Abstract
Male European starlings (Sturnus vulgaris) intermingle fresh herbs, preferably species rich in volatile compounds, into their dry nest material. In a field study, we investigated whether these herbs affect the mite and bacteria load of the nests and the condition of the nestlings either directly or via parasite control. We examined the amount of herbs and the number of plant species males carried into their nests, the variation of volatile compounds in the headspace air of the nest boxes and mite/bacteria load of the nests throughout the season. The amount of herb material and the number of plant species, the number of substances emanated by these plants and the infestation of the nests with bacteria and mites (Dermanyssus gallinae) increased with season. In a field experiment, we exchanged natural starling nests with experimental nests with or without herbs. We found that the herbs had no effect on the mites but fewer bacteria were sampled in nests with herbs than in nests without herbs. The body mass of the fledging was not related to the season or the mite/bacteria load of the nests. However, nestlings from nests with herbs fledged with higher body mass than nestlings from nests without herbs. Both bacteria and mite load were related to nestling mortality. In nests containing no herbs, the numbers of fledglings declined significantly with the increasing mite load while the mites had no effect on the number of fledglings in nests with herbs. Thus, the nest herbs counteracted the effect of the mites. In conclusion, it seems that volatile herbs can reduce bacterial but not mite infestation of the starling nests. The positive influence of herbs on nestling growth indicates that herbs either directly (perhaps as immunostimulants) improve the condition of the nestlings and help them cope with the harmful effects of mites, or they provide a nest environment beneficial for the nestlings‘ development by the reduction of germs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European starlings (Sturnus vulgaris) and several other bird species intermingle fresh herbs into their otherwise dry nest material. In a survey of 137 passerine species, Clark and Mason (1985) estimated that species such as hole-nesting starlings that reuse their nests year after year were about six times as likely to employ green nest material than species that use their nest only once. Several hypotheses accounting for the use of green nest material have been proposed (Clark 1991a, 1991b). Because reuse of the nest site (a routine in hole-nesting birds) may result in a heavy parasite load of the nests, the explanation offered most often was that birds, like herbalists, may use intrinsic properties of plants to protect their nestlings against harmful parasites and pathogens (Widmann 1922; Sengupta 1981; Wimberger 1984; Clark and Mason 1985, 1988; Rodgers et al. 1988; Clark 1991a, 1991b; Roulin et al. 1997; Petit et al. 2002). Experimental work investigating this “nest protection hypothesis” is rare. In laboratory experiments, fresh wild carrot leaves (Daucus carota), a nest herb preferred by North American starlings, inhibited bacterial growth. Put into starling nests during the nestling period, wild carrots inhibited the development of mites (Clark and Mason 1985, 1988). Three other studies with starlings, one performed in the same North American population and the other two in South Germany and in the Netherlands, failed to demonstrate the effect of the starlings’ preferred herbs on mites, the main nest ectoparasites (Fauth et al. 1991; Gwinner et al. 2000; Brouwer and Komdeur 2004). Rodgers et al. (1988) examined the effect of aromatic plants on parasites in wood stork (Mycteria americana) nests and found no effect. Other investigations emphasized the role of green plants as tools to attract females (“courtship hypothesis”) because only male starlings carry fresh plants into the nests. Males provide herbs especially when females are present and cease this behaviour with egg laying (Pinxten and Eens 1990; Fauth et al. 1991; Gwinner 1997). Nests containing green nest material are more likely to be chosen by females (Brouwer and Komdeur 2004). In a previous study, we proposed the “drug hypothesis”. We showed that herbs, like drugs, had a stimulating effect on the elements of the immune system, which might enable the nestlings to cope better or faster with stress in general and with parasites in particular (Gwinner et al. 2000).
