Abstract
Tawny owl reproduction and offspring sex ratios have been considered to depend on the abundance of small voles. We studied reproductive performance (laying date, clutch and brood size) during 1995–2003 and offspring sex ratios from 1999 to 2003 in relation to the abundance of small voles and food delivered to the nest in a tawny owl population in southern Finland. Abundance of small voles (field and bank voles) was based on trappings in the field, and estimates of food delivery was based on diet analysis of food remains in the nest boxes. In this population, reproductive output was not related to the abundance of small voles. Analysis of food delivered to the nest showed that the prey weight per offspring varied more than twofold between years and revealed that this difference was mainly related to the proportion of water voles in the diet. Only the number of water voles correlated with laying dates. Offspring sex ratios were weakly male biased (55%) but did not differ from parity. Sex ratios were not related to the abundance of small voles, and we found no evidence that parents delivered more food to nests with proportionally more offspring of the larger (female) sex. Our results underline the notion that populations may differ in their sex allocation pattern, and suggest such differences may be due to diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food availability and reproductive output are tightly linked, both ecologically and evolutionarily (Martin 1987). In many birds of prey, variations in vole abundance have been found to affect breeding success, e.g. American kestrel Falco sparverius, Wiebe and Bortolotti (1992); Eurasian kestrel Falco tinnunculus, Korpimäki et al. (2000); Ural owl Strix uralensis, Brommer et al. (2003); Tengmalm’s owl Aegolius funereus, Hipkiss and Hörnfeldt (2004). For birds of prey in Fennoscandia, small voles (especially field vole Microtus agrestis and bank vole Clethrionomys glareolus) have been viewed as the primary prey during breeding. There has been a regular cycle in vole abundance, varying from a 3-year cycle in southern Fennoscandia to a 5-year cycle in the north and northeast (Sundell et al. 2004), although the amplitude and regularity of the Fennoscandian vole cycle has, in recent years, diminished (Henttonen 2000; Hörnfeldt et al. 2005).
Trivers and Willard (1973) envisaged that parents should alter their sex allocation in response to the future fitness returns of producing sons and daughters. Such fitness returns may vary at the family level as a function of environmental conditions experienced by the parents. Variation in food supply may, therefore, alter the sex-specific value of offspring through its sex-specific effects on nestling survival or future reproductive success. Food supply, indeed, correlates with offspring sex ratios in a variety of species, although its effects clearly vary across species. For example, in years of poor food availability, Eurasian kestrels and American kestrels produce more male fledglings (Wiebe and Bortolotti 1992, Korpimäki et al. 2000), but goshawks and Tengmalm’s owls produce more female fledglings (Byholm et al. 2002; Hipkiss and Hörnfeldt 2004).
In a population of tawny owls (Strix aluco) in the UK, females that hatched in a territory with high vole abundance had higher reproductive output as adults than did females that hatched in a poor territory, whereas males experienced no such effects of natal food conditions (Appleby et al. 1997). Furthermore, tawny owls apparently responded to this fitness difference and produced more daughters than sons in territories with high vole abundance. Tawny owls are reversely size dimorphic, adult females being 15–25% larger than males (Sunde et al. 2003). One possible explanation is that daughters, because of their larger body size and energy requirements, are more affected than sons by a shortage of food provisioning to the nest. Such sex-specific sensitivity to food provisioning has been found in the closely related Ural owl, where the fledging weights of daughters are negatively affected by poor food conditions, whereas the weights of sons remain relatively unchanged (Brommer et al. 2003).
In this paper, we describe reproductive timing, reproductive output and sex ratio in response to food supply in a population of tawny owls breeding near their northern range margin in southern Finland. Using 9 years of data, we describe the relationship between reproductive output and abundance of small voles, which are considered the tawny owl’s main prey (Appleby et al. 1997; Sasvari and Nishiumi 2005). We used prey remains, taken from the nest, to study the importance of different types of prey on the level of the territory. In particular, we explored which group of prey correlated best with laying date. In birds of prey, the seasonal timing of laying is considered an adaptive response to food supply in the territory, and clutch size is thought to be a trait tightly coupled to laying date (Daan et al. 1990). We proceed to link the territory-based estimates of food provisioning also to offspring sex ratios.
