Abstract
To investigate migratory connectivity in the Reed Warbler Acrocephalus scirpaceus, we analysed (1) all available sub-Saharan ringing recoveries and (2) stable isotopes in feathers grown in Africa sampled at 17 European breeding sites across a migratory divide. A cluster analysis of ringing recoveries showed remarkable connectivity between breeding and non-breeding grounds. Two main clusters represented populations taking the two main migratory routes [southwesterly (SW) and southeasterly (SE)]. Stable isotope analysis confirmed the separation of wintering areas of SW- and SE-migrating populations. Higher δ15N values in feathers of SE-migrating birds indicated that they occupied more xeric biome types. Values of δ13C that did not differ significantly among populations were higher than those from feathers of known European origin and indicated a C4 biome. Three populations with an unknown migratory direction were assigned to the SE-migrating populations on the basis of δ15N values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migratory birds occur in different places at different times of the year, thereby representing serious challenges to researchers interested in factors affecting birds’ survival. Until recently, most studies have focused on factors limiting avian species in the breeding grounds, and only a few have acknowledged that events outside the breeding season may be equally important to the survival of such species. Adverse conditions in the wintering grounds and en route need not only negatively influence body condition (Marra et al. 1998; Gunnarsson et al. 2005) and survival (Peach et al. 1991; Szép 1995; Schaub et al. 2005), but migrants may also prolong moult and consequently arrive later at their breeding grounds (Marra et al. 1998; Saino et al. 2004). Hostile conditions in non-breeding grounds can have negative consequences for reproductive output (Lozano et al. 1996; Smith and Moore 2005) and population sizes (Newton 2006). Research into seasonal interactions and migratory connectivity (Norris 2005) is therefore of crucial importance for the effective protection of migratory birds (Newton 2004). Migratory connectivity—the links between breeding and non-breeding populations (Webster et al. 2002)—also determines the possibilities for the evolution of specific adaptations and responses to environmental changes and may eventually lead to speciation (Webster and Marra 2005).
Despite intensive ringing efforts during the past 100 years (Bairlein 2003), we still know very little about the migration and stopovers of different populations of migratory birds. Studies on the extent of population mixing in the non-breeding grounds have been hampered by extremely low recovery rates of ringed birds from the winter quarters. Because satellite tracking is suitable only for larger birds, several indirect approaches, such as the measurement of stable isotope abundance in avian tissues (reviewed by Hobson 2005), are being adopted to overcome the low number of recoveries and possible bias in ringing recovery data. The use of stable isotopes as tracers of geographical origin is based on the fact that site-specific stable isotopic profiles are passed through food webs into bird tissues (Kelly 2000). By taking a species' moult pattern and tissue-specific isotopic discrimination and elemental turnover rates into account, we can infer the breeding or wintering origin of individuals from the stable isotope signatures if the birds use isotopically distinct areas.
In the study reported here, we combined two different approaches—the analysis of stable isotope ratios in feathers and ringing recoveries—to study migratory connectivity in the Reed Warbler Acrocephalus scirpaceus. This species is a long-distance migrant, breeding in the marshlands of the Western Palaearctic and wintering in sub-Saharan Africa. During the boreal winter it occupies different types of swamp vegetation, but also tall grass and thickets on dry ground (Leisler 1981; Cramp 1992). In both Europe and Africa, its diet comprises invertebrates, with a predominance of mobile insects (Cramp 1992). Ringing recoveries have shown that there is a migratory divide in Central Europe: most European populations migrate to their winter quarters in western Africa via the Iberian Peninsula and Morocco, while birds from eastern Austria and Hungary head initially southeast (SE) entering Africa through Egypt (Zink 1973; Schlenker 1988; Cramp 1992).
Our objectives were twofold. First, using all relevant recoveries, we attempted to link the breeding and non-breeding grounds of the European Reed Warbler populations. Second, we compared stable carbon (C) and nitrogen (N) isotope ratios in feathers moulted in Africa sampled at 17 breeding sites across the migratory divide. We expected that if wintering grounds of southwestern (SW)- and southeastern (SE)-migrating populations differed geographically and were isotopically distinct, this would be reflected in the stable isotopic values in feathers grown in Africa (e.g. Chamberlain et al. 2000; Bensch et al. 2006). We should emphasise that no detailed baseline information on δ13C and δ15N values is available for most parts of Africa, but based on the current knowledge one would expect that SE-migrating birds use drier biomes (Chamberlain et al. 2000; Bensch et al. 2006). Secondly, we wanted to test the potential of stable C and N isotope measurements to infer wintering sites of three populations with an unknown migratory direction.
