Abstract
Roads have broadly adverse impacts on wildlife, including nonhuman primates. One direct effect is mortality from collisions with vehicles. While highly undesirable, roadkills provide valuable information on the health and condition of endangered species. We present a case report of a wild chimpanzee (Pan troglodytes schweinfurthii) killed crossing a road in Bulindi, Uganda, where chimpanzees inhabit forest fragments amid farmland. Details of the collision are constructed from eyewitness accounts of pedestrians. Physical examination of the cadaver indicated good overall body condition; at 40 kg, the deceased female was heavier than usual for an adult female East African chimpanzee. No external wounds or fractures were noted. Coprological assessment demonstrated infection by several gastrointestinal parasites commonly reported in living wild chimpanzees. Histopathology revealed eosinophilic enteritis and biliary hyperplasia potentially caused by parasite infection. However, eosinophilia was not widely spread into the submucosa, while egg/cyst counts suggested low-intensity parasite infections compared to healthy female chimpanzees of similar age in nearby Budongo Forest. No behavioral indicators of ill health were noted in the deceased female in the month prior to the accident. We conclude that cause of death was acute, i.e., shock from the collision, and was probably unrelated to parasite infection or any other underlying health condition. Notably, this female had asymmetrical polythelia, and, while nursing at the time of her death, had one functioning mammary gland only. In Uganda, where primates often inhabit human-dominated landscapes, human population growth and economic development has given rise to increasing motor traffic, while road development is enabling motorists to travel at greater speeds. Thus, the danger of roads to apes and other wildlife is rising, necessitating urgent strategies to reduce risks. Installation of simple speed-bumps—common on Ugandan roads—would be effective in reducing risks to wildlife, and would also make roads safer for human pedestrians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Across the globe, wild animals are impacted by human activities and processes that alter natural landscapes and threaten wildlife survival. Roads are one such impact with broadly adverse effects on animal communities and ecosystems, for example by fragmenting habitats, altering animal movements and distributions, and increasing human settlement and activities such as hunting (Trombulak and Frissell 2000; Laurance et al. 2006; Shepard et al. 2008). A direct effect of roads is mortality from collisions with vehicles. Roadkill surveys confirm that a broad range of vertebrate taxa worldwide are vulnerable to being killed by vehicles during road crossings (Lode 2000; Hobday and Minstrell 2008; Cáceres et al. 2010; Kioko et al. 2015). However, despite the negative impact of road mortalities on animal populations, roadkills can present a valuable source of information on the health and condition of endangered species (Richini-Pereira et al. 2010; Auricchio et al. 2014).
Like many wild animals, nonhuman primates (hereafter “primates”) are increasingly found in habitats modified substantially by humans, such as plantations, forest–agricultural mosaics, and periurban areas (Anderson et al. 2007; Estrada et al. 2012; Hockings et al. 2015; Maibeche et al. 2015). Life in such human-dominated landscapes is perilous for primates. Aside from direct threats to primate survival from hunting and persecution by people, potential dangers distinct to these environments include roads, predation by domestic dogs, electrocution, and exposure to human and livestock pathogens (Anderson 1986; Lokschin et al. 2007; Goldberg et al. 2008; Healy and Nijman 2014; Rodrigues and Martinez 2014; Kumar and Kumar 2015). Various primates have been recorded as roadkill, with records often involving taxa widely distributed in human-dominated environments (e.g., yellow baboon Papio cynocephalus: Drews 1995; rhesus macaque Macaca mulatta: Pragatheesh 2011; see also Cibot et al. 2015). However, road mortalities of highly threatened primates are also reported (e.g., black lion tamarin Leontopithecus chrysopygus: Valladares-Padua et al. 1995; Zanzibar red colobus Procolobus kirkii: Struhsaker and Siex 1996; lion-tailed macaque Macaca Silenus: Kumara et al. 2000).
All great ape taxa are listed as Endangered or Critically Endangered by the International Union for Conservation of Nature (IUCN 2014). Since habitat alteration and infrastructural development in great ape habitats are ongoing, conservation requires finding ways for great apes to survive in human-impacted landscapes (Hockings et al. 2015). Among great apes, chimpanzees (Pan troglodytes) have the broadest current geographic range and appear most resilient to anthropogenic impacts such as agriculture, providing they are not hunted or persecuted (Hockings and McLennan 2012; McLennan and Hockings 2014; cf. McLennan et al. 2012). In the absence of hunting, chimpanzees show little road avoidance (cf. Hicks et al. 2012). Where human population densities are high, chimpanzee home ranges are inevitably dissected by roads (e.g., Uganda: Reynolds et al. 2003; McLennan 2008; Guinea: Hockings et al. 2006), including roads which pass through protected areas (e.g., Cantanhez National Park, Guinea-Bissau: Hockings and Sousa 2013; Kibale National Park, Uganda: Cibot et al. 2015).
Studies of chimpanzee behavior on roads at Bossou in Guinea (Sakura 1994; Hockings et al. 2006; Hockings 2011) and Sebitoli in Kibale National Park (Cibot et al. 2015), demonstrate they perceive roads as dangerous, and show behavioral adjustments to reduce risks posed by vehicles and pedestrians. Although a chimpanzee is not known to have been killed or injured crossing roads at Bossou (Hockings 2011), an adult male chimpanzee was found dead on a road at Sebitoli in 2003 (Krief et al. 2008). While necropsy confirmed this individual was in good physical condition, with normal organs, research was not being conducted at Sebitoli at that time, and details of the accident are unknown (S. Krief, pers. comm., 2015). Here, we present a case report of a wild adult female chimpanzee struck and killed by a vehicle in Bulindi, Uganda, on 24 March 2015 with (1) details of circumstances surrounding the accident, and (2) an assessment of the female’s health at the time of the accident.
