Abstract
The common waxbill Estrilda astrild was first introduced to Portugal, from Africa, in 1964, from where it has spread to much of the country and to Spain. We modelled the expansion of this species on a 20×20-km UTM grid in 4-year periods from 1964 to 1999. Colonisation process on a grid was modelled as a function of several biophysical and spatio-temporal variables through the fitting of several multiple logistic equations. Variation partitioning confirmed the importance of the spatial-temporal component, explaining 33% of the total variation, followed by the combined effects of both environmental and spatial-temporal variables (around 25%). Only 11% of the total variation can be attributed strictly to the considered environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introduced species have become a major cause of concern to human societies. Species that move beyond their traditional natural range can have undesirable ecological and economical consequences (Shogren 2000). Moreover, alien species could be a major problem for biodiversity loss accounting for as much as 20% of recent extinctions (Vitousek et al. 1997). In the last decade, a growing awareness of this problem has stimulated research on the naturalisation and spread of exotic species (e.g., Hengeveld 1989; Smallwood 1994; Simberloff 1997). Environmental consequences of introduced species have also been an important issue of great concern in recent years (Rainbow 1998, Turpie and Heydenrych 2000). Moreover, realisation is growing that introduced species represents a serious economic threat with insidious and pervasive conservation problems (Simberloff 1997).
The common waxbill Estrilda astrild is one of the most successful alien birds in both the Iberian Peninsula and the Mediterranean Basin. The first documented releases occurred in coastal Portugal in the mid 1960s (Xavier 1968) and the species has since spread to most of Continental Portugal, the northwest of Spain (Galicia) and the southwest of Spain (Extremadura and Andalucia communities) (Reino and Silva 1998; Silva et al. 2002).
Several bird introductions and releases have occurred in Portugal in the last decades (Matias and Lobo 1999). Moreover, the problem is becoming of great urgency, mainly due to an almost total absence, or insufficient number, of studies, lack of supervision of the aviculture industry and importations, and insufficient monitoring of areas that are known for holding populations of certain species. Nonetheless, in 2002, the first field guide of exotic breeding birds in Continental Portugal (Matias 2002) was published. This book was written with the main goal of providing a practical reference for fieldwork recording for the forthcoming new atlas of breeding birds in Portugal, describing over 60 species that can breed in Portugal in the wild.
The aim of this study is to model the common waxbill’s expansion at the nationwide level as a function of both spatial-temporal and environmental components. However, most of the data available for this species in Portugal is qualitative, limiting the use of common frameworks in expansion, as, for example, diffusion models. Because of this, we adopted the methodology proposed by Silva et al. (2002) and based it on logistic regression analysis. However, we extended this methodology for variation partitioning, modelling the relative importance of different factors in the biological invasion of the common waxbill in Portugal. The aim of the study is the evaluation of possible underlying factors and aspects responsible for the spread of this introduced species in Portugal. We also intend to discuss the consequences of the results for conservation management of invasive introduced bird species.
Methods
Data source
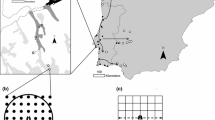
The common waxbill expansion was mapped on a 4-year basis, between 1964 and 1999. This information was plotted on 20×20-km squares using the UTM grid, covering all of Continental Portugal (Fig. 1). Data used in this paper were similar to those analysed by Silva et al. (2002).
The common waxbill’s presence/absence for time t (Pres) together with 11 independent variables were mapped for Continental Portugal (Table 1), nine being environmental variables and two spatial-temporal [number of presences in the four (Nei4) and eight (Nei8) neighbouring UTM squares in the previous time period]. Neighbouring covariates provide both spatial and temporal information. In addition, neighbouring covariates allow the explicit modelling of spatial autocorrelation (Augustin et al. 1996).
The database was constructed only for the Portuguese territory. Information on the waxbill’s occurrences in the Spanish territory was used only for an unbiased calculation of occupied neighbouring squares. Data from Spain was obtained from Reino and Silva (1998).