The plants that starlings and other bird species choose as green nest material are rich in volatile compounds, which may, when inhaled or penetrated through the skin, affect the nestlings’ condition or act as a fumigant that inhibits parasite development in the nest. For example, starlings from a South German population prefer milfoil (Achillea millefolia), cow weed (Anthriscus sylvestris), and hog weed (Heracleum spondyleum) (Gwinner 1997); starlings from a North American population favour agrimony (Agrimonia sp.), wild carrots and milfoil (Clark and Mason 1985); and Corsican blue tits (Parus caeruleus) use lavender (Lavendula stoechas), milfoil (Achillea ligustica) and mint (Mentha suaveolens) as green nest material (Lambrechts and Dos Santos 2000).
The starlings from our South German population have two breeding events, one in April/May and the second in May/June. Second-brood nestlings have often been observed to be of lower quality (Feare 1984). Among the numerous factors that could be responsible for this difference, parasite/pathogen load may be one. In the present paper, we, therefore, examined the variation in the infestation of the nests with mites and bacteria throughout the reproductive season in relation to the seasonal changes of amount and composition of the green nest material incorporated into the nests by starling males, possibly for the sake of parasite/pathogen control. Because males carry herbs only during courtship and nest building, but do not—as blue tits do—put them into the nests during the nestling period, it was questioned whether volatile plant compounds could be detected at all several weeks after their deposition when nestlings hatch. We, therefore, collected headspace air in the nest boxes during the nestling period to investigate its composition. Finally, in field experiments with artificial nests containing herbs or no herbs, we investigated the effect of herbs on nestlings and their parasites under controlled conditions.
Methods
Our study site, a starling colony of 60 nest boxes, is located close to lake Ammersee (South Germany, 48° N 11° E).
Plants
In the first year of the study, we collected the plants the males had deposited in their nests every day from the first day on which green nest material appeared in the box to the onset of laying. Plant data therefore refer to the total amount or number of species one male carried into his box. The plants were dried, weighed and identified (details: Gwinner 1997).
Experimental nests
On the basis of our collections from natural starling nests, we designed an experimental “herb nest” that we used for 5 years to investigate the effect of green nest material on various parameters of the nest and the nestlings. “Herb nests” contained 40 g dry grass and 40 g fresh, green nest material consisting of milfoil (Achilea millefolia), hogweed (Heracleum spondyleum), cow parsley (Anthriscus sylvestris), black elder (Sambucus niger), gout weed (Aegopodium podagraria) and willow (Salix alba), all of these are the preferred nest plants of the starlings of our population. Control nests, “grass nests, ”contained only grass, Brachipodium silvaticum, a species often used by our starlings as nest material. We exchanged natural starling nests for experimental ones after egg deposition to minimize nest desertion (details: Gwinner et al. 2000).
Condition of the nestlings
We measured the body mass of the nestlings close to fledging (day 18) for 5 years. The accuracy of measurement was to the nearest 0.1 g.
Parasite load of the nests
The main ectoparasite in our starlings was the red fowl mite (Dermanyssus gallinae). We scored mites collected from the lid of the nest boxes (score 1: no or few mites, score 2: clusters of mites) on day 14 for 4 years.
Bacteria were collected on day 14 for 2 years and for 1 year on days 1, 9 and 14 from the belly of two nestlings per nest. An agar paddle (Hycheck, Becton Dickinson& Co.) was pressed on the nestlings’ belly for 5 s, and the paddles were then incubated for 48 h at 38°C. The colonies appearing on the agar paddles were photographed and counted. To ascertain the seasonal development in different nests, the colonies on the whole paddle were counted. On days 1, 9 and 14, a segment of 1.5×3 cm of the paddle was counted to find out about bacterial development in individual nests from days 1 to 14. For the statistical analysis, we used the data for the chick with the higher count of the two chicks (details: Berger et al. 2003).
To identify volatile substances, headspace air was sampled for 3 years from 8 am to 4 pm from nest boxes with natural nests. Charcoal tubes (Sigma–Aldrich, MO, USA) were inserted through a small hole on the side of the nest boxes about 4 cm below the roof. The tubes were connected to plastic hoses (diameter: 0.5 cm) leading to pumps placed underneath the nest boxes. The air-flow created by the pumps was defined at 300–400 ml/h. The air samples were tightly closed, frozen at −60°C and transported on ice to the Max Planck Institute for Chemical Ecology in Jena, Germany, where they were processed and the compounds identified by chromatography and mass spectography (Gwinner and Krock in preparation). In the current presentation, we use the number of peaks appearing on the chromatograms between the retention times of 7.44–15 min as a measure of the “volatile richness” of an air sample.