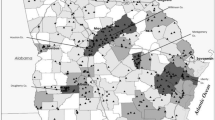
Methods
Study area
Bulindi is located at 1°28′N, 31°28′E in Hoima District in western Uganda, midway between the main Budongo and Bugoma forest reserves (Fig. 1). Outside these reserves, the landscape is densely populated by people (>150 persons per km2 in 2014; UBOS 2014) and dominated by subsistence and commercial agriculture. However, small fragments of unprotected forest inhabited by chimpanzees persist along watercourses throughout Hoima and neighboring Masindi District (McLennan 2008; McLennan and Hill 2015). A recent genetic census revealed that 256–319 chimpanzees from at least nine distinct groups (“communities”) inhabit this fragmented human-dominated landscape, across an area greater than 600 km2 (McCarthy et al. 2015). The Bulindi chimpanzees are one of these “fragment communities.” Hoima and Masindi districts are served by numerous roads that receive varying amounts of vehicle traffic. Chimpanzees are mobile throughout this forest–agricultural matrix, crossing roads and tracks to access fragmented food sources, including agricultural crops (Reynolds et al. 2003; McLennan 2008, 2013).
Map showing the main Budongo and Bugoma forest reserves (in Masindi and Hoima districts, respectively) and unprotected forest fragments in the intervening region inhabited by a population of c.256–319 chimpanzees (McCarthy et al. 2015); the Bulindi area is encircled. The region is served by numerous roads
Chimpanzees at Bulindi were first studied in 2006–2008 (McLennan and Hill 2010), and subsequently from 2012 to the present. In March 2015, the community comprised 21 individuals, including three adult males, six adult females, two subadults, and ten juveniles and infants. Their home range exceeded 20 km2; however, they usually ranged within a core area of c.5 km2 (Fig. 2a). Several chimpanzees in Bulindi have been caught in large steel “man-traps” set to protect crops or catch small game, and some probably died from their injuries (McLennan et al. 2012). However, all 21 individuals in the community at the time of the road accident were able-bodied, and none presented snare or trap injuries.
Map of Bulindi (Google Earth 7.1.5, 2015). a Outer yellow line shows the known home-range limits of the Bulindi chimpanzee community. Individuals may range outside this area occasionally; in particular, the extent of their range in hills to the east is unknown. Inner yellow polygon shows the most commonly used portion of the home range. Dark green areas indicate riverine forest, Cyperus papyrus swamp, and woodland; the surrounding matrix is dominated by farmland and village areas. b Detail showing the main Hoima–Masindi road where it dissects the chimpanzees’ home range; some minor roads are also shown. Stars on the main road indicate habitual chimpanzee crossing points, including the location of the collision. The small fragments of riverine forest and papyrus swamp visible on either side of the main road are used habitually by the chimpanzees
Extensive forest clearance for timber and farming has occurred in the past two decades in this region (McLennan and Hill 2015; Twongyirwe et al. 2015). In Bulindi, remaining forest occurs patchily on either side of a main road connecting Hoima and Masindi towns—two large urban centers with fast-growing human populations of c.100,000 persons each (UBOS 2014). This unpaved road is 12–15 m wide including shoulders (Fig. 3). It is frequently busy with motorized traffic (trucks, buses, mini-buses, taxis, cars, and motorbikes) in addition to cyclists and pedestrians, especially in morning hours (estimated at several vehicles per minute). Although the road is unpaved, vehicles travel at relatively high speeds (60–70 km/h), despite high numbers of pedestrian road-users.
Photograph of the Hoima–Masindi road showing the accident site. The arrows on either side of the road indicate a habitual chimpanzee crossing point. The star indicates where Olive’s body was found following impact with the taxi. The chimpanzees were crossing from right to left, while the taxi was approaching from the far side. Photograph by M. McLennan
Chimpanzees at Bulindi have reportedly crossed this road for generations. During some months they traverse it daily, depending on the spatiotemporal distribution of food resources (McLennan, unpubl. data) (Fig. 4). Undoubtedly, however, traffic intensity was much lower in the past compared to today. While road-crossing behavior has not been studied systematically at Bulindi, qualitative observations confirm the chimpanzees exhibit caution and vigilance as described at Bossou (Sakura 1994; Hockings et al. 2006; Hockings 2011) and Sebitoli (Cibot et al. 2015), for example by waiting at the roadside before crossing, scanning for pedestrians and vehicles, crossing quickly, and waiting for other party members. Adult males at times show “guarding” behavior while others cross (i.e., standing in a quadrupedal posture on the road for more than 5 s without moving; Hockings 2011) (Fig. 4).
Chimpanzees of the Bulindi community crossing the Hoima–Masindi road in March 2013. The female on the right is Olive; she is carrying an infant on her stomach while her juvenile son rides on her back. The adult male at center is showing “guarding” behavior by standing in the middle of the road while females and youngsters cross. Photograph by M. McLennan
We did not witness the accident directly. Instead, we use eyewitness accounts of two pedestrians, together with the first author’s (MM) field notes from the morning of the accident, to construct details of the collision. Information from the eye witnesses was gained through informal discussion at the scene within 1–2 h of the accident. Opportunistic observations of the chimpanzees, including behavioral indicators of sickness (e.g., coughing, sneezing, lethargy, injuries), were made in the month prior to the accident by MM and a long-term field assistant.
Health assessment
A field necropsy was performed onsite within 3 h of the collision by the second author (CA), a qualified veterinarian, with colleagues from Budongo Conservation Field Station (BCFS). The BCFS veterinary team is well-trained on biosafety and precautionary measures for handling animal carcasses to mitigate pathogen transfer to humans or other species. All procedures followed strict guidelines for chimpanzee health monitoring in Uganda, standardized by the Uganda Wildlife Authority veterinary unit, Jane Goodall Institute–Uganda veterinarians, and BCFS veterinarians; see also Leendertz et al. (2006) and PREDICT One Health Consortium (2013) for related protocols for performing primate necropsies under field conditions. The cadaver was subjected to an internal and external examination, and overall body condition was scored qualitatively on a 5-point scale corresponding to: very poor (index score = 1), poor (=2), fair (=3), good (=4), and fat (=5). Tissue samples c.10 mm thick were taken from liver, spleen, kidneys, gall bladder, heart, lungs, intestines (ileum, colon, and cecum), lymph nodes (mesenteric, gastric, and splenic), and brain, and fixed in 10 % neutral buffered formalin and submitted for histopathology at the Central Diagnostic Laboratory (CDL), College of Veterinary Medicine and Animal Resources, Makerere University, Uganda. The cadaver was buried immediately after examination, as was deemed appropriate following liaison with representatives of an organization involved in chimpanzee conservation regionally.
