Abstract
Predation pressure affects most aspects of primate behaviour, and is especially pronounced in the context of the use of sleeping sites, given the vulnerability of the animal at this time. Most small-bodied platyrrhines have highly systematic patterns of sleeping site choice and use. This study analyses the use of sleeping sites by a free-ranging group of titis (Callicebus coimbrai) monitored at a site in Sergipe, Brazil, between July, 2009 and June, 2010. When the subjects approached a sleeping tree their behaviour was typically cautious, including slow and silent movement, early retirement (20–162 min before sunset on 52 dry afternoons), and sleeping in a tight huddle with their tails entwined. Despite this behaviour, which has an obvious anti-predator function, the group slept in only three different trees during the course of the study, and returned to the same tree used on the previous night on a quarter of evenings (n = 56). This was despite the availability within the group’s home range of a large number of trees with similar structural characteristics (i.e. tall, open crown in the upper canopy). Surprisingly, the three trees were all members of the same species, Licania littoralis (Chrysobalanaceae). The choice of this species, which was not an important source of dietary resources, and the repeated use of a small number of sites, did not seem to be related to factors such as ranging or foraging patterns, but may have a been a response to the specific threat from capuchins, Cebus xanthosternos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arboreal primates typically spend at least half their lives at nocturnal roosts, and selection of appropriate sites seems an important aspect of individual survival (Anderson 1984). Predation pressure is probably the primary factor determining site choice and use (Ferrari and Lopes 1990; Cowlishaw 1994; Anderson 1998; Di Bitetti et al. 2000). Most sleeping sites are found in relatively inaccessible locations, for example emergents, vine tangles, or tree holes, and their use may be characterised by relatively cryptic behaviour and systematic visits to alternative sites on successive days (Ferrari and Lopes 1990; Heymann 1995). Such behaviour may be especially marked in the small-bodied platyrrhines, for example callitrichids and titis (Callicebus spp.), which are vulnerable to a wide range of potential predators (Lawrence 2003; Sampaio and Ferrari 2005; Cisneros-Heredia et al. 2005; Ferrari 2009; de Luna et al. 2010).
A number of factors may determine the use of sleeping sites, for example the availability of appropriate locations, and the ranging and foraging behaviour of the primate (Hamilton 1982; Chapman et al. 1989; Cowlishaw 1994; Anderson 1984, 1998). Although some patterns are apparent from these results, many unanswered questions remain (Fruth and McGrew 1998; Kappeler 1998), and it is clear that full understanding of the phenomenon and the selective pressures that have moulded behaviour patterns will only be possible through compilation of observations from as wide a number of species and study sites as possible.
Little is known of the roosting behaviour of the titis, a diverse group of platyrrhines widely distributed in tropical South America, although Kinzey (1978), Kinzey et al. (1977), Kinzey and Becker (1983), Neri (1997) and Heiduck (2002) provide some information on sleeping sites and their use. These studies indicate patterns typical of small-bodied platyrrhines, in terms of site choice and use, for example selection of sites with relatively dense vegetation, huddling, and extremely cautious behaviour. This study focuses on the use of sleeping sites by a free-ranging group of Callicebus coimbrai. Although much of the animals’ behaviour was typical of that of other small monkeys, some aspects were unusual. Possible explanations of the discrepancies are discussed.
Methods
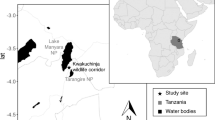
The study was conducted at the Fazenda Trapsa (11º12′S, 37º14′W) in the municipality of Itaporanga d’Ajuda in the northeastern Brazilian state of Sergipe, where a group of Coimbra-Filho’s titis (Callicebus coimbrai) was monitored in an isolated 14.4 hectare fragment of Atlantic Forest (Souza-Alves and Ferrari 2010). The fragment is typical of the forest reserve at the site, with a mosaic of habitats, including a large portion in which the undergrowth was burned off in 2008, a year before this study period, when this area was already regenerating successfully. The forest has been classified as an arboreal restinga (cf. Scarano 2002), which is characterised by a relatively low canopy, with trees rarely exceeding 15 m in height (Chagas and Ferrari 2010).