Sampling design
Our aim was to model the influence of different components on the common waxbill’s spread evolution on a 20×20-km grid basis (250 squares). For modelling purposes we used the same procedure described in Silva et al. (2002): (1) positive samples (presences) comprised records where the species was present at time t but absent at t−1, (2) conversely negative samples (absences) comprised records where the species was not present at time period t, and (3) in the present data set, once the species occupies a square it never regresses.
Thereafter we obtained a data set composed by 58 occurrences and 192 absences. Nevertheless, we decided to use a balanced set, considering an equal number of presences (58) and absences (58), removing from the analysis the remaining absences. Absences were randomly subsampled and to avoid pseudoreplication (Zar 1996) each square was selected only once and for one time period only.
Environmental data
To determine the relative importance of different components of variables on the expansion of the common waxbill in Portugal, a set of environmental characteristics, assumed to be relevant, was considered. Thereafter, a matrix was constructed under the selected covariates, considering the selected grid system for Portugal. All squares were characterized with nine independent environmental variables (six climatic, two hydrographic and one topographic). The climatic variables were extracted from 1:1,000,000 environmental digital maps published by the Portuguese Ministry of the Environment (http://www.iambiente.pt) (Table 1). Similar environmental charts were previously published as “Atlas do Ambiente” (CNA 1983). Both the digital maps and the charts represented annual environmental variables (in classes), illustrated as contours. Altitude was extracted from the US Geological Survey (http://edcwww.cr.usgs.gov/doc/edchome/datasets/edcdata/html) and used to produce a relief map. River density was extracted from IgeoE map 1/25,000 and presence of wetlands from Farinha and Trindade (1994). All data were mapped on 20×20-km squares and, to each class of variable, we attributed a different value that makes the variations occur in unitary steps.
Statistical analysis
Following Borcard et al. (1992) and Legendre and Legendre (1998), we attempted to partition the variation in the data set, based on both environmental and spatial-temporal effects. This aims to disentangle the importance of spatial-temporal and environmental components in the selected logistic models. The methodology developed here is an adaptation from that proposed by Borcard et al. (1992) and Legendre and Legendre (1998) and extended for logistic regression by Reino et al. (2002).
In our study, variation partitioning separates environmental from spatial-temporal information. Following this analysis, the total variation in the expansion model was partitioned into four independent components: (1) a pure environmental component (a), not attributed spatial-temporal component; (2) shared component, i.e., the environmental variation that is spatially and temporally structured (b); (3) a pure spatial-temporal component due to neighbouring effects (c), independently of the environmental variables; and (4) an unexplained variation (d). Therefore total variation was partitioned by the expression Vt=Σ a+b+c+d and using a simple linear system one can extract any single component.
This procedure involves calculating the following statistical models: (1) one partial multiple logistic regression with all environmental variables, obtaining the significant terms obtained by stepwise forward regression (a + b); (2) two partial univariate logistic regressions with the two spatial-temporal covariates developed separately and modelled as univariate analysis (b + c); and (3) two joint spatial-environmental models with all significant variables checking sharing effects between the two components, considering Nei4 (model A) and Nei8 (model B), separately (a+b+c). In Table 2 discriminated variables are entered in each model subset.
For calculating the variation explained by each set of variables and by the shared components we used the Nagelkerke coefficient (Nagelkerke 1991) available in SPSS 12 software. This coefficient of determination is defined as
where the maximum equals:
where l(0) = log L(0) denote the log likelihoods of the fitted and the “null” model, respectively.
In the whole of the logistic regression analysis, statistical significance of variables was tested using log likelihood’s test considering a probability level of 0.05.
The performance of the models was assessed by calculating the receiver operating characteristics curve (AUC) (Pearce and Ferrier 2002) that is not dependent on the threshold.
This approach models the colonisation process of each square at each time increment as a function of spatial, temporal and environmental factors. Our methodology was conceived to analyse the expansion process from the beginning to 1999. Our modelling strategy is not put forward to analyse the present distribution of this species.