Material and methods
Tawny owls were studied in and around the city of Lahti in southern Finland. The area consists of a mosaic of agricultural land (approximately 50%), spruce-dominated forest (40%) and lakes and streams (10%). Nest boxes were placed in mixed forest areas with a relatively high proportion of deciduous trees. Nest sites were visited at least twice during the breeding season. Because tawny owls can be sensitive to disturbance in the early stages of breeding, visits were restricted to a minimum. Nests were visited around the time of hatching and then at the time when owl young were ringed. Eggs and young were counted, and nestlings were weighed and measured. Feather samples for DNA-based sex determination were collected. We determined the timing of hatching by backdating from the nestlings’ wing length, based on daily measurements obtained by Jokinen (1975). We estimated laying dates by calculating 30 days of incubation, in the case of clear size difference between the biggest nestlings, and 32 days if the two oldest nestlings were of a similar size (typically, for clutches larger than two, a female will not start incubation until after the second egg has been laid).
Determination of offspring sex
DNA-based sex determination of tawny owl young was based on feather samples taken during 1999–2003 from all nestlings during the breeding season. The feathers were put into Eppendorf tubes filled with ethanol and frozen for preservation. DNA was extracted in 1999 and partly in 2000 by the Chelex method (Biorad), and the DNA in the rest of the samples was extracted by the salt extraction method.
In birds the female is the heterogametic sex (ZW) and the male is the homogametic sex (ZZ). Introns of the sex-chromosome linked genes CHD1Z and CHD1W were amplified using the protocol of Fridolfsson and Ellegren (1999) using polymerase chain reaction (PCR) with the primers 2178R and 2550F, adjusted for Ural owl sexing (Brommer et al. 2003). Approximately 10 μl PCR amplifications contained 1 μl of DNA extraction, 10 pmol of each primer, 10 μg non-acetylated bovine serum albumin (BSA) (New England Biolabs), 0.1 units of BioTaq DNA polymerase (Bioline), 100 μM of each dNTP in 1.5 mM MgCl2 mix containing PCR buffer according to the fabricant’s specifications. The PCR product was separated on a 2% agarose gel stained with ethidium bromide and visualised in ultraviolet light.
Analysis of prey delivered to the nest
We quantified the amount of food that parents had delivered to the nest by sorting the remains of prey the tawny owl young had consumed. We studied sex-specific food investment by relating the brood sex ratio to the amount of food that parents had brought to the nest boxes. Tawny owl young regurgitate the undigested parts of prey in pellets, which break down and mix with the layer of sawdust (c. 10 cm) in the bottom of the nest box. This material was collected after every breeding season, and sorted. Bones of the prey animals were classified as right or left side of the body and paired so that we did not overestimate the number of prey individuals. Species were determined by the unique morphological features of the bones (Siivonen and Sulkava 2002). The number of individuals was counted from the bones that were most numerous in that species. The number of individuals was multiplied by species-specific average weights or class weights (Juutilainen 1998), and the total amount of food that parents had delivered to the nest was measured. Mammals were determined by species or family level, but birds were divided into small birds, finches, thrushes and large birds. The parts of some of the smallest prey animals are not left, and females may eat some of the prey when the nestlings are young and regurgitate the remains outside the box. This method gives good estimates of the parental prey delivery for the closely related Ural owl (Brommer et al. 2003).
Estimating food supply
We estimated the food supply by snap-trapping voles in autumn (late September to early October) and summer (early June). Because of extensive snow cover, we could not obtain a comparable index of vole abundance during the time of egg formation (January–March), and we therefore used the trapping results from the previous year’s autumn as a best description of the situation in spring (cf. Brommer et al. 2003). Voles were trapped by the small-quadrate method (Myllymäki et al. 1971; Hanski et al. 1994), where traps baited with rye bread were placed in 15 m × 15 m quadrates. Each quadrate corner had three traps 1–2 m apart. Traps were activated for two consecutive nights. The trapped voles were removed after the first night. Altogether, 25 quadrates (i.e. 300 traps) were located in three separate areas approximately 40 km NE of the centre of the study area. Voles are spatially synchronous over large distances (Hanski et al. 1994), and these trappings are therefore representative of the entire study area. Trapping sites contained major vole habitats (spruce-dominated forest, field and clearcut). The vole index was expressed as the number of field voles (Microtus agrestis) and bank voles (Clethrionomys glareolus) caught per 100 trap-nights (50 traps activated for two nights equals 100 trap-nights).