The dry season in the study area typically encompasses the period of the austral summer, between September and March. Total precipitation recorded during the 12 months of this study period (July, 2009 to June, 2010) was almost 50% higher than the 10-year average (2223 mm vs. a mean of 1482.4 mm between 1998 and 2007), with an atypical peak in February.
The site was prepared with a standard 50 m × 50 m trail grid covering the home range of the study group. Behavioural monitoring involved following for complete days, with observation beginning before the animals left the sleeping tree in the morning and until 30 min after they retired to a tree at the end of the daily activity period. At the beginning of the study period, in July, 2009, the group was composed of an adult pair, a subadult male, and a juvenile (already independent at the beginning of the study period). However, the behaviour of the subadult became increasingly peripheral during the course of the study. This subadult was absent in May, 2010, and presumably emigrated in the interim.
Quantitative behavioural data were collected in scan samples (1-min scans at 5-min intervals) and behaviour sampling, following standard procedures for the study of titis (Palacios et al. 1997; Heiduck 2002). In the scan samples, activity states were divided into five basic categories (move, forage, feed, rest, miscellaneous); behaviour sampling recorded all social and interspecific interactions, and feeding events involving items not recorded during scan sampling. With the exception of August and September, 2009 (4 days), the group was monitored on 5 days per month, resulting in a total sample of 58 days, and 72 recorded sleeping sites. All data collection was non-invasive and satisfied the legal requirements of the Brazilian Environment Institute (IBAMA).
At the end of each day, the site to which the group members retired for the night was recorded and marked. Any specific behaviour patterns relevant to understanding of the use of the site—e.g., mode of locomotion, choice of branch—were recorded ad libitum (Altmann 1974). The sites were plotted on a map of the study area, according to their position in relation to the trail grid.
The availability of suitable sleeping trees was evaluated using the approach of Di Bitetti et al. (2000). The first step was to classify the habitats available within the study group’s home range according to the scheme of Chagas and Ferrari (2010), in which three main categories were recognised: mature forest (continuous canopy 12–15 m tall), secondary forest (continuous canopy 8–10 m tall), and disturbed forest (discontinuous canopy 5–10 m tall). Subsequently, four 50 × 50 m plots representing each habitat category were chosen randomly within the trail grid (using the random number function of a pocket calculator), resulting in a sample of 1 hectare per habitat type. Within each plot, all trees with a diameter at breast height (DBH) of at least 25 cm (which is slightly smaller than the DBH of the smallest sleeping tree) were identified and measured. In addition to the DBH, which was measured with a surveyor’s tape, the height and crown diameter of each tree was estimated qualitatively, although these data are not presented here.
Results
General behaviour patterns
Only three different sleeping sites were used during the course of the study (Table 1), and the most used tree was chosen more than twice as frequently as the least visited. All three trees had relatively open crowns and dense foliage, but no lianas. Although relatively small in stature in absolute terms, the crowns of all three trees were located in the upper canopy. The frequency of use correlates with tree size, although perhaps the most remarkable aspect of site choice is the exclusive use of a single species, Licania littoralis (Chrysobalanaceae). This is despite the fact that many other appropriate trees belonging to other species, e.g., Tapirira guianensis, Parkia pendula, and Inga sp., were available within the group’s home range (see below).
The species was not an important source of food for the titis, although tree 2 did produce fruit during the study period, and some of these fruits were consumed by group members on one morning in August, after spending the night in this tree. They also returned to the tree to feed later in the day, but the species accounted for only 10.4% of feeding records in this month, and did not contribute to the group’s diet in any other month.
The three trees were evenly spaced within the group’s home range (Fig. 1), in the better-preserved western portion of the area. The smallest tree (#3) was visited half as often as the two larger trees, although it is not clear whether or to what extent its location may have affected the frequency of use. Trees 1 and 2 were both relatively close to the locations of the group’s main fruit-feeding trees throughout most of the study, whereas tree 3 was located in an area of highly disturbed forest, characterised by very dense undergrowth and few large trees.
