hakkarainen H, Koivunen V, Korpimäki E (1997) Reproductive success and parental effort of Tengmalm’s owls: effect of spatial and temporal variation in habitat quality. Ecoscience 4:35–42
Hakkarainen H, Korpimäki E (1994) Does feeding effort of Tengmalm’s owls reflect offspring survival prospects in cyclic food conditions? Oecologia 97:209–214. doi:10.1007/BF00323151
Hakkarainen H, Korpimäki E (1995) Contrasting phenotypic correlations in food provision of male Tengmalm’s owls (Aegolius funereus) in a temporally heterogenous environment. Evol Ecol 9:30–37. doi:10.1007/BF01237694
Hakkarainen H, Korpimäki E, Koivunen V, Ydenberg R (2002) Survival of male Tengmalm’s owls under temporally varying food conditions. Oecologia 131:83–88. doi:10.1007/s00442-001-0865-5
Hörnfeldt B, Carlsson BG, Löfgren O, Eklund U (1990) Effects of cyclic food suply on breeding performance in Tengmalm’s Owl (Aegolius funereus). Can J Zool 68:522–530
Jacobsen BV, Sonerud GA (1987) Home range of Tengmalm’s Owl: a comparison between nocturnal hunting and diurnal roosting. USDA Forest Service General Technical Report RM 142:189–192
Jaksic FM, Braker HM (1983) Food–niche relationships and guilds structure of diurnal birds of prey: competition versus opportunism. Can J Zool 61:2230–2241
Jönsson KI, Wiehn J, Korpimäki E (1999) Body reserves and unpredictable breeding conditions in the Eurasian kestrel, Falco tinnunculus. Ecoscience 6:406–414
Klaus S, Mikkola H, Wiesner J (1975) Aktivität und Ernährung des Rauhfusskauzes Aegolius funereus (L.) während der Fortpflanzungsperiode. Zool Jb Syst 102:485–507
Koivunen V, Korpimäki E, Hakkarainen H, Norrdahl K (1996) Prey choice of Tengmalm’s owls (Aegolius funereus funereus): preference for substandard individuals? Can J Zool 74:816–823. doi:10.1139/z96-094
Korpimäki E (1981) On the ecology and biology of Tengmalm’s Owl Aegolius funereus in Southern Ostrobothnia and Soumenselkä, western Finland. Acta Univ Oul A 118 Biol 13:1–84
Korpimäki E (1984) Population dynamics of birds of prey in relation to fluctuations in small mammal populations in western Finland. Ann Zool Fenn 21:287–293
Korpimäki E (1986a) Seasonal changes in the food of the Tengmalm’s Owl Aegolius funereus in western Finland. Ann Zool Fenn 23:339–344
Korpimäki E (1986b) Diet variation, hunting habitat and reproductive output of the Kestrel Falco tinnunculus in the light of the optimal diet theory. Ornis Fenn 63:84–90
Korpimäki E (1986c) Gradients in population fluctuations of Tengmalm’s owl Aegolius funereus in Europe. Oecologia 69:95–201. doi:10.1007/BF00377621
Korpimäki E (1987) Clutch size, breeding success and brood size experiments in Tengmalm’s Owl Aegolius funereus: a test of hypotheses. Ornis Scand 18:277–284. doi:10.2307/3676896
Korpimäki E (1988) Diet of breeding Tengmalm’s Owls Aegolius funereus: long-term changes and year-to-year variation under cyclic food conditions. Ornis Fenn 65:21–30. doi:10.2307/3676522
Korpimäki E (1989) Mating system and mate choice of Tengmalm’s Owls Aegolius funereus. Ibis 131:41–50. doi:10.1111/j.1474-919X.1989.tb02742.x
Korpimäki E (1992) Diet composition, prey choice and breeding success of Long-eared Owls: effects of multiannual fluctuations in food abundance. Can J Zool 70:2373–2381
Korpimäki E (1994) Rapid or delayed tracking of multi-annual vole cycles by avian predators? J Anim Ecol 63:619–628. doi:10.2307/5228
Korpimäki E, Hakkarainen H (1991) Fluctuating food supply affects the clutch size of Tengmalm’s owl independent of laying date. Oecologia 85:543–552. doi:10.1007/BF00323767
Korpimäki E, Marti CD (1995) Geographical trends in the trophic characteristics of mammal- and bird-eating raptors in Europe and North America. Auk 112:1004–1023
Korpimäki E, Norrdahl K (1989) Predation of Tengmalm’s owls—numerical responses, functional responses and dampening impact on population fluctuations of microtines. Oikos 54:154–164. doi:10.2307/3565261
Korpimäki E, Norrdahl K (1991) Numerical and functional responses of Kestrels, Short-eared owls and Long-eared owls to vole densities. Ecology 72:814–826. doi:10.2307/1940584
Korpimäki E, Oksanen L, Oksanen T, Klemola T, Norrdahl K, Banks PB (2005) Vole cycles and predation in temperate and boreal zones of Europe. J Anim Ecol 74:1150–1159. doi:10.1111/j.1365-2656.2005.01015.x
Korpimäki E, Rita H (1996) Effects of brood size manipulations on offspring and parental survival in the European kestrel under fluctuating food conditions. Ecoscience 3:264–273
Korpimäki E, Sulkava S (1987) Diet and breeding performance of Ural Owls Strix uralensis under fluctuating food conditions. Ornis Fenn 64:57–66