Statistics
The effects of herbs (nest type: herb nest versus grass nest), mites [mite load: low (1) versus high (2)] and season as covariate on fledgling mass were tested with a general linear model (GLM) of SPSS Version 12.0.1. The same model was used to investigate the effect of nest type and season on bacteria colonies. In this analysis, data were pooled for the May and June nests respectively. The number of fledglings was analysed by multiple regression with backward selection. Factors (number of hatchlings, bacteria load, mite load and season) were retained in the model when standardized coefficients were significant (p≤0.05). The differences between groups were tested by the Mann Whitney U-test and the correlations by Spearman’s non-parametric correlation when the data were not normally distributed and otherwise by t-tests. All data are expressed as means of nests with standard errors.
Results
Herb mass, herb species and season
The mass of green nest material and the numbers of plant species male starlings carried into their nests during courtship and nest building increased with season, as follows: April nests (n=58) contained 1.7±0.3 g (dry weight) herbs of 8.6±0.7 species. May nests (n=34) contained 17±3.3 g of herbs and 12.3±0.8 plant species (herb mass: Z=−6.5, p=0.0001, plant species: Z=−3.4, p=0.001; Fig. 1).
Volatile substances in headspace air samples
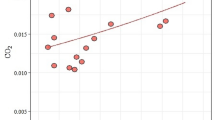
We sampled air for 8 h from nest boxes containing natural starling nests at the nestlings’ age of 8 or 9 days. The numbers of volatile substances, defined as peaks depicted on the chromatograms, correlated positively with the date during 2002 and 2004 but not during 2003 (2002: ρ =0.5, n=18, p=0.05; 2003: ρ =−0.4, n=9, p=0.3; 2004: ρ =0.6, n=15, p=0.02; Fig. 2). In 2003, a very hot and dry year, most clutches from nests were deserted late in the season and, therefore, data for those nests are lacking.
The correlation of the date and the peaks of all the 3 years combined was also significant: ρ =0.4, n=42, p=0.01. The number of substances in air samples from nest boxes containing standardized experimental herb nests increased as well when data for all the 3 years were combined (ρ =4, n=29, p=0.027).
Mites, herbs and season
We tested the effect of “nest type”(nests with/without herbs) and season (May/June) on the mite load. More mites were detected in June than in May nests, but the herbs were ineffective in controlling the mite load (GLM: nest type: F1,112=2.25, p=0.14, season: F1,112=21.7, p= 0.0001; Fig. 3).
Bacteria, herbs and season
We tested the effect of nest type and season on a number of bacteria colonies (GLM: nest type: F 1,76=5, p=0.029, season F 1,76=34, p=0.0001). The infestation of nestlings with bacteria was higher in the June nests than in the May nests and the herbs affected this increase. In May, the bacteria load was not different in the nests with or without herbs (t-test: t=−0.16, n=34, p=0.9). In June, however, there were fewer bacteria in nests containing herbs (t-test: t=−2.4, n=45, p=0.02; Fig. 4).
Within a nest, the number of bacteria colonies counted for individual nestlings at days 1, 9 and 14 increased with nestling age. The increase was the same for nests with and without herbs in the first week. In the second week of life, nestlings in nests without herbs suffered an equally high bacterial increase. In herb nests, however, bacterial growth seemed to be suppressed; some nests even showed a decline in infestation (Z=−1.9, n=25, p=0.06; Fig. 5).