Statistical analyses
We characterised the timing of laying in a given year by its median laying date, because of differences in the distribution of laying dates across years. We used parametric tests whenever data approximated a normal distribution. In order to be able to compare the contributions of different prey classes directly with the variation in laying date, we standardized the data on prey weights per class to zero mean and unit standard deviations. Intervals around means and estimates reported are standard errors unless indicated otherwise.
In analyses of sex ratios, we used a generalised linear model (GLM) with binomial errors (logistic regression), where the significance of explanatory variables was tested by likelihood ratio tests and F statistics (e.g. Crawley 2002). Analyses were carried out with SPSS (SPSS Inc.) and Statistix (Analytical Software). Analyses linking food provisioning to sex ratio considered only nests where no nestling mortality had occurred, because the estimated prey weight delivered per offspring throughout the nestling season would only be accurate for such nests.
Results
Reproductive output
During 1995–2003, tawny owls produced 475 eggs and 401 young in 127 breeding events. On average, a tawny owl pair produced 3.7 ± 0.08 eggs and 3.2 ± 0.13 hatchlings. Average clutch and brood sizes varied between years (clutch size F8,118 = 4.5, P = 0.0001; brood size F8,118 = 3.7, P = 0.0008). The largest clutches and broods were produced in 1997, when there were, on average, 5.0 ± 0.25 eggs and 4.3 ± 0.31 nestlings per pair. In 2003 only 3.3 ± 0.20 eggs and 2.2 ± 0.36 nestlings were produced (Table 1). Of the eggs, 15.6% never hatched or the young died as nestlings. Clutch size and laying date correlated strongly (GLM—year F8,106 = 2.1, P = 0.04; laying date F1,106 = 14.0, P < 0.001), and, hence, we focused on the seasonal timing of laying as a proxy for reproduction.
Timing of laying varied substantially between years (Table 1; F8,107 = 11.7, P < 0.0001). The yearly variation in laying dates was not driven by the abundance of small voles in the field in the previous autumn (b = −0.403 ± 0.345, t = −1.2, df = 8, P = 0.28).
Food delivered to nests and the seasonal timing of laying
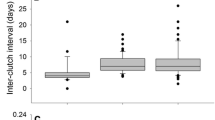
There was much variation in the total mass of food that the tawny owl parents brought to the nests during 1995–2003 (Fig. 1A). During 1995–1997, generally more food was delivered than during 1998–2003. Brood size explained most of the variation in the amount of food brought to the nests [analysis of variance (ANOVA)—year F8,77 = 14.2, P < 0.001; brood size F1,77 = 38.6, P < 0.001; interaction not significant (n.s.), R 2 = 0.72], and there were clear differences in the food mass supplied per nestling across years (Fig. 1B).
a The proportion of different prey animals in the food brought per nest. Prey items are divided into five categories: water voles, small voles (field voles and bank voles), other mammals, birds and frogs. Bars present average weights of categories per nest each year. The total weights differ across years (Kruskal–Wallis, χ2 = 41.4, df = 8, P < 0.001). b Variations in food delivered per nestling (in grammes) in different years (ANOVA F8,79 = 13.0, P < 0.001). Boxes show the median, with upper and lower quartiles (50%) of observations. The lines from the boxes are the highest and lowest quartiles. Sample sizes (for both panels) are reported above the x-axis
Tawny owls clearly had a varied diet, including plenty of prey other than small voles. In terms of prey mass, the proportion of small voles was minor (Fig. 1a). Most of the diet consisted of water voles and birds. In 1995–1997, when nestlings obtained most food, water voles were delivered to the nests substantially more than in later years (Fig. 1a). Birds formed a large proportion of the diet every year. Not surprisingly, the yearly mean mass of food provided by parents did not correlate with small vole abundances (F1,8 = 0.019, r 2 = 0.3%, P = 0.894), although there was a clear tendency for more small voles to be delivered in years with a higher abundance of small voles, as established by the trapping (r s = 0.64, n = 9, P = 0.07). A multivariate analysis that compared the effects of the prey mass of all classes found in the nest boxes showed that only water voles had a significant effect on tawny owl laying dates, while the effects of small voles and other prey classes were insignificant (Table 2).