Methods
Ringing recoveries
Recoveries of ringed birds were obtained from the EURING data bank; for countries underrepresented in this data set, we also requested information from individual ringing schemes. Loxodromic (rhumb-line) distance and direction of recoveries were calculated according to Imboden and Imboden (1972). From a total of 11,342 long-distance (>100 km) recoveries spanning the period between 1931 and 2006, we retrieved 242 encounters from sub-Saharan Africa. In further analyses, we used only long-distance recoveries that involved the breeding population (n = 91). Based on the timing of the migration of the species (Cramp 1992), the breeding season was confined to the period between 1 June and 31 July. Postbreeding migration of Reed Warblers may, however, commence earlier. Data after 15 July may thus include some transient migrants, but the majority of birds start their journey in August (Cramp 1992). Because Reed Warblers overwinter north of the Sahara only exceptionally (Cramp 1992), we used all long-distance recoveries from sub-Saharan Africa (below 20°N) for the delineation of wintering grounds. For a more strict demarcation of the wintering sites, we considered recoveries from December to February, but for most analyses we used all sub-Saharan records on the breeding populations because of the paucity of recoveries from true winter months.
The migratory divide in Central Europe (Central Europe is defined here as 7°–22°E and 45°–55°N) determines a west–east migratory pattern in the study species. For convenience, we refer to SW- and SE-migrating Reed Warblers throughout the paper. This denotes the two general migratory flyways through the western and eastern Mediterranean, respectively, that Reed Warblers use when heading from the European continent to Africa (Cramp 1992; Fig. 1). We are aware that the migratory direction differs among populations according to the relative position of the breeding and wintering grounds and that the initial heading may change after entering Africa—for example, from the southeast to the south–southwest (Zink 1977; Helbig et al. 1989).
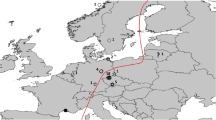
Feather sampling sites. Blue arrows schematically indicate southwestern (SW)-migrating populations of the Reed Warbler Acrocephalus scirpaceus, red arrows southeastern (SE)-migrating populations, question marks migratory direction unknown (see Methods). Site abbreviations: CZ1 Litomyšl, CZ2 Lužice, Czech Republic; DE Lankersee, Germany; ES Barrañán, Spain; FR Etang du Trunvel, France; HR1 Vransko Lake, HR2 Draganić fishponds, HR3 Neretva delta, Croatia; HU1 Fenékpuszta, HU2 Kolon Lake, HU3 Hortobágy, Hungary; IT Lagnone, Italy; MT Simar, Malta; NL Almere, The Netherlands; RU Petergoff, Russia; SK Trnava fishponds, Slovakia; UK Llangorse Lake, UK
To explore the migratory connectivity across the migratory divide we employed a cluster analysis (complete linkage, Euclidean distances) using breeding longitude and wintering longitude for each of 91 sub-Saharan recoveries of breeding birds (Fig. 2b). Note that some birds were ringed in sub-Saharan Africa and recovered later breeding in Europe; in such cases, wintering longitude refers to the place of ringing and breeding longitude to the place of recovery.
a All sub-Saharan recoveries (n = 242) of the Reed Warbler. Dots True winter records (December–February, n = 33). b Recoveries of birds with known breeding origin (June–July, n = 91). Dots Ringing and recovery places. c Recoveries of birds with known breeding origin reported from December–February in sub-Saharan Africa (n = 15). Dots Ringing and recovery places. Lines connect ringing and recovery places and do not reflect the real migratory route
Stable isotopes
During breeding seasons 2004 and 2005, we plucked the left innermost primaries of 165 local adult Reed Warblers at 17 sites across Europe. The feathers were grown in Africa during the previous winter (Jenni and Winkler 1994). The sampling sites were grouped into SW-migrating and SE-migrating populations, respectively, based on the two distinct initial migratory directions. For three sites, however, there were no recovery data for the breeding population. We therefore treated these as populations with an unknown migratory direction (question marks, Fig. 1).
The feathers were stored dry in plastic bags until analysis. Upon analysis, they were first rinsed in a 2:1 chloroform:methanol solution and air-dried in a fume hood. Subsamples of approximately 1 mg were weighed into small tin cups and then analysed on a Europa 20:20 continuous-flow isotope-ratio mass spectrometer (CFIRMS) interfaced with a RoboPrep elemental analyser. Measurements are reported in conventional δ-notation relative to the PDB (carbon) and atmospheric N2 standards in parts per thousand (‰). Replicate assays of internal laboratory standards (albumen) included in each run indicated measurement errors (SD) of ±0.1 and ±0.3‰ for δ13C and δ15N values, respectively. All samples were run at the Department of Soil Science, University of Saskatchewan in Saskatoon, Saskatchewan, Canada.