Fledgling condition, mites, bacteria, herbs and season
The body mass of the fledglings was similar in the May and June nests (May: 68.8±0.44 g, June: 69.2±0.61 g; Z=−3.9, n=155, p=0.56). The bacteria load had no influence on the body mass: fledglings in highly infested nests weighed 71.05 g and in lowly infested nests they weighed 71.3 g (t-test: t=0,26, n=70, p=0.7). Also, the mite load of the nest boxes had no effect on the body mass but the herbs had a favourable influence on the fledging mass of the nestlings. Over the years, the fledglings from nests with herbs were 2 g heavier; pooled data on fledging mass as dependent variable, mite load as factor and season as covariate revealed a highly significant effect of herbs (“nest type”) on fledgling mass (GLM: mite load: F 1,120=0.2, p=0.4, season: F 1,120=1.4, p=0.24, nest type: F 1,120=12.0, p=0.001; Fig. 6). The interactions between nest type and mite load and mite load and season were not significant (p>0.5).
The number of hatchlings and fledglings decreased with season. In May 4.55±0.1chicks hatched and in June 3.8±0.14 chicks hatched (Z=−4.6, n=183, p=0.0001). In May, nests fledged 3.8±0.12 chicks and in June 2.9±0.19 chicks fledged (Z=−4.3, n=171, p=0.0001).
Both bacteria and mite load were correlated with the number of chicks that died during the nestling phase (dead chicks/mite load: ρ =0.19, n=129, p=0.029; dead chicks/bacteria load: ρ =0.27, n=77, p=0.017).
We investigated in a multiple regression analysis with backward selection whether fledging success was related to season, bacteria load and the number of hatchlings. We introduced hatchlings as a factor because mortality might be different in nests with higher clutch size, for example, due to the escalating mite load in a nest with many nestlings.
The number of fledglings (n=77) was positively correlated with the number of hatchlings (β : 0.4, p=0.0001 and negatively correlated with bacteria load (β = −0.27, p=0.008), while season was excluded as a relevant factor in reducing fledging success (β = −0.056, p=0.65) in this analysis.
For mite load, the same analysis (with a different data set, n=114) showed a positive effect of number of hatchlings (β =0.6, p=0.0001) and a negative effect of season (β = −0.2, p=0.004) on fledging success while mite load had no effect. (β = −0.07, p=0.3). When we analysed the data separately for herb and grass nests, the relations were similar for herb nests. However, in nests without herbs (n=52) the numbers of fledglings declined significantly with increasing mite load (β = −0.3, p=0.012), the relationship between the number of hatchlings and fledglings was the same and season was excluded (β = −0.17, p=0.2).
Similar results came from a comparison of the May and June nests. In nests with high mite load but without herbs fewer nestlings hatched/fledged in June than in May (Z=−2.9/−2.5, n=29, p=0.005/0.02). In nests with herbs hatching/fledging success was not different in May and June nests when the mite load was high (Z=−1.4/−1.2, n=33, p=0.2/0.3). The seasonal and mite-related decrease in the number of fledglings was counteracted by the herbs in the nests. The same analysis for the bacteria data was not possible, because the sample size of the high bacteria load category of early nests was too small.
Discussion
The seasonal increase in nest ectoparasites and bacteria is accompanied by an increasing amount of green nest material carried into the nests by the males. This phenomenon may partly be explained by the greater effort males have to expend in order to attract females later in the season (Gwinner 1997). However, a greater quantity of volatile herbs incorporated later in the season may also be a male’s response to increased infestation of the nests with parasites and pathogens. Because the mite load of the nests was not affected by the herbs, we suggested (Gwinner et al. 2000) that males may protect their nestlings from unfavourable influences of ectoparasites and other stressors by strengthening the immunity with herbs incorporated in the nests.
The question whether volatile substances are traceable several weeks after plant deposition can be answered with a yes, there are substances dispersed from plants in the nest box air. The greater variety of substances emanated by the nest–herbs later in the season may be the result of more herbs added to nests by the males. In addition, it may be caused by the changing chemical properties of the plants later in season, because in our standardized herb nests, the number of substances also increased with season when the data of the 3 years were combined. Depending on the years, nestlings from later nests may, therefore, have contacted a greater variety of volatile compounds than nestlings from earlier nests. The higher numbers of substances in the June samples was not due to higher temperatures later in the year. With higher temperature substances with lesser volatility vaporize which should show up late on the chromatogram, at higher retention-times. However, the additional peaks of our samples occurred mainly at early retention-times.