Sex ratios
During 1999–2003, mortality from egg to fledging was 7.2% (20/278) (Table 3). There were 258 young sexed, including those in nests where deaths of nestlings had occurred. In a sub-sample of nests without nestling mortality, there were 218 young. Overall, for 1999–2003, the sex ratio of tawny owl offspring was male biased [54.7% (141/258) males], although this bias was not statistically significant (chi-square test, χ2 = 2.2, df = 1, P = 0.14). The most pronounced bias was in 2002, when the proportion of males was 63%. Not all years were male biased, with 1999 and 2003 being female biased (Table 3), but this difference across years was not statistically significant (logistic regression: year F4,68 = 2.1, P = 0.09). Yearly sex ratios changed little when nestlings from nests with mortalities were included (Table 3).
There was no relationship between offspring sex ratios and laying date (logistic regression on 73 nests with known sex ratios and laying dates: b = −0.009 ± 0.016, F1,71 = 0.3, P = 0.6). Sex ratios did not change with the amount of food delivered per offspring to the nest (GLM on 51 nests without offspring mortality: year F4,46 = 2.7, P = 0.04; prey weight per offspring F1,45 = 0.04, P = 0.85).
Discussion
The amount of food delivered to the offspring in our tawny owl population in southern Finland varied substantially across years. In the first 3 years of the study, a single offspring received much more food than in later years. These differences were not because the voles were small, but, instead, appeared to be largely due to the number of water voles. Water voles are traditionally thought to be one of the tawny owls’ alternative prey to small voles (Solonen and Karhunen 2002), but it has been mainly birds that have been emphasised as an important alternative prey (Solonen 2005; Sasvari et al 2000). On the basis of food delivered to the nest, we found that only the number of water voles correlated with the seasonal timing of laying. The timing of laying is a reasonable proxy for tawny owl reproduction, as early laying correlates with the production of larger clutches. Despite the fact that small voles are thought to be the species’ main prey, neither the abundance of small voles in the field nor the food delivered to the nests correlated with the seasonal timing of reproduction in this population. Our results imply that water vole abundance is the most important factor for tawny owl reproductive decisions in this population.
The use of prey remains collected from nests to describe the diet of owls has a long history in ornithology. This method has also been used extensively to compare the relative abundance of different groups of animals in the diet. We here distinguish between birds, frogs, small voles, water voles, and ‘other mammals’. The probability of finding bones may differ across these five categories. For example, skeletal parts of voles and frogs are typically well ossified and gather in the prey remains intact, which makes the assigning of unique bones to individuals unambiguous. Bird bones are hollow and are often in the prey remains as fragments, which makes it more difficult for one to count how many individuals were caught and delivered. In addition, the bones of young birds (e.g. nestling thrushes) are not ossified, and their remains cannot be detected reliably. Mostly small prey items will be difficult to detect, making the total mass of prey probably a fairly robust estimate, although a minimal one. Nevertheless, prey analysis based on food remains has been shown to correlate with directly observed prey delivery rate in Tengmalm’s owls (Hakkarainen and Korpimäki 1995). Most importantly, even if the detection probability differs across prey categories; any bias in detecting a particular category of prey is systematic and will thus be random with respect to annual variation and the reproductive parameters, laying date and sex ratio that we have focused on here. Hence, detection bias has not influenced our conclusions.
Water vole populations vary cyclically in their numbers, although the cycles are longer than the population cycles of small voles. Korpimäki et al. (2005) found evidence of 8–10 year water vole cycles in western Finland, and water vole population cycles also occur in Switzerland (Saucy 1988). Such a cycle may have occurred in our study area as well, since water voles were abundant again in more recent years (2005–2006), which are not covered in this paper (personal observation). Nevertheless, quantitative assessment of such a cycle is currently unavailable. Long-eared owls in western Switzerland exhibit a strong numerical response without a time lag to the abundances of a 7-year water vole cycle (Weber et al. 2002). Quantitative assessments of water vole densities are, to our knowledge, not available in Finland, despite the strong tradition of small mammal research (e.g. Sundell et al. 2004 and references therein). Hence, we cannot establish a direct relationship between abundance of water voles and tawny owl laying date in our study population. Nevertheless, it seems intuitive that the dramatic differences across years in the number of water voles that the tawny owls deliver to their nests are, indeed, reflecting changes in the abundance of the water voles themselves. Intriguingly, Finnish water voles are still overwintering underground during the time of tawny owl egg formation (which occurs during the winter months before laying, see Hirons et al. 1984). It therefore remains unclear how water vole abundance in spring can directly affect tawny owl reproduction in our population, but it seems possible that water vole abundance in the preceding autumn and early winter affects the owls’ overwintering conditions and timing of reproduction in the next spring. Our findings suggest that assessing water vole abundance in the field will be an important aspect for understanding tawny owl reproductive decisions.