As stable isotope values were not normally distributed, we applied non-parametric statistics. All tests were computed in STATISTICA ver. 6.0 (StatSoft, Tulsa, OK 2001).
Results
Ringing recoveries
A cluster analysis revealed two main groups of sub-Saharan recoveries (Fig. 3): one cluster represented four birds from SE-migrating populations (Serbia, Hungary, eastern Austria and SE Czech Republic) that were recovered within a relatively restricted area around the Lake Chad basin, the other cluster consisted of 87 West African recoveries of SW-migrating Reed Warblers breeding from Iberia and Britain east to Lithuania and north to Sweden (Figs. 2b, 3). The same result was obtained from the analysis of all 242 sub-Saharan recoveries (Fig. 2a) (data not shown).
Result of a cluster analysis (complete linkage) based on breeding and wintering longitude of 91 Reed Warbler sub-Saharan recoveries of birds ringed or recovered in Europe during June–July. Abbreviations (EURING codes) denote European countries of breeding—AU Austria, BL Belgium, CZ Czech Republic, DE Germany, ES Spain, FR France, GB United Kingdom, HG Hungary, LI Lithuania, NL The Netherlands, PL Poland, PO Portugal, RU Russia, SV Sweden, YU Serbia—and sub-Saharan countries of occurrence—EM Mali, FT Chad, GH Ghana, GQ Guinea Bissau, GY Guinea, NM Mauritania, NU Senegal, NV Nigeria, PQ Liberia, PY Ivory Coast, QQ Cameroon, VU Burkina Faso, XH Togo
The cluster of SW-migrating birds showed further structuring: one group comprised birds breeding in Eastern and Central Europe, while the other group consisted of birds from Western and Central Europe. Within the latter group, there was a pronounced cluster of British and Iberian populations recovered in westernmost Africa (Senegal, Guinea-Bissau and Mauritania; Figs. 2b, 3). An arbitrary subdivision of the recoveries into two parts (west of 12°W, 12°W–10°E) further confirmed the high migratory connectivity of the SW-migrating populations. Of 63 British and Iberian birds, 60 were found west of 12°W, whereas 20 of 24 other European breeders using the SW route were reported east of 12°W, revealing a highly significant non-random distribution of SW-migrating birds in the non-breeding grounds (χ 2 = 55.17, P < 0.0001). Moreover, the longitude of sub-Saharan recoveries positively correlated with breeding longitude (breeding data: r = 0.623, n = 91, P < 0.001; all sub-Saharan data: r = 0.690, n = 242, P < 0.001), suggesting that the W–E distribution of Reed Warbler populations found on the breeding grounds is also generally maintained in the winter quarters.
Only 15 recoveries enabled a link to be established between breeding birds with true winter records in sub-Saharan Africa (Fig. 2c). Seven Iberian birds wintering in Senegal were leapfrogged by three British Reed Warblers staying in Guinea Bissau, while five birds from the rest of the European continent spent the winter south of 10°N from Liberia to Nigeria.
Stable isotopes
There was a large variation in both stable C and N isotope values that considerably overlapped among sampling sites (Table 1). Values of δ13C did not differ significantly among the sites (Kruskal–Wallis H 16,165 = 9.99, P = 0.867), but δ15N values did differ among sites (Kruskal–Wallis H 16,165 = 28.66, P = 0.026).
To explore whether the SW-migrating population used isotopically different wintering areas than SE-migrating birds, we divided the sites into three groups based on the initial migratory directions of the studied Reed Warbler populations [SW, SE and unknown (U); see Methods and Fig. 1)]. Again, there were no differences in δ13C values (Kruskal–Wallis H 2,165 = 0.62, P = 0.733), but the feathers again differed in their δ15N values among the three groups (Kruskal–Wallis H 2,165 = 13.04, P = 0.0015). Feathers of SE-migrating birds were significantly enriched in 15N compared to those of SW-migrating birds (Mann–Whitney z 54,83 = 2.36, P = 0.018; Fig. 4).
Feathers of SW-migrating Reed Warblers differed from those of birds with an unknown migratory direction (Mann–Whitney z 83,28 = −3.29, P = 0.001), whereas the differences between SE-migrating Reed Warblers originating from breeding populations with an unknown migratory direction were not significant (Mann–Whitney z 54,28 = −1.50, P = 0.133). The same result was obtained when the three westernmost populations (which are expected to winter in westernmost Africa according to the results of ringing recoveries) were excluded from the analysis: the remaining SW-migrating Reed Warbler populations differed significantly from populations with an unknown migratory direction (Mann–Whitney z 54,28 = −3.55, P = 0.0004).