Study group members would normally begin to retire to the night’s roost soon after the day’s last feeding bout. The final approach to the sleeping tree was characterised by slow, silent movements in single file, during which the animals apparently chose the route which would cause least disturbance, i.e., primarily horizontal branches, located close together. Progress was interrupted frequently, when the animals would remain silent and absolutely still for up to 5 min before continuing. This whole sequence of behaviour normally lasted 20–30 min.
Sites were reached well before dusk on some evenings (see below), and group members would typically engage in social interactions, for example grooming, before retiring for the night. The animals invariably roosted on horizontal branches positioned centrally within the tree crown, in dense foliage. Vine tangles—as observed by Kinzey (1978) for Callicebus moloch, and Neri (1997) for Callicebus personatus—were not used.
Group members slept in a tight huddle with their tails entwined (Fig. 2), with the juvenile invariably at the centre. Between July and November, the group always roosted on the same branches in trees 1 and 2, in the top of the crown, and always in the same position, facing east. In December, however, when both trees had lost some of their foliage, presumably as a result of hydrological stress during the course of the dry season, the group used branches slightly lower down in the crown, and slept facing west. The same branches were used throughout the rest of the study period. Use of specific locations within tree 3 was more variable.
The morning descent was sometimes delayed for up to an hour after sunrise, although on just over a third (36.8%) of mornings the tree was vacated before sunrise. On most occasions, the difference was only a few minutes, but on four mornings in February, March, and May, the sleeping tree was left 21–23 min before sunrise, i.e. at the beginning of the crepuscular period (but never in the dark). In contrast with the ascent, the animals normally left sleeping trees rapidly, but moved out independently, one at a time, rather than in a cohesive group.
Seasonal variation in the use of the sites
Although the group used a small number of sleeping trees in a somewhat homogeneous fashion throughout the study period, some variation was observed among months. Although tree 3 was not visited in July, August, and November, for example, it was used on half the nights in October, and 57.1% in May (Fig. 3). Tree 1 was the only site used by the group in every month, and tree 2 was overlooked in October.
Overall, tree 1 was the most frequently used during the wet season, with 50.0% of the total roosts (n = 36), followed by tree 2 (33.3%), and tree 3 (16.7%). In the dry season, by contrast, tree 2 was visited most frequently (44.4%; n = 36), followed by trees 1 (38.9%) and 3 (16.7%). There was no statistical difference between seasons in the relative use of different sites (χ² = 1.071, df = 2, p = 0.585).
This shift in preference is also reflected in visitation patterns. Whereas the group returned to the same tree used on the previous night on four occasions in each month between June and August (wet season), it almost invariably alternated between sites during the rest of the study, using the same tree on consecutive nights on two occasions only, in the dry season months of January and October. Although this implies a seasonal pattern (z = 5.246, p < 0.001) possibly related to shifts in foraging behaviour (e.g. July was the maximum month for fruit-feeding), the group rarely ranged more than a few hundred metres from the central portion of its home range at any time, so choice of one or other site on a given night may have been determined more by proximate or random factors than by foraging behaviour.
One other marked pattern in the use of sleeping sites was early retirement and, to a lesser extent, delayed departure in relation to sunset and sunrise times (obtained from Time and Date 2010), respectively. The group retired between 20 and 162 min before sunset on the 52 dry afternoons monitored, and between 74 and 174 min on the six rainy afternoons. By way of contrast, the site was left 3–23 min before sunrise on 22 days, including two rainy mornings. On the two other rainy mornings, the group left the sleeping tree 2 and 151 min after sunrise. On other dry mornings, the tree was left at 0–91 min after sunrise.
Considering the mean monthly intervals between sunrise and the onset of activity (Fig. 4), there was a marked shift to relatively early rising between January and May, coinciding with the late dry season. In February and March, this coincided with a tendency to late retirement, which resulted in an increase of over an hour in the mean activity period during these 2 months (11 h 35 min) in comparison with the remaining 10 months (10 h 29 min).
Mean monthly difference between the start (sunrise-first activity) and end (retirement-sunset) of the daily activity period of the C. coimbrai study group. Negative values (first activity only) refer to mornings on which the group left the tree because of dawn, on average. Rain-affected events were excluded
Availability of sleeping sites
The inventory of the sample plots returned 51 trees of DBH ≥25 cm in the mature forest, 51 in the secondary forest, and 36 in the disturbed habitat. Assuming that these values are representative of each habitat type, and given that mature and secondary forest cover an estimated 48.3% of the study group’s 14.4 ha home range, a conservative estimate of the total number of potential sleeping trees within the home range would be at least 500 trees, on the basis of size alone.