Results
As shown in Fig. 1 the common waxbill has colonised most of the country since the first birds were introduced in the 1960s.
Stepwise regression on environmental variables selected Pret, Radi and Alti as the only significant and negative covariates associated with the expansion of this species in Portugal (Table 3). In both models, neighbourhood effects are also significant and positively associated. Decomposition models (considering all significant variables in forward stepwise regression) managed to explain 69.2% (model A) and 68.3% (model B) of the variation of the data set (Fig. 2). The environmental component, a+b, accounted for 35.7% of the total data set variation, but a alone (pure environmental) only explained 10.9% for model A and 11% for model B of the total variation. The b+c component explained 58.4% for model A and 57.3% for model B; while the c component alone (pure spatial-temporal) explained 33.5% (model A) and 32.6% (model B) of the variation. Thus, the joint environmental and spatial-temporal portion of variation was 24.9 and 24.7% for models A and B, respectively (b). Finally, unexplained variation accounts for 30.8% (model A) and 31.7% (model B) of the total amount.
The results suggested a good adjustment of the different models to the data (0.799<AUC<0.931) (Table 4). Moreover, spatial-temporal models (AUC A=0.870 and AUC B=0.888) had a better discrimination and accuracy when compared with the environmental model (AUC=0.799). Joint models corroborate the good adjustment of the models to the data.
Discussion
The common waxbill is known to be a widespread and adaptable estrildid with a natural range throughout sub-Saharan Africa in many mesic habitats (Hall and Moreau 1970). In southern Africa it is known to be a nearly ubiquitous species, avoiding only areas without surface water or rank vegetation (Bernard 1997). This suggests that the common waxbill is a rather eclectic species and this could be one of the major reasons for its success in many places where it has been introduced around the world.
The results of this study show that both environmental and spatial-temporal variables have influenced the expansion wave and success of this passerine in Portugal. Nevertheless, variation partitioning suggested that spatial-temporal effects were the major cause for shaping the expansion process. Variation partitioning shows that only 11% of the total variation can be attributed strictly to the environmental factors, Pret, Radi and Alti (Table 3). Although the environmental component (a) is relatively small, the combined component (b) also includes the spatially structured environmental factors. The combined effects are ca. 25% in both models. This component could also include historical events, such as the locations where the species was introduced and autocorrelation in both environmental and spatial-temporal variables.
On the other hand, variation attributed exclusively to the spatial-temporal variable, independently of environmental covariates, accounted for over 30% in both models. These results show that differences between models A and B are not significant. This may suggest that the expansion process is mainly made by continuous front (see Silva et al. 2002). We should also consider scale effects, i.e. these results should be regarded in the context of this rather coarse scale.
The large value of pure neighbourhood or spatial-temporal effects (c) could have two main causes. First, it might mirror the real importance of the exclusively spatial nature of the biological wave process due to an expansion mainly by continuous front, instead of jump dispersal, where neighbourhood areas are the most likely to be colonised and occupied (see Silva et al. 2002). Second, it might be due to the existence of spatially structured environmental factors not included in this analysis, but which have been represented by the spatial variable, for example due to local environmental factors not included in this analysis (e.g. habitat variables) and most likely accounting for expansion to new areas.
These results suggest that both environmental and spatial-temporal covariates have affected the spread of this species. This could be due to a landscape heterogeneity and composition along a spatial gradient. Reino and Silva (1998) reported that for the Iberian Peninsula the expansion rate for the north front was 13.02 km/year, while for the south front it was 5.84 km/year, and that the expansion process to the east is faster in the south compared to the north, suggesting that colonisation rates are spatially and environmentally dependent. Different rates of expansion suggest an anisotropic nature of the spreading process, indicating that colonisation rate is direction dependent. Different spread rates may be explained by differences in habitat conditions that are not constant across space. In the case of the common waxbill it seems that we have two main spatial-environmental gradients: (1) south-north; (2) west-east. This could be due to the different effects of environmental factors that are not constant across all the territory. For example, the different spread on the two fronts could be due to differences in habitat availability in a north-south gradient. Moreover, characteristics of landscape like altitude and climate also vary in the same gradient, affecting habitat availability. Higher radiation values occurred both inland and in the south, regions also exhibiting gaps in the distribution throughout 1999. Combined higher-altitude and higher-radiation areas characterise the inland regions with a more continental climate (Silva et al. 2002) and with less suitable habitat for this species. This climatic influence featuring colder winters and with unsuitable habitat could be decisive factors in shaping the colonisation process. Due to this spatial heterogeneity of the environmental information in the different directions (e.g. north vs south and coastal areas vs inland areas), the probability that the common waxbill will move from one place to another is spatial-temporally structured. Thus, because the waxbills spread through a mosaic (and in different fronts) at multiple rates they may perceive the landscape structure at different scales.