Discussion
Ringing recoveries
The comprehensive survey of African non-breeding records of Eurasian Reed Warblers by Dowsett-Lemaire and Dowsett (1987) outlined the main migration routes and the African winter range of the species. In this study we went one step further and identified two wintering areas which correspond well with the two main migratory routes taken by the SW- and SE-migrating European populations. Our analysis of all available recovery data suggests that the two areas lie close to each other, and although there have been no indications for admixture (Fig. 2a), we presume some overlap, for example, in Nigeria, because of the paucity of recoveries and the expected continuous distribution in West Africa. Both the cluster analysis and the correlation of breeding and wintering longitudes indicated a parallel migration pattern when the west–east distribution on the breeding grounds is also maintained in the winter quarters (Salomonsen 1955). In addition, we found further sub-structuring within the cluster of SW-migrating birds. Recoveries of birds from westernmost Europe (British and Iberian breeders) clustered together, clearly converging on the western coast of Africa, whereas the rest of the SW-migrating populations wintered further east. This distribution suggests a relatively high migratory connectivity of the species.
The high reporting rate from Senegal relative to the rest of West Africa results from intensive netting by French and British ringers (Redfern and Alker 2002). It should be noted that most of the British birds were captured in Senegal outside the winter months, which may indicate a connectivity between breeding and stopover sites rather than to the final stationary winter grounds (all birds of known breeding origin ringed during December–February in the Djoudj National Park were later recovered during breeding in Iberia; British Reed Warblers winter more southerly; Fig. 2c). The presence of the westernmost populations in Senegal is furthermore supported by biometry data. Short-winged individuals, typical of Iberian and British breeders (Cramp 1992), predominate in Senegal both on passage and during the winter (Morel 1987; Cramp 1992).
Southeastern-migrating populations initially head on a broad front for the southeast, crossing the eastern Mediterranean (Schlenker 1988). After entering Africa via northern Egypt and northeastern Libya, the migratory direction changes to the south (based on recoveries in Egypt interior and the Sudan; Schlenker 1988) and then probably turns southwest, as indicated by recoveries in the Lake Chad basin (Schlenker 1988; this study) and by the absence of the nominate subspecies in East Africa (Pearson 1982; Dowsett-Lemaire and Dowsett 1987).
The disparity in the initial migratory directions of Central European breeders (SW vs. SE, with almost no recoveries in a southern direction) is quite remarkable and strongly supports the existence of a migratory divide (Schlenker 1988). However, there is some evidence that Reed Warblers of unknown provenance do cross the central Mediterranean region. A few non-breeding recoveries between Italy and Malta (R. Galea, personal communication) and several autumn records of transient birds in central Libya (Toschi 1969) indicate that some Reed Warblers (of central Mediterranean origin?) take a central route, as suggested by Dowsett-Lemaire and Dowsett (1987). A detailed discussion of this aspect, however, is beyond the scope of this paper and will be treated in a future study. To shed more light on the migration pattern of the species, it would be desirable to assess the relative proportion of Reed Warblers passing through the eastern and central Mediterranean areas and discover the breeding origin of the birds using intense targeted bird ringing, orientation experiments and measurements of stable hydrogen isotopes in feathers of transient first-year birds (which would provide information on breeding latitude in Europe; Hobson et al. 2004).
Stable isotopes
Nitrogen
Our study showed that SW- and SE-migrating Reed Warbler populations differ in feather δ15N values. This result is in agreement with the analysis of ringing recoveries, which demonstrated that European Reed Warbler populations spend the boreal winter in two different areas. Stable N measurements from Reed Warbler feathers sampled in Portugal (9.4‰, n = 8; Neto et al. 2006) and the Kaliningrad region, Russia (9.9‰, n = 15; E. Yohannes and V. Kosarev, unpublished), both supposedly moulted in western Africa, fall well within the range of values reported for the SW-migrating populations we sampled (Table 1). Moreover, feather δ15N values helped to assign the two Croatian and the Maltese populations with an unknown migratory direction to the SE-migrating populations.