As tree 3 was used much less frequently than the others, this estimate might be further refined by applying a minimum DBH of 40 cm, rather than 25 cm, which is approximately the diameter of the smaller of the two other trees. In this case, the estimate would be reduced by just over half, considering that only 48.6% of the 138 trees recorded in the sample plots had a DBH of at least 40 cm.
These findings suggest that the study group selected trees on the basis of a criterion other than size. As mentioned above, the three sleeping trees also had no noticeably distinct characteristics in terms of variables such as crown density or the presence of lianas. The exclusive use of Licania littoralis certainly does not seem to be related to the abundance of the species at the site, given that only four (2.9%) of the 138 individuals identified in the sample plots belonged to this species, in contrast with 62 Tapirira guianensis (44.9%), for example. This species was one of the most important sources of plant foods for the study group, providing both fruit and leaves.
Discussion
While a relatively large number of potential sleeping sites were available within the 14.4 hectares of the study group’s home range, only three were used during the course of the study, and during some months the group returned to the site used on the previous night on a third of the days monitored. This seems to contradict patterns observed in titis (Kinzey et al. 1977; Kinzey and Becker 1983; Neri 1997) and other small-bodied platyrrhines (Ferrari and Lopes 1990; Heymann 1995; Smith et al. 2007), which tend to use a large number of sites and alternate frequently among them, so they are rarely used on consecutive nights. However, Heiduck (2002) reported the use of only six different sites by a group of Callicebus melanochir over a 12-month period in a home range twice the size of that of the C. coimbrai study group.
Because it is likely to reduce the probability of a predator locating a site, the alternating use of a large number of trees seems to be an important behavioural strategy (Heymann 1995; Zhang 1995; Di Bitetti et al. 2000), and it is unclear why the members of the study group did not adopt such a tactic, especially as other aspects of their behaviour were consistent with the antipredator strategies observed in other species. These strategies included slow and cautious approach to the sleeping tree, early retirement, and the relatively wary descent in the morning.
Repeated use of sleeping sites, apparently in response to relatively harsh winter or dry season conditions, has been recorded for a number of catarrhines (Raemaekers and Chivers 1980; Zhao 1999; Liu and Zhao 2004; Wang et al. 2011). In our study, in contrast, sleeping sites were most often repeated during the wet season months of June, July, and August, when preferred resources such as fruit were apparently most abundant (Souza-Alves 2010), although the small number of sites used overall meant that the same ones were used frequently in all months.
What is perhaps even more intriguing than the small number of sites, is the fact that the group used trees of only a single species. This seems to be a unique pattern, and assuming active selection of the species, it would at least partly account for the small number of sites recorded, i.e. because of the availability of suitable trees of this species, although no obvious reason could be discerned for the choice of the species in question. It may thus be a form of local social tradition, similar to those recorded in other platyrrhines (Tabacow et al. 2009).
Overall, then, although the behaviour patterns recorded in this study seem to reflect the evolutionary effect of predation pressures (Caine et al. 1992; Kappeler 1998; Miller and Treves 2007; Ferrari 2009), the repeated use of a small and very specific selection of the available sleeping sites seems contradictory. However, there is evidence that site selectivity may be moulded by specific predation pressures at some locations. For example, Duarte and Young (2010) concluded that the selection and use of sleeping sites by marmosets (Callithrix penicillata) in an urban park was determined by extreme pressure from a single predator, the domestic cat (Felis cattus). In this case, the marmosets used a relatively small selection of the available trees that were virtually inaccessible to cats (tall-crowned, with either smooth or thorny bark).