To assess gastrointestinal parasite infections, c.5 g feces was taken directly from the dead female’s jejunum and colon, preserved in 10 % formalin, and subjected to the modified Wisconsin Sugar Flotation (Cox and Todd 1962) and sedimentation (Gillespie 2006) methods at the BCFS laboratory. In the former method, formalin was decanted after centrifugation at 2000 rpm for 5 min. The sediment was then mixed with water and centrifuged again at 2000 rpm. Three grams were weighed out and subjected to the modified Wisconsin sugar floatation method. We assumed that 2 g were fecal material while 1 g was water, since not all the water is poured out as supernatant. Therefore, all eggs/cysts counted were assumed to be contained in 2 g of fecal material. Parasite eggs and cysts were viewed under a binocular microscope at ×10 and ×40 magnifications and identified to genus level according to their color, shape, content, and size. Direct counts were done to estimate the intensity for each parasite independently and the total was divided by two to give an egg/cyst count per gram (Cox and Todd 1962). Use of egg counts to assess infection intensity is discouraged by some authors since it is not a reliable index of adult worm burden (Gillespie 2006). Therefore, egg counts reported here should be considered a qualitative measure of intensity.
For comparison, we analyzed fecal samples from three adult multiparous females of the nearby Sonso community in Budongo Forest Reserve, 25 km north of Bulindi (see Fig. 1). These females [Kutu (KU), Melissa (ML), and Janie (JN)] were of similar age to the deceased female and in good body condition with no record of deformities. Fecal samples from these females were also collected in March 2015 immediately after defecation, preserved in 10 % formalin, and analyzed as described above. Molecular identification of gastrotintestinal parasites observed post-mortem was beyond the scope of the present study. However, DNA sequence analysis of nematode and cestode parasites infecting chimpanzees of the small Bulindi community has been performed in a separate study (Ota et al. 2015; Hasegawa and McLennan, unpublished data).
This research adhered fully to the legal requirements of Uganda and the research protocol of BCFS. It was approved by Uganda Wildlife Authority, National Forestry Authority, and Uganda National Council of Science and Technology. Additionally, it complies with ethical guidelines detailed by the Association for the Study of Animal Behavior (UK), and had full ethical approval of the Oxford Brookes University Research Ethics Committee.
Results
Circumstances of the accident
The chimpanzees spent the morning of 24 March 2015 in a stretch of Phoenix reclinata swamp forest north of the road (Fig. 2b), where MM and a field assistant left them at midday. Most community members (N = 17) were observed in the party. Shortly before 1500 we received news that a chimpanzee had been hit and killed by a vehicle on the road; the accident probably happened between 1430 and 1445. When MM arrived (c.1 h following the collision), a crowd of passers-by had gathered at the scene but the cadaver was apparently untouched. The dead chimpanzee was identified as Olive (OL), an adult female of the Bulindi community, estimated at 25–30 years old (Fig. 5). OL had four known offspring, of which three were alive at the time of her death: a subadult male (estimated age in 2015: 10–11 years); a juvenile male (estimated age: 6–7 years); an older female infant, which disappeared for unknown reasons in October 2014 aged 3 years; and a 4-month-old infant born in November 2014. In the month prior to her death, OL was observed in large mixed parties comprising most community members on 8 days, including 4 days before the accident. On five other days, identification of all party members could not be achieved owing to limited visibility (e.g., in dense swamp forest); however, on no occasion during this 4-week period was OL confirmed absent from large parties. On all occasions that she was observed, she behaved normally, feeding and traveling, with no indicators of ill health such as coughing, sneezing, obvious lethargy, or wounds.
The cadaver was lying inside the road at one of the chimpanzees’ habitual crossing points (Figs. 2b, 3), c.150 m from the swamp where they spent the morning. Two adult pedestrians, including a schoolteacher, claimed to have witnessed the collision from an estimated distance of c.75–100 m. They provided the following account. Chimpanzees were crossing the road from north to south and some had already crossed. OL, carrying an infant, had crossed or was in the process of crossing when she turned back and was struck by a taxi car, which was reportedly traveling fast and did not stop. A “small” chimpanzee had apparently waited behind at the roadside; the schoolteacher suggested OL was returning to collect this younger individual. Afterwards, chimpanzees reportedly spent several minutes at, or near, the roadside vocalizing. Individuals that had already crossed the road crossed back again and the party returned to the swamp on the northern side, from where a chimpanzee vocalized at 1710. At 1835 the chimpanzees vocalized from a forest patch c.250 m south of the road, having used an alternative crossing point to avoid people still gathered at the accident site.
OL’s 4-month-old infant was observed neither with the cadaver nor subsequently with other chimpanzees. Cases of young chimpanzees captured for sale and/or use in traditional medicine have occurred regionally (McLennan 2008). Therefore, the infant might have been taken by a person(s) before we arrived. However, no reports or rumors have surfaced to suggest that was the case. More likely, it was picked up by another chimpanzee at the roadside.