Marti CM, Korpimäki E, Jaksic FM (1993) Trophic structure of raptor communities: a three-continent comparison and synthesis. Curr Ornithol 10:47–137
Norrdahl K, Korpimäki E (2002) Changes in individual quality during a 3-year population cycle of voles. Oecologia 130:239–249
Pokorný J (2000) The diet of the Tengmalm’s Owl (Aegolius funereus) in Northbohemian mountain areas damaged by immissions. Buteo 11:107–114
Pokorný J, Kloubec B, Obuch J (2003) Comparison of Tengmalm’s Owl Aegolius funereus diet in several Czech mountain areas. Vogelwelt 124:313–323
Recher HF (1990) Specialist or generalist: avian response to spatial and temporal changes in resources. Stud Avian Biol 13:333–336
Reif V, Tornberg R (2006) Using time-lapse digital video recording for a nesting study of birds of prey. Eur J Wildl Res 52:251–258. doi:10.1007/s10344-006-0039-1
Reif V, Tornberg R, Jungell S, Korpimäki E (2001) Diet variation of common buzzards in Finland supports the alternative prey hypothesis. Ecography 24:267–274. doi:10.1034/j.1600-0587.2001.240304.x
Riegert J, Fuchs R (2004) Insects in the diet of urban kestrels from central Europe: an alternative prey or constant component of the diet? Ornis Fenn 81:23–32
Riegert J, Dufek A, Fainová D, Mikeš V, Fuchs R (2007) Increased hunting effort buffers against vole scarcity in an urban Kestrel Falco tinnunculus population. Bird Study 54:353–361
Rutz C, Bijlsma RG (2006) Food-limitation in a generalist predator. Proc Royal Soc B 273:2069–2076
Salafsky SR, Reynolds RT, Noon BR, Wiens JA (2007) Reproductive responses of northern goshawks to variable prey populations. J Wildl Manage 71:2274–2283. doi:10.2193/2006-357
Salamolard M, Butet A, Leroux A, Bretagnolle V (2000) Responses of an avian predator to variations in prey density at a temperate latitude. Ecology 81:2428–2441
Solheim R (1983) Bigyny and biandry in the Tengmalm’s owl Aegolius funereus. Ornis Scand 14:51–57. doi:10.2307/3676251
StatSoft, Inc. (2003) STATISTICA (data analysis sofware system), version 6.1. http://www.statsoft.com
Steenhof K, Kochert MN (1988) Dietary responses of three raptor species to changing prey densities in a natural environment. J Anim Ecol 57:37–48. doi:10.2307/4761
Tolonen P, Korpimäki E (1994) Determinants of parental effort: a behavioural study in the Eurasian kestrel, Falco tinnunculus. Behav Ecol Sociobiol 35:355–362. doi:10.1007/BF00184424
Tolonen P, Korpimäki E (1996) Do kestrels adjust their parental effort to current or future benefit in a temporally varying environment? Ecoscience 3:165–172
Tornberg R, Korpimäki E, Jungell S, Reif V (2005) Delayed numerical response of goshawks to population fluctuations of forest grouse. Oikos 111:408–415. doi:10.1111/j.0030-1299.2005.14066.x
Underhill-Day JC (1993) The foods and feeding rates of Montagus Harriers Circus pygargus breeding in arable farmland. Bird Study 40:74–80
Valkama J, Korpimäki E, Arroyo B, Beja P, Bretagnolle V, Bro E et al (2005) Birds of prey as limiting factors of gamebird populations in Europe: a review. Biol Rev Camb Philos Soc 80:171–203. doi:10.1017/S146479310400658X
Valkama J, Korpimäki E, Tolonen P (1995) Habitat utilization, diet and reproductive success in the Kestrel in temporally and spatially heterogeneous environment. Ornis Fenn 72:49–61
Village A (1981) The home range and density of Kestrels in relation to vole abundance. J Anim Ecol 51:413–428. doi:10.2307/3974
Village A (1987) Numbers, territory-size and turnover of Short-eared Owls Asio flammeus in relation to vole abundance. Ornis Scand 18:198–204. doi:10.2307/3676767
Wiehn J, Korpimäki E (1997) Food limitation on brood size: experimental evidence in the Eurasian kestrel. Ecology 78:2043–2050
Acknowledgments
Many thanks belong to Jan Zárybnický, Karel Št’astný, Michael Griesser, Jorma Nurmi and Rauno Varjonen for their help in the field. We are grateful to Vladimír Bezouška and Pavel Děd for their technical assisstance. We also thank Vincenzo Penteriani and an anonymous referee for their comments on the manuscript. The work of the first two authors has been supported by grants of the Czech University of Life Sciences Prague (CIGA 41110-1313-3156) and the Ministry of Education, Youth and Sports of the Czech Republic (LC 06073, MSM 0021620828 and FRVS 41110-1161-1625). This research complies with the laws of the countries in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Friedl.
Rights and permissions
About this article
Cite this article
Zárybnická, M., Sedláček, O. & Korpimäki, E. Do Tengmalm’s Owls alter parental feeding effort under varying conditions of main prey availability?. J Ornithol 150, 231–237 (2009). https://doi.org/10.1007/s10336-008-0342-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-008-0342-6