Concluding remarks
Logistic regression and variation partitioning proves to be useful in the modelling of the common waxbill expansion, in spite of their limited power. The approach developed here is coherent with the results of Silva et al. (2002). However, these results should be regarded with care due to certain limitations, for example: (1) the environmental data considered are static; and (2) most of the environmental data considered could be unsuitable to study this problem, mostly because fine-habitat data are not considered due to unavailability of such data for this study.
Therefore, further research is needed to evaluate environmental and spatial correlates at different scales. For example, the incorporation of habitat descriptors could be more appropriate than climatic data to study this expansion process. Contrariwise, the utilisation of distinct approaches may also be useful. For example, an approach based on the concept of ecological niches as a constraint on the distributional potential of species (Grinnell 1917, 1924), involving a logical extension of the basic niche concept, i.e. that species will be able to establish populations only in areas that match the set of ecological conditions to which they are currently limited on native range areas (Peterson 2003).
This study may also be useful for the implementation of management prescriptions for the assessment of newly introduced species in Iberia before they become naturalised. Moreover, in the future it would be interesting to understand the reasons for the shared component on the distribution of this waxbill. Today, expansion process of this species is quite well known in Iberia. There is no evidence of harmful impacts of this species on native species or on agriculture. However, the common waxbill is very abundant in the habitat types of species with restricted breeding distribution in the area (e.g. Savi’s warbler Locustella lusciniodes and reed bunting Emberiza schoeniclus) and our knowledge about the interaction between the exotic waxbill and indigenous species is very small.
Zusammenfassung
Faktorenanalyse zur Ausbreitung des in Portugal eingeführten Wellenastrild Estrilda astrild
Zusammenfassung Faktorenanalyse zur Ausbreitung des in Portugal eingeführten Wellenastrild Estrilda astrild Der Wellenastrild Estrilda astrild wurde erstmals 1964, von Afrika kommend, in Portugal eingeführt und hat sich seither über die meisten Regionen Portugals und nach Spanien ausgebreitet. Wir analysierten die Ausbreitung in 4-Jahres Abschnitten zwischen 1964 und 1999 auf der Basis von 20’20 km UTM Rasterquadraten. Die Besiedlung eines Quadrates wurde als Funktion verschiedener biophysikalischer und räumlich-zeitlicher Variablen mit einer multivariaten logistischen Regression untersucht. Als wichtigste Faktoren mit 37,8 % der erklärten Varianz erwiesen sich die räumlich-zeitlichen Variablen, gefolgt von der Kombination aus Umwelt- und räumlich-zeitlichen Faktoren mit 20,6% der erklärten Varianz. Nur etwa ein Zehntel der gesamten Variation erklärt sich durch die untersuchten Umweltfaktoren allein.
References
Augustin NH, Mugglstone MA, Buckland ST (1996) An autologistic model for the spatial distribution of wildlife. J Appl Ecol 33:339–347
Bernard P (1997) Common waxbill Estrilda astrild. In: Harrison JA, Allan DG, Underhill LG, Herremans M, Tree AJ, Parker V, Brown CJ (eds) The atlas of Southern African birds, vol 2, passerines. Birdlife South Africa, Johannesburg, pp 612–613
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
CNA (1983) Atlas do Ambiente. Comissão Nacional do Ambiente (CNA). Direcção Geral do Ambiente, Ministério do Ambiente e dos Recursos Naturais (85 environmental charts), Lisboa
Farinha JC, Trindade A (1994) Contribuição para o Inventário e caracterização de zonas húmidas em Portugal Continental. MedWet/Instituto da Conservação da Natureza, Lisbon.