Health assessment
External examination
OL’s body condition was rated 4 (i.e., in good condition). She had a body weight of 40 kg with moderate subcutaneous body fat. By the time of necropsy, the cadaver was bleeding from the anus with a minor rectal prolapse. No external wounds, bruises, or fractures or limb deformities were noted. There was a depression on the dorsal lateral aspect of the cadaver, and the last two right ribs curved inwards slightly. The site of impact suggested OL was crossing the road in a south–north direction, consistent with the eyewitness account that she had turned back, having initially crossed from the northern side. There were minor bruises on the right oral mucosa, probably inflicted during the collision. Lymph nodes were not enlarged on palpation, suggesting cause of death was likely to have been acute.
A notable gross finding was a unilateral supernumerary nipple in the pectoral region (Fig. 6). The left mammary gland was of normal size and functional with signs of recent lactation while, on the right side, two nipples were noted. The upper was of normal size, with areola and mammary glands but no sign of recent lactation. About 8 cm below and medially located was a nonfunctional smaller nipple, with no areola and mammary gland (asymmetrical polythelia). There was evidence of recent suckling on both normal nipples, but not on the supernumerary one.
Olive had asymmetrical polythelia, a minor congenital malformation consisting of an extra nipple to the two normally appearing on the pectoral region. The condition was unilateral, with the supernumerary nipple located below the normal right one. Although she was nursing at the time of her death, only the left mammary gland showed signs of recent lactation. Unlike the supernumerary nipple, both normal nipples were recently suckled, as evidenced by their paleness. Photograph by M. McLennan
Internal examination
In a supine position, the cadaver was well hydrated, as evinced by ease in detachment of the skin from the abdominal musculature. There was a moderate amount of fat covering the mesentery. The abdominal and thoracic viscera were normal in their positions, sizes, texture, and consistency. When the cadaver was placed on the left position, the lateral right lobe was congested with blood. There were no obvious pathological lesions on the viscera when the cadaver was placed on the right lateral position. However, there was clotted blood in the atrial cavities, suggesting a ruptured (undetermined) blood vessel. On opening the digestive tract, there was evidence of digestive functionality, with food present throughout the tract—fruit and non-fruit fibrous plant material. Multiple nodular lesions (granulomas) were observed covering the distal end of the colon (Fig. 7a), consistent with infection by nodular worms Oesophagostomum spp. (cf. Krief et al. 2008; Terio et al. 2011).
a Colon with multifocal nodular lesions consistent with infection with nodular worms (Oesophagostomum spp.). Two nodules opened during field necropsy contained a brown pus-like substance, but larvae were not seen. b Eosinophilic infiltration of the mucosa and submucosa of the colon (H–E 10×). c Eosinophilic enteritis magnified (40×). d Eosinophilic infiltration in the hepatic triad causing biliary hyperplasia (H–E 10×). While eosinophilic enteritis and biliary hyperplasia are potentially attributable to gastrointestinal parasite infection, the pathogenic agent that would explain these pathological lesions could not be established in the present study (see “Discussion”). Photographs b–d by M. Afayoa
Gastrointestinal parasite assessment
Three pathogenic parasite taxa were isolated in the sample collected from OL at post-mortem, comprising a nematode (Strongyloides sp.), a protozoan (Balantidium sp.), and a cestode (probably Bertiella sp.); additionally, the commensal entodiniomorphid ciliate Troglodytella abrassarti was detected (Table 1). Similar parasite faunas were isolated in each of the three Sonso females from nearby Budongo, along with Oesophagostomum sp. Although this pathogenic nematode was not detected in OL’s sample post-mortem, it was isolated in a fecal sample collected from her 4 months earlier, in November 2014. Egg counts were overall similar or low in OL’s fecal sample as compared to samples from the Sonso females (Table 1).
Histopathological findings
There was infiltration of the eosinophilic cells and macrophages into the mucosa and submucosa of the intestines (eosinophilic enteritis) (Fig. 7b, c), and infiltration of macrophages, lymphocytes, and some eosinophils in the hepatic triad, leading to biliary hyperplasia (Fig. 7d). Unidentified worms with a cestode-like structure were observed in the lumen. In the spleen, hemorrhages and an increase in hemosiderin were observed—probably an impact of the collision. No pathological lesions were observed in other organs submitted to the laboratory.
Discussion
This is the first detailed report of a wild chimpanzee hit and killed by a vehicle (although a previous case occurred at Kibale National Park; Krief et al. 2008). Whether OL was born in Bulindi or immigrated into the community at adolescence is unknown. Regardless, she had certainly navigated the road safely on a regular basis throughout her adult life (Fig. 4). A physical examination of the cadaver indicated no underlying health conditions to help explain her failure to cross safely on this occasion. Parasitological assessment of OL’s feces demonstrated infection by several pathogenic parasites (Table 1). However, these taxa are commonly detected in parasitological surveys of wild chimpanzees (e.g., Huffman et al. 1997; Krief et al. 2005; Gillespie et al. 2010; Ebbert et al. 2015), and have been recorded previously in living chimpanzees from both Bulindi and Budongo (Huffman et al. 2009; McLennan and Huffman 2012; Zommers et al. 2013; BCFS, unpubl. data). Moreover, egg/cyst counts in OL’s feces were either comparable or low when compared to what was found in apparently healthy adult females of the nearby Sonso community during the same month. While Oesophagostomum eggs were not isolated in OL’s fecal sample post-mortem, they were detected in her feces 4 months previously. Moreover, intestinal nodular lesions (granulomas) consistent with infection by this nematode were noted during necropsy (cf. Krief et al. 2008; Terio et al. 2011). This might mean that the Oesophagostomum was not mature for egg production, or else eggs were simply undetected in this particular fecal sample. A previous molecular analysis of adult and larval Oesophagostomum from chimpanzee feces in Bulindi demonstrated infections by two species of nodular worm, O. stephanostomum and O. bifurcum (Ota et al. 2015). A high prevalence of Oesophagostomum sp. (predominantly O. stephanostomum) is common in wild chimpanzee populations (e.g., Gillespie et al. 2010), and infected individuals may develop nodular oesophagostomiasis in the absence of severe clinical signs of the disease, i.e., weight loss and diarrhea (Krief et al. 2008; Terio et al. 2011). Krief et al. (2008) also noted numerous intestinal nodules consistent with multinodular oesophagostomiasis in the apparently healthy individual killed on the road at Sebitoli.