Food supply and sex ratios
Sex ratio manipulation according to food supply has been observed in several studies of birds of prey. For example, in years of good food availability, Montagu’s harriers produce more daughters (Arroyo 2002), while Tengmalm’s owls produce more sons (Hipkiss and Hörnfeldt 2004). Tawny owl sex ratios in our population, based on molecular sexing over 5 years, show a tendency to have more males (55%) and have not differed significantly across years. This finding is in qualitative accordance with results on studies of the closely related Ural owl, which shows a consistent male offspring bias of 56% (Brommer et al. 2003). A similar result was found in a Danish population of tawny owls, where the offspring sex ratios over 4 years were consistently male biased (Desfor et al. 2006). In contrast, tawny owls in a Hungarian population produced female-biased broods in years when environmental conditions (snow cover) during laying were favourable (Sasvari and Nishiuma 2005).
Appleby et al. (1997) found that tawny owls in the UK produce more daughters in territories with a high abundance of small voles. Although those authors did not provide estimates of food delivery, their findings imply a correlation between sex ratio and food delivered to the nest. Furthermore, tawny owl females are larger than males, and the energy requirements are expected to be higher for female nestlings (cf. Anderson et al. 1993; Riedstra et al. 1998; Vedder et al. 2005). Contrary to this expectation, the sex ratio of the Finnish tawny owl offspring does not correlate with food delivered to the nest. Despite this lack of correlation, energy requirements of male and female nestlings may still differ, but parents do apparently not adjust their feeding rate accordingly. Apparently, feeding investment by parents has little effect on offspring mortality, given that both the per-capita weight of prey delivered and the overall mortality were low during the years in which we studied sex ratios. If anything, sex ratios appeared to be female biased during those years (1999 and 2003) when food supply was poorest (Fig. 1, Table 3). Nevertheless, our results on offspring sex ratios had moderate sample sizes (10–19 broods per year) and stem from years that apparently form a prolonged ‘low’ in the water vole cycle. Hence, more data from years with high water vole densities are needed, so that sex ratios may be studied under good food conditions. Furthermore, we currently lack information on whether food supply in the box has any sex-specific fitness consequences in this population, and we can therefore not assess whether there is any selection pressure on making sex-ratio adjustments in this population.
Our results demonstrate that sex allocation patterns found in one population of tawny owls need not apply to other populations. Differences between populations in sex allocation patterns have been observed previously in birds of prey (e.g. Smallwood and Smallwood 1998; Byholm et al. 2002) and appear to occur also across tawny owl populations (Appleby et al. 1997; Sasvari and Nishiuma 2005; Desfor et al. 2006, this paper). We have further documented a difference in the main prey species (water vole versus small voles) between our Finnish population and the UK population studied by Appleby et al. (1997). Predictability of food supply may play a role for sex allocation. A review of sex allocation studies pointed out that when predictability decreases the selection for sex ratio manipulation diminishes (West and Sheldon 2002). Small vole populations in the tawny owl study area in the UK (Kielder Forest) are a stable food resource that show spatial synchrony in their numbers across all suitable habitat patches (MacKinnon et al. 2001). Water voles are, presumably, a less predictable food supply than small voles are, because water vole populations are highly patchily distributed in the landscape, are frequently absent from suitable habitat and are characterised by frequent population turnovers (Aars et al. 2001; Telfer et al. 2001). Studies of food supply, diet and sex allocation in tawny owls in additional populations may, therefore, contribute to our general understanding of sex allocation patterns.