Grinnell J (1917) Field tests of theories concerning distributional control. Am Nat 51:115–128
Grinnell J (1924) Geography and evolution. Ecology 5:225–229
Hall BP, Moreau RE (1970) An atlas of speciation in African passerine birds. British Museum (Natural History), London
Hengeveld R (1989) Dynamics of biological invasions. Chapman & Hall, London
Legendre L, Legendre P (1998) Numerical ecology, developments in environmental modeling, 3. Elsevier, Amsterdam. The Netherlands
Matias R (2002) Aves exóticas que nidificam em Portugal continental. Instituto da Conservação da Natureza, Lisboa
Matias R, Lobo F (1999) Aves exóticas que nidificam em Portugal continental. Sociedade Portuguesa para o Estudo das Aves. Unpublished Report for Instituto da Conservação da Natureza, Lisboa
Nagelkerke NJD (1991) A note on a general definition of the coefficient of determination. Biometrika 78:691–692
Pearce J, Ferrier S (2000) Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Model 132:225–245
Peterson AT, (2003) Predicting the geography of species’ invasions via ecological niche modeling. Q Rev Biol 78:419–433
Rainbow P (1998) Impacts of invasions by alien species. J Zool Lond 246:247–248
Reino LM, Silva T (1998) The distribution and expansion of the Common waxbill Estrilda astrild in the Iberian Peninsula. Biol Cons Fauna 102:16–22
Reino LM, Beja P, Gordinho LO, Heitor AC (2002) Influências espaciais e ambientais na distribuição do Pardal-espanhol (Passer hispaniolensis) em Portugal. Airo 12:45–49
Shogren JF (2000) Risk reduction strategies against “explosive invader”. In: Perrings C, Williamson M, Dalmazzone S (eds) The economics of biological invasions. Elgar, Cheltenham, pp 56–69
Silva T, Reino LM, Borralho R (2002) A model for range expansion of an introduced species: the common waxbill Estrilda astrild in Portugal. Divers Distrib 8:319–326
Simberloff D (1997) The biology of invasions. In: Simberloff D, Schmitz DC, Brown TC (eds) Stranglers in paradise: impact and management of nonindigenous species in Florida. Island Press, Washington, DC, pp 3–17
Smallwood KS (1994) Site invasibility by exotic birds and mammals. Biol Conserv 69:251–259
Turpie J, Heydenrych B (2000) Economic consequences of alien infestation of the Cape Floral Kingdom’s Fynbos vegetation. In: Perrings C, Williamson M, Dalmazzone S (eds) The economics of biological invasions. Elgar, Cheltenham, pp 152–182
Vitousek PM, Mooney HA, Lubchenco J, Melillo LM (1997) Human domination of earth’s ecosystems. Science 277:494–499
Xavier A (1968) Bicos de Lacre em Óbidos. Cyanopica 1:77–81
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice Hall, N.J.
Acknowledgements
The author wishes to thank Tiago Silva and Miguel Araújo for their helpful comments on an earlier version of this paper. I am also grateful to A. Townsend Peterson, Carsten Rahbek and an anonymous referee for many comments and suggestions. Iam also grateful to Helena Simões (ERENA) for her help on the map production and to Wanda Meulemberg for her careful help and review of the English. Finally, to Tiago Silva, for all these years working together since we started in 1994 on the introduction and expansion of the common waxbill in Iberia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Rahbek
Rights and permissions
About this article
Cite this article
Reino, L. Variation partitioning for range expansion of an introduced species: the common waxbill Estrilda astrild in Portugal. J Ornithol 146, 377–382 (2005). https://doi.org/10.1007/s10336-005-0093-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-005-0093-6