Histopathology revealed OL had eosinophilic enteritis and biliary hyperplasia, both potentially caused by gastrointestinal parasite infection. However, the eosinophilia was not widely spread into the submucosa. Eosinophil cells reside in the lamina propria of the stomach and intestines, and their release may be stimulated by allergic reactions and parasitic infections, although many cases are reportedly idiopathic (Triantafillidis et al. 2002). Thus, their presence in the intestinal mucosa and partially in the submucosa and hepatic triad was not altogether surprising. Biliary hyperplasia associated with chronic irritation of the bile ducts is potentially associated with parasitic infestation, commonly trematodes (i.e., flukes); however, flukes were not observed in the bile ducts (Afayoa, pers. comm.). While numerous cestode-like worms were observed in the lumen, unfortunately these could not be identified on the basis of morphology. Cestode eggs resembling Bertiella sp. were isolated in OL’s feces at necropsy. While Bertiella infections are common in wild chimpanzees in Uganda (Wrangham 1995; Huffman et al. 2009; McLennan and Huffman 2012), recognizable clinical signs of infection have not been reported, suggesting tapeworm infections are usually tolerated. Overall, these findings suggest that parasite infection or some other underlying health condition was probably not a factor in OL’s death; rather, cause of death was most likely acute, caused by shock from the collision.
Visibility on the Hoima–Masindi road is generally lower during the main dry season (December to early March) compared to wet months because of the thick dust clouds stirred up by passing vehicles (Fig. 8). By late March, when the accident occurred, the rainy season in Hoima District has normally started, but in 2015 it was still dry. Moreover, c.50 m from the accident site—in the direction from which the taxi was approaching—the road curves (see Fig. 3). Therefore, low visibility could potentially have caused OL to fail to see the approaching vehicle in time. The schoolteacher’s eyewitness account suggests she crossed the road, but then turned back, which might indicate she panicked upon seeing the taxi and/or feared for the safety of a younger individual (potentially her juvenile offspring) which had reportedly waited behind, and went to deter it from crossing.
While highly undesirable, OL’s death presented a rare opportunity to assess the physical condition of a wild chimpanzee surviving in exceptionally degraded habitat. OL’s overall body condition was good and she was well fed when she died. At 40 kg, she was heavier than is usual for wild adult females of the East African subspecies (P. t. schweinfurthii), which average 30–35 kg (Rahm 1967; Uehara and Nishida 1987; Pusey et al. 2005). OL was not the largest adult female at Bulindi, and at least two females are likely heavier. Body mass of great apes is expected to be reduced in degraded versus less disturbed habitats because of low food availability, greater parasite loads, and/or heightened stress (Rayadin and Spehar 2015). As noted above, however, chimpanzees in Bulindi and elsewhere in this region supplement a natural diet with agricultural crops (Reynolds et al. 2003; McLennan 2008, 2013), as do chimpanzees in human-impacted habitats across tropical Africa (Hockings and McLennan 2012; McLennan and Hockings 2014). Since agricultural foods are often nutritionally dense, apes that forage frequently on crops (e.g., sugary fruits) might experience improved physical condition as a result. Notably, Eley et al. (1989) reported overall better body condition in olive baboons Papio anubis that fed on human crops and garbage compared to those that did not.
Also notable was OL’s asymmetrical polythelia (Fig. 6), a rarely reported congenital malformation in chimpanzees (Coolidge 1933) among other primates (e.g., Zuckerman 1935; Speert 1942; Coolidge 1943; Hsu et al. 2006). The etiology of polythelia is poorly understood. In humans, the condition has been linked anecdotally to multiple births and increased fertility, probably erroneously (Grossl 2000). Still, in Formosan macaques (Macaca cyclopis), polythelia was associated with a high incidence of twinning (Hsu et al. 2000, 2006). The estimated ages of OL’s offspring suggest she experienced relatively short inter-birth intervals of 3–4 years (inter-birth intervals in wild chimpanzees average >5 years; Emery-Thompson et al. 2007). Whether OL’s high prolificacy and polythelia were related is unknown. Hsu et al. (2000) suggested that isolation and inbreeding might be a potential cause of high rates of polythelia in one population of Formosan macaques. It remains to be established if the Bulindi chimpanzees have become isolated from other “fragment communities” regionally. However, recent widespread deforestation, agricultural intensification, and infrastructural development (see McLennan and Hill 2015) inevitably have increased the challenges to migrating females in this human-dominated landscape.
Conclusions and recommendations
This study confirms that roads may pose a significant mortality risk to chimpanzees in anthropogenic environments, further to other known human-induced causes of mortalities such as hunting (including retaliatory killings), snares, and traps. Like other great apes, chimpanzees have slow reproductive rates and life histories, making them vulnerable to such anthropogenic pressures (Hockings et al. 2015). While chimpanzee road mortalities appear uncommon, the loss of a prime-aged breeding female weakens the viability of small threatened populations such as those in Bulindi and surrounding areas (McLennan 2008; McCarthy et al. 2015). By contrast, the human populations of Hoima and Masindi districts are growing rapidly; in the towns, the per annum growth rate between 2002 and 2014 censuses was 10.7 and 10.1 %, respectively (UBOS 2014). Population growth coupled with economic development and migration have given rise to increasing motorized traffic in western Uganda. Additionally, with the development of petroleum production in adjacent Lake Albert, major transport routes, including the Hoima–Masindi road, are currently being widened and upgraded to asphalt (OIU 2013), enabling motorists to travel at higher speeds. As a cautionary illustration, roadkills of endangered Zanzibar red colobus increased following the paving of a major road (Struhsaker and Siex 1996).