Zusammenfassung
Reproduktionserfolg und Geschlechterverhältnis der Nachkommen beim Waldkauz unter verschiedenen Nahrungsbedingungen
Man nahm an, dass Reproduktionserfolg und Geschlechterverhältnis der Jungen beim Waldkauz von der Abundanz kleiner Wühlmäuse abhängen. An einer Waldkauzpopulation in Süd-Finnland haben wir von 1995 bis 2003 die Reproduktionsleistung (Legedatum, Gelegegröße und Anzahl Junge) und von 1999 bis 2003 das Geschlechterverhältnis der Jungen in Bezug zur Häufigkeit kleiner Wühlmäuse und der zum Nest gebrachten Nahrung untersucht. Die Abundanzbestimmung kleiner Wühlmäuse (Erd- und Rötelmäuse) stützte sich auf Fänge im Gelände, und Abschätzungen der Menge herangeschaffter Nahrung erfolgten anhand von Nahrungsanalysen der Beutereste in den Nistkästen. In unserer Population gab es keinen Zusammenhang zwischen Reproduktionsleistung und der Abundanz kleiner Wühlmäuse. Bei der Analyse der zum Nest gebrachten Nahrung zeigte sich, dass das Beutegewicht pro Jungem um mehr als das Zweifache zwischen den Jahren schwankte und es wurde deutlich, dass dieser Unterschied hauptsächlich mit dem Anteil von Schermäusen an der Nahrung zusammenhing. Lediglich die Menge an Schermäusen korrelierte mit den Legedaten. Die Geschlechterverhältnisse waren bei den Nachkommen schwach männchenlastig (55%), unterschieden sich statistisch aber nicht von einer Gleichverteilung. Es gab keinen Zusammenhang zwischen Geschlechterverhältnissen und der Abundanz kleiner Wühlmäuse und wir fanden keinerlei Hinweis darauf, dass Eltern mehr Nahrung zu Nestern mit einem höheren Anteil an Nachkommen des größeren Geschlechts (Weibchen) gebracht hätten. Unsere Ergebnisse unterstreichen, dass sich Populationen im Muster ihrer Geschlechter-Allokation unterscheiden könnten und deuten darauf hin, dass solche Unterschiede möglicherweise an der Nahrung liegen.
References
Aars J, Lambin X, Denny R, Griffin AC (2001) Water vole in the Scottish uplands: distribution patterns f disturbed and pristine populations ahead and behind the American mink invasion front. Anim Conserv 4:187–194
Anderson DJ, Reeve J, Martinez-Gomez JE, Whethers WW, Hutson S, Cunnigham HV, Bird DM (1993) Sexual size dimorphism and food requirements of nestling birds. Can J Zool 71:2541–2545
Appleby BM, Petty SJ, Blakey JF, Rainey P, MacDonald DW (1997) Does variation of sex ratio enhance the reproductive success of offspring in tawny owls? Proc R Soc Lond B 264:1111–1116
Arroyo B (2002) Sex-biased nestling mortality in the Montagu’s harrier Circus pygargus. J Avian Biol 33:455–460
Brommer JE, Karell P, Pihlaja T, Painter JN, Primmer CR, Pietiäinen H (2003) Ural owl sex allocation and parental investment under poor food conditions. Oecologia 137:140–147
Byholm P, Ranta E, Kaitala V, Linden H, Saurola P, Wikman M (2002) Resource availability and goshawk offspring sex ratio variation: a large scale ecological phenomenon. J Anim Ecol 71:994–1001
Crawley MJ (2002) An Introduction to data analysis using S-Plus. Wiley, New York
Daan S, Dijkstra C, Tinbergen JM (1990) Family planning in the kestrel (Falco tinnunculus): the ultimate control in covariation of laying date and clutch size. Behaviour 114:83–116
Desfor KB, Boomsma JJ, Sunde P (2007). Tawny owls Strix aluco with reliable food supply produce male-biased broods. Ibis 149:98–105
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Hakkarainen H, Korpimäki E (1995) Contrasting phenotypic correlations in food provisioning of male Tengmalm’s owls (Aegolius funereus) in a temporally heterogeneous environment. Evol Ecol 9:30–37
Hanski I, Henttonen H, Hansson L (1994) Temporal variability and geographical pattern in the population-density of microtine rodents. J Anim Ecol 66:353–367
Henttonen H (2000) Long-term dynamics of the bank vole Clethrionomys glareolus at Pallasjärvi, Northern Finnish taiga. Pol J Ecol 48:87–96
Hipkiss T, Hörnfeldt B (2004) High interannual variation in the hatching sex ratio of Tengmam’s owl broods during a vole cycle. Popul Ecol 46:263–268
Hirons GJM, Hardy AR, Stanley PI (1984) Body weight gonad development and moult in the tawny owl (Strix aluco). J Zool (Lond) 202:145–164
Hörnfeldt B, Hipkiss T, Eklund U (2005) Fading out of vole and predator cycles? Proc R Soc Lond B Biol Sci 272:2045–2049