Many potential predators, for example large raptors, are absent from the Fazenda Trapsa, and the principal current threat to the titis seems to be the local capuchins (Cebus xanthosternos). Capuchins are known to prey on titis (Lawrence 2003; Cisneros-Heredia et al. 2005; Sampaio and Ferrari 2005), and were observed approaching the study group on two occasions (once in the early morning, when the animals were still in the sleeping tree), when they provoked incisive avoidance behaviour. In the light of Duarte and Young’s (2010) results, one possibility is that the study group members had foregone the potential advantages of using a variety of well-protected sites in favour of a small number of trees with specific characteristics advantageous to the avoidance of capuchins, e.g., relatively open crowns (Fig. 2), which enable efficient detection of approaching predators, and familiar escape routes. Obviously, a more definitive interpretation of any such strategy would require a more ample set of data on both the study group and the species, but, whatever the eventual conclusions, the results of this study reinforce the potential complexities of the use of sleeping sites by small-bodied platyrrhines.
References
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49:227–267
Anderson JR (1984) Ethology and ecology of sleep in monkeys and apes. In: Rosenblatt JS, Beer C, Busnel MC (eds) Advances in the study of behavior. Elsevier, Philadelphia, pp 165–229
Anderson JR (1998) Sleep, sleeping sites, and sleep-related activities: awakening to their significance. Am J Primatol 46:63–75
Caine NG, Potter MP, Mayer KE (1992) Sleeping site selection by captive tamarins (Saguinus labiatus). Ethology 90:63–71
Chagas RRD, Ferrari SF (2010) Habitat use by Callicebus coimbrai (Primates: Pitheciidae) and sympatric species in the fragmented landscape of the Atlantic Forest of southern Sergipe, Brazil. Zoologia 27:853–860
Chapman CA, Chapman LK, McLaughlin RL (1989) Multiple central place foraging by spider monkeys: travel consequences of using many sleeping sites. Oecologia 79:506–511
Cisneros-Heredia DF, Leon-Reyes A, Seger S (2005) Boa constrictor predation on a titi monkey, Callicebus discolor. Neotrop Primates 13:11–12
Cowlishaw G (1994) Vulnerability to predation in baboon populations. Behaviour 131:293–304
de Luna AG, Sanmiguel R, Di Fiore A, Fernandez-Duque E (2010) Predation and predation attempts on red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador. Folia Primatol 81:86–95
Di Bitetti MS, Vidal EML, Baldovino MC, Benesovsky V (2000) Sleeping site preferences in tufted capuchin monkeys (Cebus apella nigritus). Am J Primatol 50:257–274
Duarte MHL, Young RJ (2010) Sleeping site selection by urban marmosets (Callithrix penicillata) under conditions of exceptionally high predator density. Int J Primatol. doi:10.1007/s10764-010-9468-5
Ferrari SF (2009) Predation risk and antipredator strategies. In: Garber P, Estrada A, Bicca-Marques JC, Heymann E, Strier KB (eds) South American Primates—comparative perspectives in the study of behavior, ecology and conservation. Springer, New York, pp 251–277
Ferrari SF, Lopes MA (1990) Predator avoidance behaviour in the buffy-headed marmoset, Callithrix flaviceps. Primates 31:323–338
Fruth B, McGrew WC (1998) Resting and nesting in primates: behavioral ecology of inactivity. Am J Primatol 46:3–5
Hamilton WJI (1982) Baboon sleeping site preferences and relationships to primate grouping patterns. Am J Primatol 3:41–53
Heiduck S (2002) The use of disturbed and undisturbed forest by masked titi monkey Callicebus personatus melanochir is proportional to food availability. Orix 36:133–139
Heymann EW (1995) Sleeping habits of tamarins, Saguinus mystax and Saguinus fuscicollis (Mammalia; Primates; Callitrichidae), in north-eastern Peru. J Zool 237:211–226
Kappeler PM (1998) Nests, tree holes, and the evolution of primate life histories. Am J Primatol 46:7–33
Kinzey W (1978) Feeding behaviour and molar features in two species of titi monkey. In: Chivers DJ, Herbert J (eds) Recent advances in primatology. Academic Press, London, pp 373–385