Higher δ15N values in SE-migrating populations indicate that these birds moulted their feathers in drier sites, since several African studies have shown that higher δ15N values of plant and animal tissues reflect areas with relatively low rainfall (Heaton et al. 1986; Heaton 1987; Sealy et al. 1987; Ambrose 1991; Van der Merwe et al. 1990). Similar to our results, Chamberlain et al. (2000) and Bensch et al. (2006) showed that feathers of SE-migrating subspecies of the Willow Warbler Phylloscopus trochilus, a species with a migratory divide in central Scandinavia, had higher δ15N values than SW-migrating birds of the same species. Feathers of Aquatic Warblers A. paludicola (Pain et al. 2004) and Barn Swallows Hirundo rustica (Evans et al. 2003), however, did not differ in δ15N values among their studied European breeding sites. An alternative explanation for the differences between SW- and SE-migrating Reed Warblers may be that they rely on foods of different trophic levels in their non-breeding grounds. The species is known to feed almost exclusively on invertebrate prey during the winter (Cramp 1992); unfortunately, no detailed studies from the relevant regions of sub-Saharan Africa are available to examine possible differences in food composition.
Detailed regional feather isotopic base maps are needed for a sound interpretation of δ15N values (Pain et al. 2004). To date, however, very little information on stable N patterns in animal tissues across Africa is available. Van der Merwe et al. (1990) found lower δ15N values in elephant ivory in western Africa than in that from eastern or southern Africa, which is in accordance with our results. Bensch et al. (2006) reported higher δ15N values in Willow Warbler feathers sampled in southern Africa, although feathers from western and Central Africa did not differ significantly. To infer a more precise location of the winter quarters from the δ15N measurements alone is, however, very difficult, and the use of additional isotope tracers (such as deuterium or strontium) and/or trace elements could potentially provide much stronger evidence for differences in sites or habitats used.
Carbon
An abundance of stable C isotopes in nature depends on the prevailing type of photosynthesis (O’Leary 1981), and it is relatively easy to trace isotopically whether the consumer depends on a C3- or C4-based foodweb (Alisauskas et al. 1998; Féret et al. 2003). In addition, plants in arid regions generally show enrichment in 13C (Farquhar and Richards 1984); therefore, as with N, stable C isotopes also have the potential to discern xeric and mesic environments.
δ13C values in Reed Warbler feathers moulted in Africa indicated a biome dominated by C4 plants (mean −13‰; Smith and Epstein 1971) and differed significantly from those in feathers of first-year birds grown at known breeding sites (Mann–Whitney U test, z 165,90 = 12.50, P < 0.001; Fig. 5). The values corresponded with the main local vegetation types in the breeding and wintering grounds and were similar to the results of Neto et al. (2006). Lower δ13C values from Europe (mean −24.3‰) indicated a C3-dominated biome, while values from feathers moulted in Africa (mean −14.6‰) reflected C4-dominated foodwebs. This is in agreement with predominantly grassland habitats occupied by Reed Warblers in Africa where primary winter quarters encompass the wet Guinea savannah zone (Dowsett-Lemaire and Dowsett 1987).
The fact that δ13C values did not differ significantly between SW- and SE-migrating Reed Warblers may have been caused by the zonal distribution of C3 and C4 vegetation (Still et al. 2003) and the relatively narrow west–east belt of Reed Warbler wintering distribution in West and Central Africa (Urban et al. 1997). In other words, the non-breeding grounds of SW- and SE-migrating populations most probably lie in the same isoscape band, with predominant C4 vegetation around 10°N (Still et al. 2003) and, therefore, show similar δ13C values. Stable C isotope measurements of feathers have proven to be useful in several avian migration studies in Africa (Chamberlain et al. 2000; Evans et al. 2003; Yohannes et al. 2005, 2007). But again, rather than delimiting the wintering area, δ13C helped to deduce whether the birds moulted their feathers in a relatively C3 or C4 (xeric or mesic, respectively) biomes.
Our analyses additionally revealed a remarkably high variance in both feather δ13C and δ15N values within populations. This variance indicates that individual Reed Warblers from the same population may use very different habitats during the winter. This may well be so, since Reed Warblers have been reported from both wet and dry sites in sub-Saharan Africa (reviewed by Leisler 1981). With respect to the substantial variation in individual δ13C and δ15N values, it would be interesting to explore whether wintering in dry habitats has fitness consequences for the Reed Warbler, as has been shown for the American Redstart Setophaga ruticilla (Norris et al. 2004; Studds and Marra 2005).