Inevitably, these developments will increase the danger of roads to chimpanzees and other wildlife regionally (Epps et al. 2015). Recently, Cibot et al. (2015) called for measures to mitigate the risk to chimpanzees of a major paved road which passes through the Sebitoli area of Kibale National Park (c.180 km southwest of Hoima town). Proposed measures include bridges, underpasses, reduced speed limits, speed bumps, signage, and/or police controls, which have variously proved effective in reducing primate road mortalities (e.g., Valladares-Padua et al. 1995; Kanga and Heidi 1999/2000; Teixeira et al. 2013). While a combination of approaches would undoubtedly have greatest impact, we suggest that installation of simple speed bumps would be effective in reducing the danger to chimpanzees from motor traffic where roads pass through wildlife habitats. We doubt many drivers would observe reduced speed limits and signage alone without significant police enforcement. Compared to smaller-bodied arboreal primates, bridges may be less effective for a large-bodied semi-terrestrial ape. Moreover, constructing bridges or underpasses is likely to be costly relative to speed bumps, which are already common on roads throughout Uganda. Speed bumps installed at known chimpanzee crossing points would additionally benefit local people by making roads safer for human pedestrians.
References
Anderson JR (1986) Encounters between domestic dogs and free-ranging non-human primates. Appl Anim Behav Sci 15:71–86
Anderson J, Rowcliffe JM, Cowlishaw G (2007) Does the matrix matter? A forest primate in a complex agricultural landscape. Biol Conserv 135:212–222
Auricchio P, Catenacci LS, Santos KR, Britto FB (2014) A protocol for the use of roadkill or stranded animals as material for research and teaching. Sitientibus série Ciências Biológicas 14:10.13102/scb237
Cáceres NC, Hannibal W, Freitas DR, Silva EL, Roman C, Casella J (2010) Mammal occurrence and roadkill in two adjacent ecoregions (Atlantic Forest and Cerrado) in south-western Brazil. Zoologia 27:709–717
Cibot M, Bortolamiol S, Seguya A, Krief S (2015) Chimpanzees facing a dangerous situation: a high-traffic asphalted road in the Sebitoli area of Kibale National Park, Uganda. Am J Primatol 77:890–900
Coolidge HJ (1933) Symmetrical supernumerary mammae in a chimpanzee. J Mammal 14:66–67
Coolidge HJ (1943) Three new cases of an accessory nipple in anthropoid apes. J Mammal 24:353–356
Cox DD, Todd AC (1962) Survey of gastrointestinal parasitism in Wisconsin dairy cattle. J Am Vet Med Ass 141:706–709
Drews C (1995) Roadkills of animals by public traffic in Mikumi National Park, Tanzania, with notes on baboon mortality. Afr J Ecol 33:89–100
Ebbert MA, McGrew WC, Marchant LF (2015) Differences between chimpanzee and baboon gastrointestinal parasite communities. Parasitology 142:958–967
Eley RM, Strum SC, Muchemi G, Reid GDF (1989) Nutrition, body condition, activity patterns, and parasitism of free-ranging troops of olive baboons (Papio anubis) in Kenya. Am J Primatol 18:209–219
Emery-Thompson M, Jones JH, Pusey AE, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y, Wrangham RW (2007) Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol 17:2150–2156
Epps CW, Nowak K, Mutayoba B (2015) Unfenced reserves, unparalleled biodiversity and a rapidly changing landscape: roadways and wildlife in East Africa. In: van der Ree R, Smith DJ, Grilo C (eds) Handbook of road ecology. Wiley, New York, pp 448–454
Estrada A, Raboy BE, Oliveira LC (2012) Agroecosystems and primate conservation in the tropics: a review. Am J Primatol 74:696–711
Gillespie TR (2006) Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. Int J Primatol 27:1129–1143
Gillespie TR, Lonsdorf EV, Canfield EP, Meyer DJ, Nadler Y, Raphael J, Pusey AE, Pond J, Pauley J, Mlengeya T, Travis DA (2010) Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. Am J Phys Anthropol 143:534–544
Goldberg TL, Gillespie TR, Rwego IB (2008) Health and disease in the people, primates, and domestic animals of Kibale National Park: implications for conservation. In: Wrangham R, Ross E (eds) Science and conservation in African forests: the benefits of long-term research. Cambridge University Press, Cambridge, pp 75–87
Grossl NA (2000) Supernumerary breast tissue: historical perspectives and clinical features. South Med J 93:29–32
Healy A, Nijman V (2014) Pets and pests: vervet monkey intake at a specialist South African rehabilitation centre. Anim Welf 23:353–360
Hicks TC, Roessingh P, Menken SB (2012) Reactions of Bili-Uele chimpanzees to humans in relation to their distance from roads and villages. Am J Primatol 74:721–733
Hobday AJ, Minstrell ML (2008) Distribution and abundance of roadkill on Tasmanian highways: human management options. Wildl Res 35:712–726
Hockings KJ (2011) Behavioral flexibility and division of roles in chimpanzee road-crossing. In: Matsuzawa T, Humle T, Sugiyama Y (eds) The chimpanzees of Bossou and Nimba. Springer, Tokyo, pp 221–229
Hockings KJ, McLennan MR (2012) From forest to farm: systematic review of cultivar feeding by chimpanzees—management implications for wildlife in anthropogenic landscapes. PLoS One 7:e33391
Hockings KJ, Sousa C (2013) Human–chimpanzee sympatry and interactions in Cantanhez National Park, Guinea-Bissau: current research and future directions. Primate Conserv 26:57–65