We think that the significant effect of herbs on the bacteria load of the nests later in the season may be due to the greater variety of compounds. Some evidence from other studies corroborates such an assumption. Blue tits, like starlings, provide their nests with aromatic plants. Lambrechts and Dos Santos (2001) found an increase of odour classes in blue tit nests in the breeding season. They hypothesize that a cocktail of odours may enhance the protection against parasites and pathogens. In a subsequent study, Lafuma et al. (2001) tested the mosquito repellent effect of the plants that they had identified in the blue tit’s nests with chicks and found a greater efficiency in plant mixtures when compared with individual plants.
The seasonal increase of bacteria and mites coinciding with the seasonal decrease in number of fledgings suggests that parasites may manipulate fledging success. However, in the multiple regression analysis mite load was negatively correlated with the number of fledglings, but there was no relationship with season. Whether the frequently observed lower quality of second brood nestlings is related to the parasite load of the nests therefore remains uncertain.
In field experiments, in which natural nests were exchanged for experimental nests with or without fresh herbs, we investigated the effect of nest herbs on parasites and nestlings. The detrimental effects of parasites and pathogens on their hosts can reduce the growth and survival of the nestlings (Richner et al. 1993; Merino and Potti 1995). In our starling colony, mite or bacteria load of the nests was not related to the nestlings’ fledging mass (Berger et al. 2003; this study) but correlated positively with the number of chicks that died during the nestling period. The analysis of the large data set collected over 5 years revealed a significantly positive relationship between fledging success, number of hatchlings and season, but no relation to the mite load of the nests. However, when we analysed the data separately for nests with and without herbs, we found that mites diminished fledging success in nests without herbs but not in nests with herbs. Because the mite load in the two nest types was the same over the years, we assume that the nest herbs had a direct beneficial effect on nestlings’ resistance against the detrimental effects of the mites.
The seasonal increase of bacteria (Berger et al. 2003) was less evident in the nests containing herbs. Herbs reduced bacterial infestation and bacterial infestation was related to fledging success. The expected effect of herbs on body mass as shown in the data set of 1995–2001 was not visible in the 2 years of bacteria investigation. We do not know whether the bacteria we collected from the nestlings were detrimental or even beneficial (see Berger et al. 2003). Also, it is unknown at what level of bacteria infestation the growth of the nestlings becomes impaired; therefore, the reduction of bacteria that we observed may have been meaningless for the nestlings’ condition.
The growth of bacteria within individual nests tended to be inhibited by nest herbs. Because we took our bacteria samples from the belly of the nestlings, it is also conceivable that nestlings from herb nests mounted a greater resistance against bacteria.
The strongest effect of herbs over the years was their positive influence on the body mass of the fledglings. In the experimental herb nests, the fledglings were heavier than the ones in nests without herbs during 5 years; the body mass pooled over the years differed with a high significance between the groups. This effect was not related to the mite load of the nests and did not change with season.
How could the herbs have operated in a beneficial way on the nestlings’ condition? Plants have evolved secondary compounds as weapons against plant-damaging parasites. These compounds may be less effective in repelling blood- feeding arthropods. Nevertheless, it is conceivable that herbs—though not reducing the number of mites—may affect their feeding behaviour. In chickens, it was shown that when they were induced with stress the corticosterone levels in the blood increased which inhibited blood sucking by the red fowl mite (Gross 1975; cited in Clark 1991b). Some plants, on the other hand, are known to stimulate corticosterone secretion (Steinegger and Hänsel 1988). In addition, herb compounds may stimulate elements of the immune system, which are important for parasite defence. In a previous study, we found a raise of basophile leukocytes in nestlings from herb nests (Gwinner et al. 2000). In a second study, two years later, this increase was not noticed (Berger 2002). In birds, basophils increase the capacity to cope with stress induced by malnutrition or severe climatic conditions (Maxwell et al. 1990; Maxwell and Robertson 1995), and in humans also they play a role in immunity against parasites (Roitt et al. 1996).