Conclusion
The results of this study show that although ringing recovery data necessarily contain an inherent bias, they undoubtedly represent a very useful and spatially accurate source of information that can be used when studying migratory connectivity. The results of our stable isotope analyses are in agreement with the pattern found in recoveries and clearly demonstrate that much work still has to be done before we can identify wintering grounds more precisely. One of the aims of future studies in Africa should be to compile detailed stable isotope base maps for different elements, such as those available in North America (Lott and Smith 2006). Researchers using stable isotopes to infer the origin of migratory birds should exploit all additional sources of information, such as ringing recoveries (Remisiewicz 2002) and remotely sensed data (Szép and Møller 2006), and combine isotope tracers with other markers, such as molecular markers (Clegg et al. 2003; Kelly et al. 2005; Boulet et al. 2006) and/or trace element profiles (Szép et al. 2003; Donovan et al. 2006).
Zusammenfassung
Gleich und Gleich gesellt sich gern auch im Winter: Konnektivität zwischen Brut- und Überwinterungsgebiet beim Teichrohrsänger Acrocephalus scirpaceus
Um die Konnektivität im Überwinterungsgebiet beim Teichrohrsänger (Acrocephalus scirpaceus) zu untersuchen, analysierten wir (1) alle zugänglichen Ringfund-Daten südlich der Sahara und (2) stabile Isotopen im Winterquartier in Afrika gewachsenen Federn bei Vögeln von 17 verschiedenen europäischen Brutgebieten über eine Zugroutenscheide hinweg. Eine Clusteranalyse der Ringfunde zeigte eine bemerkenswerte Konnektivität zwischen Brut- und Überwinterungsgebiet. Zwei Cluster repräsentierten die Populationen, die jeweils eine der zwei Hauptzugrouten nehmen (Südwest bzw. Südost). Darüber hinaus bestätigten die Isotopenanalysen die Trennung der Überwinterungsgebiete der südwest- und der südostziehenden Populationen. Höhere δ15N-Werte in den Federn südöstlich ziehender Vögel weisen darauf hin, dass sie in trockeneren Biomen überwintern. Die Werte für δ13C unterschieden sich nicht signifikant zwischen den Populationen und waren höher als derjenigen Federn, die im Brutgebiet gebildet wurden, was auf ein C4-Biom deutete. Drei Populationen mit unbekannter Zugrichtung konnten mit Hilfe der δ15N-Werte der südöstlichen Zugroute zugeordnet werden.
References
Alisauskas RT, Klaas EE, Hobson KA, Ankney CD (1998) Stable-carbon isotopes support use of adventitious color to discern winter origins of lesser snow geese. J Field Ornithol 69:262–268
Ambrose SH (1991) Effects of diet, climate and physiology on nitrogen isotope abundance in terrestrial food webs. J Arch Sci 18:293–317
Bairlein F (2003) The study of bird migrations—some future perspectives. Bird Study 50:243–253
Bensch S, Bengtsson G, Åkesson S (2006) Patterns of stable isotope signatures in willow warbler feathers collected in Africa. J Avian Biol 37:323–330
Boulet M, Gibbs HL, Hobson KA (2006) Integrated analysis of genetic, stable isotope, and banding data reveal migratory connectivity and flyways in the northern yellow warbler (Dendroica petechia; aestiva group). Ornithol Monogr 61:29–78
Chamberlain CP, Bensch S, Feng X, Åkesson S, Andersson T (2000) Stable isotopes examined across a migratory divide in Scandinavian willow warblers (Phylloscopus trochilus trochilus and Phylloscopus trochilus acredula) reflect their African winter quarters. Proc R Soc B 267:43–48
Clegg SM, Kelly JF, Kimura M, Smith TB (2003) Combining genetic markers and stable isotopes to reveal population connectivity and migration patterns in a Neotropical migrant, Wilson’s warbler (Wilsonia pusilla). Mol Ecol 12:819–830
Cramp JS (1992) The birds of the Western Palearctic. In: Warblers, vol 6. Oxford University Press, Oxford
Donovan T, Buzas J, Jones P, Gibbs HL (2006) Tracking dispersal in birds: assessing the potential of elemental markers. Auk 123:500–511
Dowsett-Lemaire F, Dowsett RJ (1987) European reed and marsh warblers in Africa: migration patterns, moult and habitat. Ostrich 58:65–85
Evans KL, Waldron S, Bradbury RB (2003) Segregation in the African wintering ranges of English and Swiss swallow Hirundo rustica populations: a stable isotope study. Bird Study 50:294–299
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Féret M, Gauthier G, Béchet A, Giroux J-F, Hobson KA (2003) Impact of a spring hunt on nutrient storage by greater snow geese in southern Québec. J Wildl Manage 67:796–807