Hockings KJ, Anderson JR, Matsuzawa T (2006) Road crossing in chimpanzees: a risky business. Curr Biol 16:R668–R670
Hockings KJ, McLennan MR, Carvalho S, Ancrenaz M, Bobe R, Byrne RW, Dunbar RIM, Matsuzawa T, McGrew WC, Williamson EA, Wilson ML, Wood B, Wrangham RW, Hill CM (2015) Apes in the Anthropocene: flexibility and survival. Trends Ecol Evol 30:215–222
Hsu MJ, Moore J, Lin JF, Agoramoorthy G (2000) High incidence of supernumerary nipples and twins in Formosan macaques (Macaca cyclopis) at Mt. Longevity, Taiwan. Am J Primatol 52:199–205
Hsu M, Lin J, Lin T, Agoramoorthy G (2006) Primate polythelia puzzle. Curr Sci 90:275–276
Huffman MA, Gotoh S, Turner LA, Hamai M, Yoshida K (1997) Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates 38:111–125
Huffman MA, Pebsworth P, Bakuneeta C, Gotoh S, Bardi M (2009) Chimpanzee–parasite ecology at Budongo Forest (Uganda) and the Mahale Mountains (Tanzania): influence of climatic differences on self-medicative behavior. In: Huffman MA, Chapman CA (eds) Primate parasite ecology: the dynamics and study of host–parasite relationships. Cambridge University Press, Cambridge, pp 331–350
IUCN (2014) The IUCN red list of threatened species, version 2014.2. http://www.iucnredlist.org
Kanga EM, Heidi CM (1999/2000) Survey of the Angolan black-and-white colobus monkey (Colobus angolensis palliatus) in the Diani forests, Kenya. Afr Primates 4:50–54
Kioko J, Kiffner C, Jenkins N, Collinson WJ (2015) Wildlife roadkill patterns on a major highway in northern Tanzania. Afr Zool 50:17–22
Krief S, Huffman MA, Sévenet T, Guillot J, Bories C, Hladik CM, Wrangham RW (2005) Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. Int J Primatol 26:467–490
Krief S, Jamart A, Mahé S, Leendertz FH, Mätz-Rensing K, Crespeau F, Bain O, Guillot J (2008) Clinical and pathologic manifestation of oesophagostomosis in African great apes: does self-medication in wild apes influence disease progression? J Med Primatol 37:188–195
Kumar V, Kumar V (2015) Seasonal electrocution fatalities in free-range rhesus macaques (Macaca mulatta) of Shivalik hills area in northern India. J Med Primatol 44:137–142
Kumara HN, Sharma AK, Kumar A, Singh M (2000) Roadkills of wild fauna in Indira Gandhi Wildlife Sanctuary, Western Ghats, India: implications for management. Biosphere Conserv 3:41–47
Laurance WF, Croes BM, Tchignoumba L, Lahm SA, Alsonso A, Lee ME, Campbell P, Ondzeano C (2006) Impacts of roads and hunting on central African rainforest mammals. Conserv Biol 20:1251–1261
Leendertz FH, Pauli G, Maetz-Rensing K, Boardman W, Nunn C, Ellerbrok H, Jensen SA, Junglen S, Boesch C (2006) Pathogens as drivers of population declines: the importance of systematic monitoring in great apes and other threatened mammals. Biol Conserv 131:325–337
Lode T (2000) Effect of a motorway on mortality and isolation of wildlife populations. Ambio 29:163–166
Lokschin LX, Printes RC, Cabral JNH, Buss G (2007) Power lines and howler monkey conservation in Porto Alegre, Rio Grande do Sul, Brazil. Neotrop Primates 14:76–80
Maibeche Y, Moali A, Yahi N, Menard N (2015) Is diet flexibility an adaptive life trait for relictual and peri-urban populations of the endangered primate Macaca sylvanus? PLoS One 10:e0118596
McCarthy MS, Lester JD, Howe EJ, Arandjelovic M, Stanford CB, Vigilant L (2015) Genetic censusing identifies an unexpectedly sizeable population of an endangered large mammal in a fragmented forest landscape. BMC Ecol 15:21
McLennan MR (2008) Beleaguered chimpanzees in the agricultural district of Hoima, western Uganda. Primate Conserv 23:45–54
McLennan MR (2013) Diet and feeding ecology of chimpanzees (Pan troglodytes) in Bulindi, Uganda: foraging strategies at the forest–farm interface. Int J Primatol 34:585–614
McLennan MR, Hill CM (2010) Chimpanzee responses to researchers in a disturbed forest–farm mosaic at Bulindi, western Uganda. Am J Primatol 72:907–918
McLennan MR, Hill CM (2015) Changing agricultural practices and human–chimpanzee interactions: tobacco and sugarcane farming in and around Bulindi, Uganda. In: Arcus Foundation (ed) State of the apes. Volume II: Industrial agriculture and ape conservation. Cambridge University Press, Cambridge, pp 29–31
McLennan MR, Hockings KJ (2014) Wild chimpanzees show group differences in selection of agricultural crops. Sci Rep 4:5956
McLennan MR, Huffman MA (2012) High frequency of leaf swallowing and its relationship to intestinal parasite expulsion in “village” chimpanzees at Bulindi, Uganda. Am J Primatol 74:642–650
McLennan MR, Hyeroba D, Asiimwe C, Reynolds V, Wallis J (2012) Chimpanzees in mantraps: lethal crop protection and conservation in Uganda. Oryx 46:598–603
OIU (2013) Oil sparks road upgrade. Oil in Uganda. http://www.oilinuganda.org/features/infrastructure/oil-sparks-off-roads-upgrade.html. Accessed 5 June 2015
Ota N, Hasegawa H, McLennan MR, Kooriyama T, Sato H, Pebsworth PA, Huffman MA (2015) Molecular identification of Oesophagostomum spp. from ‘village’ chimpanzees in Uganda and their phylogenetic relationship with those of other primates. R Soc Open Sci 2:150471
Pragatheesh A (2011) Effect of human feeding on the road mortality of rhesus macaques on National Highway-7 routed along Pench Tiger Reserve, Madhya Pradesh, India. J Threat Taxa 3:1656–1662