The effect of herbs on the body mass of the fledglings was particularly distinct under unfavourable conditions, for example, when the mite load was high, the ambient temperature was low and prolonged rainfalls occurred during the early nestling phase or when in polygynous pairings only the females took care of the nestlings (Gwinner in press). The changing environmental conditions from year to year, therefore, may create changing effects of herbs on the nestlings’ condition. Further investigations of immunological and metabolic parameters are being considered, in order to cast more light on the mode of herb activity in starling nests.
Zusammenfassung
Kondition der Nestlinge, Parasiten, Kräuter und Jahreszeit beim Europäischen Star
Starenmännchen (Sturnus vulgaris) weben frische Kräuter, bevorzugt ätherische Ölpflanzen, in ihr trockenes Nestmaterial ein. Wir prüften im Freiland, ob diese Pflanzen in der Lage sind, den Milben- und Bakterienbefall der Nester zu vermindern und ob sie die Kondition der Nestjungen entweder direkt oder durch die Kontrolle von Parasiten beeinflussen können. Wir untersuchten die Menge Pflanzenmaterial und die Anzahl von Pflanzenarten, die von den Männchen in ihre Nester getragen wurden, die ätherischen Öle, die von den Pflanzen in die Nistkastenluft abgegeben werden und das Vorkommen von Milben und Bakterien in den Nestern im Laufe der Brutsaison. Die Menge an Pflanzenmaterial, die Anzahl der Pflanzenarten, die Anzahl ätherischer Substanzen, der Milben- (Dermanyssus gallinae) und Bakterienbefall der Nester nahmen im Laufe der Saison zu. In einem Feldexperiment tauschten wir natürliche Starennester gegen experimentelle Nester aus, die entweder Kräuter oder keine Kräuter enthielten. Die Nestkräuter bewirkten keine Reduktion von Milben, doch fanden wir weniger Bakterien in Nestern mit Kräutern. Die Körpermasse der Nestjungen kurz vor dem Ausfliegen war unverändert im Verlauf der Saison und konnte auch nicht zum Milben- oder Bakterienbefall der Nester in Beziehung gesetzt werden. Allerdings hatten die Jungen aus Nestern mit Kräutern eine höhere Ausflugsmasse als Junge aus Nestern ohne Kräuter. Mehr Starenjunge starben in Nestern mit hohem Bakterien- oder Milbenbefall. In Nestern ohne Kräuter nahm die Anzahl der ausfliegenden Nestjungen signifikant mit zunehmendem Milbenbefall ab, während in den Nestern mit Kräutern dieser Effekt nicht zu sehen war. Der schädliche Milbeneinfluss wurde demnach von den Nestkräutern gemildert. Unsere Ergebnisse erlauben den Schluss, dass Kräuter zwar Bakterien aber nicht Milben in den Starennestern reduzieren können und dass Kräuter einen direkten günstigen Einfluss auf das Wachstum der Jungen haben könnten, indem sie relevante Elemente des Immunsystems fördern und so den Nestjungen helfen, besser mit den ungünstigen Einflüssen von Milben fertig zu werden. Sie könnten aber auch durch ihre bakterizide Wirkung ein für die Entwicklung der Jungen günstiges Nestklima bereiten.