Gunnarsson TG, Gill JA, Newton J, Sutherland WJ (2005) Seasonal matching of habitat quality and fitness in a migratory bird. Proc R Soc B 272:2319–2323
Heaton THE (1987) The 15N/14N ratios of plants in South Africa and Namibia: relationship to climate and coastal/saline environments. Oecologia 74:236–246
Heaton THE, Vogel JC, von la Chevallerie G, Gollet G (1986) Climatic influence on the isotopic composition of bone nitrogen. Nature 322:822–823
Helbig AJ, Berthold P, Wiltschko W (1989) Migratory orientation of blackcaps (Sylvia atricapilla): population-specific shifts of direction during the autumn. Ethology 82:307–315
Hobson KA (2005) Using stable isotopes to trace long-distance dispersal in birds and other taxa. Divers Distrib 11:157–164
Hobson KA, Bowen GJ, Wassenaar LI, Ferrand Y, Lormee H (2004) Using stable hydrogen and oxygen isotope measurements of feathers to infer geographical origins of migrating European birds. Oecologia 141:477–488
Imboden C, Imboden D (1972) Formel für Orthodrome und Loxodrome bei der Berechnung von Richtung und Distanz zwischen Beringungs- und Wiederfundort. Vogelwarte 26:336–346
Jenni L, Winkler R (1994) Moult and ageing of European passerines. Academic Press, London
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Kelly JF, Ruegg KC, Smith TB (2005) Combining isotopic and genetic markers to identify breeding origins of migrant birds. Ecol Appl 15:1487–1494
Leisler B (1981) Die ökologische Einnischung der mitteleuropäischen Rohrsänger (Acrocephalus, Sylviinae). I. Habitattrennung. Vogelwarte 31:45–74
Lott CA, Smith JP (2006) A geographic-information-system approach to estimating the origin of migratory raptors in North America using stable hydrogen isotope ratios in feathers. Auk 123:822–835
Lozano GA, Perreault S, Lemon RE (1996) Age, arrival date and reproductive success of male American redstarts Setophaga ruticilla. J Avian Biol 27:164–170
Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282:1884–1886
Morel M-Y (1987) Acrocephalus scirpaceus et Acrocephalus baeticatus dans la région de Richard-Toll (Sénégal). Malimbus 9:47–55
Neto JM, Newton J, Gosler AG, Perrins CM (2006) Using stable isotope analysis to determine the winter moult extent in migratory birds: the complex moult of Savi’s warblers Locustella luscinioides. J Avian Biol 37:117–124
Newton I (2004) Population limitation in migrants. Ibis 146:197–226
Newton I (2006) Can conditions experienced during migration limit the population levels of birds? J Ornithol 147:146–166
Norris DR (2005) Carry-over effects and habitat quality in migratory populations. Oikos 109:178–186
Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliff LM (2004) Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc R Soc B 271:59–64
O’Leary MH (1981) Carbon isotope fractionation in plants. Phytochemistry 20:553–567
Pain DJ, Green RE, Giessing B, Kozulin A, Poluda A, Ottosson U, Flade M, Hilton GM (2004) Using stable isotopes to investigate migratory connectivity of the globally threatened aquatic warbler Acrocephalus paludicola. Oecologia 138:168–174
Peach WJ, Baillie SR, Underhill L (1991) Survival of British sedge warblers (Acrocephalus schoenobaenus) in relation to west African rainfall. Ibis 133:300–305
Pearson DJ (1982) The migration and wintering of Palaearctic Acrocephalus warblers in Kenya and Uganda. Scopus 6:49–59
Redfern CPF, Alker P (2002) Eurasian Reed Warbler (Acrocephalus scirpaceus). In: Wernham CV, Toms MP, Marchant JH, Clark JA, Siriwardena GM, Baillie SR (eds) The migration atlas: movements of the birds of Britain and Ireland. T & AD Poyser, London, pp 548–551
Remisiewicz M (2002) The spatio-temporal pattern to robin Erithacus rubecula migration—evidence from ringing recoveries. Ardea 90(special issue):489–502
Saino N, Szép T, Romano M, Rubolini D, Spina F, Møller AP (2004) Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol Lett 7:21–25
Salomonsen F (1955) The evolutionary significance of bird migration. Det Kgl Danske Vidensk Selsk Biologiske Medd 22:1–62
Schaub M, Kania W, Koppen U (2005) Variation of primary production during winter induces synchrony in survival rates in migratory white storks Ciconia ciconia. J Anim Ecol 74:656–666
Schlenker R (1988) Zum Zug der Neusiedlersee (Österreich)-Population des Teichrohrsängers (Acrocephalus scirpaceus) nach Ringfunden. Vogelwarte 34:337–343