PREDICT One Health Consortium (2013) Protocol for primate sampling methods. http://www.vetmed.ucdavis.edu/ohi/predict/PREDICT_Publications.cfm#Protocols. Accessed 23 Dec 2015
Pusey AE, Oehlert GW, Williams JM, Goodall J (2005) Influence of ecological and social factors on body mass of wild chimpanzees. Int J Primatol 26:3–31
Rahm U (1967) Observations during chimpanzee captures in the Congo. In: Starck D, Schneider R, Kuhn HJ (eds) Neue ergebnisse der primatologie. Gustav Fischer Verlag, Stuttgart, pp 195–207
Rayadin Y, Spehar SN (2015) Body mass of wild Bornean orangutans living in human-dominated landscapes: implications for understanding their ecology and conservation. Am J Phys Anthropol 157:339–346
Reynolds V, Wallis J, Kyamanywa R (2003) Fragments, sugar, and chimpanzees in Masindi District, western Uganda. In: Marsh LK (ed) Primates in fragments: ecology and conservation. Springer, New York, pp 309–320
Richini-Pereira VB, Bosco SMG, Theodoro RC, Barrozo L, Bagagli E (2010) Road-killed wild animals: a preservation problem useful for eco-epidemiological studies of pathogens. J Venom Anim Toxin Trop Dis 16:607–613
Rodrigues NN, Martinez RA (2014) Wildlife in our backyard: interactions between Wied’s marmoset Callithrix kuhlii (Primates: Callithrichidae) and residents of Ilhéus, Bahia, Brazil. Wildl Biol 20:91–96
Sakura O (1994) Factors affecting party size and composition of chimpanzees (Pan troglodytes verus) Bossou, Guinea. Int J Primatol 15:167–183
Shepard DB, Kuhns AR, Dreslik MJ, Phillips CA (2008) Roads as barriers to animal movement in fragmented landscapes. Anim Conserv 11:288–296
Speert H (1942) Supernumerary mammae, with special reference to the rhesus monkey. Q Rev Biol 17:59–68
Struhsaker TT, Siex KS (1996) The Zanzibar red colobus monkey, Procolobus kirkii: conservation status of an endangered island endemic. Afr Primates 2:54–61
Teixeira FZ, Printes RC, Fagundes JCG, Alonso AC, Kindel A (2013) Canopy bridges as road overpasses for wildlife in urban fragmented landscapes. Biota Neotrop 13:117–123
Terio KA, Kinsel MJ, Raphael J, Mlengeya T, Lipende I, Kirchhoff CA, Gilagiza B, Wilson ML, Kamenya S, Estes JD, Keele BF, Rudicell RS, Liu W, Patton S, Collins A, Hahn BH, Travis DA, Lonsdorf EV (2011) Pathologic lesions in chimpanzees (Pan troglodytes schweinfurthii) from Gombe National Park, Tanzania, 2004–2010. J Zoo Wildl Med 42:597–607
Triantafillidis JK, Parasi A, Cherakakis P, Sklavaina M (2002) Eosinophilic gastroenteritis: current aspects on etiology, pathogenesis, diagnosis and treatment. Ann Gastroenterol 15:106–115
Trombulak SC, Frissell CA (2000) Review of ecological effects of roads on terrestrial and aquatic communities. Conserv Biol 14:18–30
Twongyirwe R, Bithell M, Richards KS, Rees WG (2015) Three decades of forest cover change in Uganda’s northern Albertine Rift landscape. Land Use Pol 49:236–251
UBOS (2014) National population and housing census 2014: provisional results. Uganda Bureau of Statistics, Kampala
Uehara S, Nishida T (1987) Body weights of wild chimpanzees (Pan troglodytes schweinfurthii) of the Mahale Mountains National Park, Tanzania. Am J Phys Anthropol 72:315–321
Valladares-Padua C, Cullen L Jr, Padua S (1995) A pole bridge to avoid primate roadkills. Neotrop Primates 3:13–15
Wrangham RW (1995) Relationship of chimpanzee leaf-swallowing to a tapeworm infection. Am J Primatol 37:297–303
Zommers Z, Macdonald DW, Johnson PJ, Gillespie TR (2013) Impact of human activities on chimpanzee ground use and parasitism (Pan troglodytes). Conserv Lett 6:264–273
Zuckerman S (1935) Supernumerary nipples in monkeys. J Mammal 16:229–230
Acknowledgments
For assistance on the day of the accident and necropsy, we thank Geoffrey Muhanguzi, Tom Sabiiti, Gerald Majanda, Eric Okwir, Pawel Fedurek, Thibaud Gruber, Jess Hartel, and the Kibale Snare Removal Project team (Mugisha Paul, Okwilo John, Nyesiga Godfrey, Tweheyo John, Friday Charles, and Twinamasiko Amos), the BCFS snare removal team (Ofeni Azima, Dominic Andi, Lemi Moses, Seteson Ocukuru, Julius Adriko, and Emmanuel Wambe), local field staff of the Chimpanzee Sanctuary and Wildlife Conservation Trust, and local people of the Bulindi and Kibugubya parishes. We are grateful for the laboratory work by Mathias Afayoa (CDL) and Paul Zziwa, Timothy Mugabe and Jacob Aliyo, Andrew Wange and Walter Akankwasa (BCFS). We are grateful to the President’s Office, the Uganda National Council for Science and Technology, and Uganda Wildlife Authority for permission to study the chimpanzees of Bulindi. MM’s fieldwork was supported by the Leverhulme Trust. We further thank the Arcus Foundation for supporting the Chimpanzee Health Monitoring Programme, Oakland Zoo for supporting the BCFS snare removal team, and the Royal Zoological Society of Scotland for core funding to BCFS. Comments from Kim Hockings, Sabrina Krief, and an anonymous reviewer helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
McLennan, M.R., Asiimwe, C. Cars kill chimpanzees: case report of a wild chimpanzee killed on a road at Bulindi, Uganda. Primates 57, 377–388 (2016). https://doi.org/10.1007/s10329-016-0528-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-016-0528-0