References
Berger S (2002) Warum tragen Starenmännchen frische Kräuter in ihre Nester ein? Diplomarbeit Universität Würzburg
Berger S, Disko R, Gwinner H (2003) Bacteria in starling nests. J Ornithol 144:317–322
Brouwer L, Komdeur J (2004) Green nesting material has a function in mate attraction in the European starling. Anim Behav 67:539–548
Clark L (1991a) The nest protection hypothesis: the adaptive use of plant secondary compounds by European starlings. In: Loye JE, Zuk M (eds) Bird-parasite interactions: ecology, evolution, and behaviour. Oxford Ornithology Series, Oxford, pp 205–221
Clark L (1991b) Countering parasites and pathogens. Parasitol Today 6:358–360
Clark L, Mason JR (1985) Use of nest material as insecticidal and anti-pathogenic agents by the European starling. Oecologia 67:169–176
Clark L, Mason JR (1987) Olfactory discrimination of plant volatiles by the European starling. Anim Behav 35:227–235
Clark L, Mason JR (1988) Effect of biologically active plants used as nest material and the derived benefit to starling nestlings. Oecologia 77:174–180
Fauth PT, Krementz DG, Hines JE (1991) Ectoparasitism and the role of green nesting material in the European starling. Oecologia 88:22–29
Feare CJ (1984) The starling. Oxford University press, Oxford
Gwinner H (1997) The function of green plants in nests of European starlings (Sturnus vulgaris). Behaviour 134:337–351
Gwinner H (in press) Parasite defense in birds. The role of volatiles. In: Proceedings of the 23rd international IOC. Acta Zool Sinica
Gwinner H, Oltrogge M, Trost L, Nienaber U (2000) Green plants in starling nests: effects on nestlings. Anim Behav 59:301–309
Lafuma L, Lambrechts MM, Raymon M (2001) Aromatic plants in bird nests as a protection against blood-sucking flying insects? Behav Processes 56:113–120
Lambrechts MM, Dos Santos A (2000) Aromatic herbs in Corsican blue tit nests: the ‘Potpourri’ hypothesis. Acta Oecol 21:175–178
Maxwell MH, Robertson GW (1995) The avian basophilic leucocyte. A review. Worlds Poultry Sci J 51:307–325
Maxwell MH, Robertson GW, Spence S, Mcorquodale CC (1990) Comparison of hematological values in restricted and ad libitum-fed domestic fowls white blood cells and thrombocytes. Br Poultry Sci 314:881–886
Merino S, Potti J (1995) Mites and blowflies decrease growth and survival in nestling pied flycatchers. Oikos 73:95–103
Petit C, Hossaert-McKey M, Perret P, Blondel J, Lambrechts MM (2002) Blue tits use selected plants and olfaction to maintain an aromatic environment for nestlings. Ecol Lett 5:585–589
Pinxten R, Eens M (1990) Polygyny in the European starling: effect on female reproductive success. Anim Behav 40:1035–1047
Richner H, Oppliger A, Christe P (1993) Effect of an ectoparasite on reproduction in great tits. J Anim Ecol 62:703–710
Rodgers JA, Werner AS, Schwikert ST (1988) The use and function of green nest material by wood storks. Wilson Bull 100:411–423
Roitt IM, Brostoff J, Male DK (1996) Immunology. Mosby, London
Roulin A, Jeanmonod J, Blanc T (1997) Green plant material on common buzzard’s (Buteo buteo) nests during the rearing of chicks. Alauda 65:251–257
Sengupta S (1981) Signifance of the use of margosa leaves in the nest of house sparrows passer domesticus. Emu 81:114–115
Steinegger E, Hänsel R (1988) Lehrbuch der pharmakognosie und phytopharmazie. Springer-Verlag, Berlin
Widmann O (1922) Extracts from the diary of Otto Widmann. Academy of Science, St. Louis 24:1–77
Wimberger P (1984) The use of green plant material in bird nests to avoid ectoparasites. Auk 101:615–618
Acknowledgements
Ebo Gwinner contributed in many ways to this study: he assisted in the field, initiated co-operations and guided us through the years of the investigation with many suggestions and enthusiasm. Discussions and questions were possible at all times.
B. Krock, Jena, analysed the head space air samples. W. Jensen, E. Koch and S. Gwinner, developed the air sampling method ingeniously. M. Suchomel gave bacteriological and B. Helm statistical advice. L. Trost performed a great deal of field work. F. Bairlein gave fruitful comments. We are very grateful to all of them.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein
Rights and permissions
About this article
Cite this article
Gwinner, H., Berger, S. European starlings: nestling condition, parasites and green nest material during the breeding season. J Ornithol 146, 365–371 (2005). https://doi.org/10.1007/s10336-005-0012-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-005-0012-x