Sealy JC, Van der Merwe NJ, Lee-Thorp JA, Lanhamm JL (1987) Nitrogen isotopic ecology in southern Africa: implications for environmental and dietary tracing. Geochim Cosmochim Acta 51:2707–2717
Smith BN, Epstein S (1971) Two categories of 13C/12C ratios for higher plants. Plant Physiol 47:380–384
Smith RJ, Moore FR (2005) Arrival timing and seasonal reproductive performance in a long-distance migratory landbird. Behav Ecol Sociobiol 57:231–239
StatSoft Inc. (2001) STATISTICA (data analysis software system), version 6. http://www.statsoft.com
Still CJ, Berry JA, Collatz GJ, DeFries RS (2003) Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochem Cycles 17:1006–1029
Studds CE, Marra PP (2005) Nonbreeding habitat occupancy and population processes: an upgrade experiment with a migratory bird. Ecology 86:2380–2385
Szép T (1995) Relationship between West African rainfall and the survival of central European bank swallows Riparia riparia. Ibis 137:162–168
Szép T, Møller AP (2006) Searching for potential wintering and migration areas of a Danish barn swallow population in South Africa by correlating NDVI with survival estimates. J Ornithol 147:245–253
Szép T, Møller AP, Vallner J, Kovács B, Norman D (2003) Use of trace elements in feathers of sand martin Riparia riparia for identifying moulting areas. J Avian Biol 34:307–320
Toschi A (1969) Introduzione alla ornitologia della Libia. Suppl Ric Zool Appl Caccia Bologna 6:1–381
Urban EK, Fry CH, Keith S (eds) (1997) The birds of Africa, vol 5. Academic Press, London
Van der Merwe NJ, Lee-Thorp JA, Thackeray JF, Hall-Martin A, Kruger FJ, Coetzee H, Bell RH, Lindeque M (1990) Source-area determination of elephant ivory by isotopic analysis. Nature 346:744–746
Webster MS, Marra PP (2005) The importance of understanding migratory connectivity and seasonal interactions. In: Greenberg R, Marra PP (eds) Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press, Baltimore, pp 199–209
Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83
Yohannes E, Hobson KA, Pearson DJ, Wassenaar LI (2005) Stable isotope analyses of feathers help identify autumn stopover sites of three long-distance migrants in northeastern Africa. J Avian Biol 36:235–241
Yohannes E, Hobson KA, Pearson DJ (2007) Feather stable-isotope profiles reveal stopover habitat selection and site fidelity in nine migratory species moving through sub-Saharan Africa. J Avian Biol 38:347–355
Zink G (1973) Der Zug europäischer Singvögel. Ein Atlas der Wiederfunde beringter Vögel. Lieferung 1. Vogelzug-Verlag, Möggingen
Zink G (1977) Richtungsänderungen auf dem Zuge bei europäischen Singvögeln. Vogelwarte 27:44–54
Acknowledgments
Thanks are due to an untold army of ringers in reed beds who have sampled a respectable number of recoveries over several decades. We are grateful to the EURING schemes which made their recoveries available and to Chris du Feu who sent us the data. Julia Bojarinova, Jaroslav Cepák, Alan Crabtree and Ray Galea kindly provided additional recoveries. Javier R. Álvarez (Grupo Phylloscopus), Bruno Bargain, Stefan Bräger, Froukje and Kees Breek, Charles Coleiro, Vladimir Fedorov, Ray Galea, Jiří Reif, Sergio Scebba (Gruppo Inanellamento Limicoli), Rob J. Thomas and Alfréd Trnka selflessly sampled feathers at their sites, Luka Jurinović helped with netting in Croatia. Eliza Yohannes and Vladislav Kosarev provided unpublished feather stable isotope measurements of birds from Rybachy. Mano Benjamin and Blanca Mora Alvarez assisted with the preparation of samples for stable isotope analyses. Jan Zárybnický and several other people made various contributions. Suggestions of Milica Požgayová and anonymous referees helped to improve earlier versions of the manuscript. Sampling adhered to the legal and ethical requirements in all the countries involved. PP was supported by GA AV CR (grant no. KJB600930508), ESF BIRD Programme and Croatian-Czech inter-academy exchange, and KAH was supported by an operating grant from Environment Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Procházka, P., Hobson, K.A., Karcza, Z. et al. Birds of a feather winter together: migratory connectivity in the Reed Warbler Acrocephalus scirpaceus . J Ornithol 149, 141–150 (2008). https://doi.org/10.1007/s10336-007-0250-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-007-0250-1