Jokinen M (1975) Lehtopöllöpoikasten kasvu ja energiakäyttö ja niiden vaikutus pesimätuloksen määrittämiseen. M.Sc. thesis, University of Turku
Juutilainen T (1998) Alueellisten erojen, poikasmäärän ja pesinnän ajankohdan vaikutus viirupöllön poikasajan ravintoon. M.Sc. thesis, University of Helsinki
Korpimäki E, May CA, Parkin DT, Wetton JH, Wiehn J (2000) Environmental- and parental condition-related variation in sex ratios of kestrel broods. J Avian Biol 31:128–134
Korpimäki E, Norrdahl K, Huitu O, Klemola T (2005) Predator-induced synchrony in population oscillations of coexisting small mammal species. Proc R Soc Lond B Biol Sci 272:193–202
Mackinnon JL, Petty SJ, Elston DA, Thomas CJ, Sherratt TN, Lambin X (2001) Scale invariant spatio-temporal patterns of field vole density. J Anim Ecol 70:101–111
Martin TE (1987) Food as a limit on breeding birds—a life history perspective. Ann Rev Ecol Syst 18:453–487
Myllymäki A, Paasikallio A, Pankakoski E, Kanera K (1971) Removal experiments on small quadrats as a means rapid assessment of the abundance of small mammals. Ann Zool Fenn 8:177–185
Riedstra B, Dijkstra C, Daan S (1998) Daily energy expenditure of male and female marsh harrier nestlings. Auk 115:635–641
Sasvari L, Nishiumi I (2005) Environmental conditions affect offspring sex ratio variation and adult survival in tawny owls. Condor 107:321–326
Sasvari L, Hegyi Z, Csörgo T, Hahn I (2000) Age-dependent diet change, parental care and reproductive cost in Tawny owls Strix aluco. Acta Oecol 21:267–275
Saucy F (1988) Dynamique de population, dispersion et organisation sociale de la forme fouisseuse du campagnol terrestre (Arvicola terrestris scherman (Shaw), Mammalia, Rodentia). Ph.D. thesis, University of Neuchâtel, Neuchâtel, Switzerland
Siivonen L, Sulkava S (2002) Pohjolan nisäkkäät. Ottava, Hesinki
Smallwood PD, Smallwood JA (1998) Seasonal shifts in sex ratios of fledgling American kestrels (Falco sparverius paulus): the early bird hypothesis. Evol Ecol 12:839–853
Solonen T (2005) Breeding of the tawny owl Strix aluco in Finland: responses of a southern colonist to the highly variable environment of the north. Ornis Fenn 82:97–106
Solonen T, Karhunen J (2002) Effects of variable feeding conditions on the Tawny owl Strix aluco near the northern limit of its range. Ornis Fenn 79:121–131
Sunde P, Bolstad MS, Moller JD (2003) Reversed sexual dimorphism in Tawny Owls, Strix aluco, correlates with duty division in breeding effort. Oikos 101:265–278
Sundell J, Huitu O, Henttonen H, Kaikusalo A, Korpimäki E, Pietiäinen H, Saurola P, Hanski I (2004) Large-scale spatial dynamics of vole populations in Finland revealed by the breeding success of vole-eating predators. J Anim Ecol 73:167–178
Telfer S, Holt A, Donaldson R, Lambin X (2001) Metapopulation processes and persistence in remnant water vole populations. Oikos 95:31–42
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary sex ratio of offspring. Science 179:90–92
Vedder O, Dekker AL, Visser GH, Dijkstra C (2005) Sex-specific energy requirements in nestlings of an extremely size dimorphic bird, the European sparrowhawk (Accipiter nisus). Behav Ecol Sociobiol 58:429–236
Weber J-M, Aubry S, Ferrari N, Fischer C, Lachat Feller N, Meia J-S, Meyer S (2002) Population changes of different predators during a water vole cycle in central European mountainous habitat. Ecography 25:95–101
West SA, Sheldon BC (2002) Constraints in the evolution of sex ratio adjustment. Science 295:1685–1688
Wiebe KL, Bortolotti GR (1992) Facultative sex ratio manipulation in American kestrels. Behav Ecol Sociobiol 30:379–386
Acknowledgements
We thank those students who have accompanied us during our field work and have helped in sorting the prey remains. The sampling of tawny owl feathers and the snap-trapping of voles were approved by the Ethics Board for Approval of Animal Experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Kekkonen, J., Kolunen, H., Pietiäinen, H. et al. Tawny owl reproduction and offspring sex ratios under variable food conditions. J Ornithol 149, 59–66 (2008). https://doi.org/10.1007/s10336-007-0212-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-007-0212-7