Kinzey WG, Becker M (1983) Activity pattern of the masked titi monkey, Callicebus personatus. Primates 24(3):337–343
Kinzey WG, Rosenberger AL, Heisler PS, Prowse DL, Trilling JS (1977) A preliminary field investigation of the yellow handed titi monkey, Callicebus torquatus torquatus, in northern Peru. Primates 18:159–181
Lawrence JM (2003) Preliminary report on the natural history of brown titi monkeys (Callicebus brunneus) at the Los Amigos Research Station, Madre de Díos, Peru. Am J Physiol Anthropol. (Suppl 36):136
Liu H-Z, Zhao Q-K (2004) Sleeping site of Rhinopithecus bieti at Mt. Fuhe, Yunnan. Primates 45:241–248
Miller LE, Treves A (2007) Predation on primates. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK (eds) Primates in perspective. Oxford University Press, New York, pp 525–543
Neri FM (1997) Manejo de Callicebus personatus, Geoffroy 1812, resgatados: Uma tentativa de reintrodução e estudos ecológicos de um grupo silvestre na Reserva do Patrimônio Natural Galheiro—Minas Gerais. Dissertation, Belo Horizonte: Universidade Federal de Minas Gerais
Palacios E, Rodriguez A, Defler TR (1997) Diet of group of Callicebus torquatus lugens (Humboldt, 1812) during the annual resource bottleneck in Amazonian Colombia. Int J Primatol 18:503–522
Raemaekers JJ, Chivers DJ (1980) Socio-ecology of Malayan forest primates. In: Chivers DJ (ed) Malayan forest primates: ten years’ study in tropical rain forest. Plenum Press, New York, pp 279–316
Sampaio DT, Ferrari SF (2005) Predation of an infant titi monkey (Callicebus moloch) by a tufted capuchin (Cebus apella). Folia Primatol 76:113–115
Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic Rainforest. Ann Bot-London 90:517–524
Smith AC, Knogge C, Huck M, Löttker P, Buchanan-Smith HM, Heymann EW (2007) Long-term patterns of sleeping sites in wild saddleback (Saguinus fuscicollis) and mustached tamarins (S. mystax): effects of foraging, thermoregulation, predation, and resource defense constraints. Am J Phys Anthropol 134:340–353
Souza-Alves JP (2010) Ecologia alimentar de um grupo de Guigó-de-Coimbra-Filho (Callicebus coimbrai Kobayashi & Langguth, 1999): perspectivas para a conservação da espécie na paisagem fragmentada do sul de Sergipe. Dissertation, São Cristóvão: Universidade Federal de Sergipe. p 108
Souza-Alves JP, Ferrari SF (2010) Responses of wild titi monkeys, Callicebus coimbrai (Primates: Platyrrhini: Pitheciidae) to the habituation process. Zoologia 27:861–866
Tabacow FP, Mendes SL, Strier KB (2009) Spread of a terrestrial tradition in an arboreal primates. Am Anthropol 111:238–249
Time and Date (2010) [15:32:40] http://www.timeanddate.com/worldclock/sunrise.html
Wang S, Luo Y, Cui G (2011) Sleeping site selection of François’s langur (Trachypitecus francoisi) in two habitats Mayangue National Nature Reserve, Guizhou, China. Primates 52:51–60
Zhang S-Y (1995) Sleeping habits of brown capuchin monkeys (Cebus apella) in French Guiana. Am J Primatol 36:327–335
Zhao QK (1999) Responses to seasonal changes in nutrient quality and patchiness of food in a multigroup community of Tibetan macaques at Mt. Emei. Int J Primatol 20:511–524
Acknowledgments
This study was supported by the Deutscher Akademischer Austausch Dienst (DAAD), CNPq (processes no. 302747/2008-7 and 476064/2008-2), and the Fundação O Boticário de Proteção à Natureza (project # 0846_20092), and received logistic support from the Sergipe state environment secretariat (SEMARH) and the Brazilian National Primate Center (CPB-ICMBio). We would also like to thank Sr. Ary Ferreira, owner of Fazenda Trapsa, for supporting our research and José Elias “Bóia” and Renata Chagas for their assistance during fieldwork. We are also grateful to two anonymous reviewers for their helpful comments on the first version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Souza-Alves, J.P., Fontes, I.P. & Ferrari, S.F. Use of sleeping sites by a titi group (Callicebus coimbrai) in the Brazilian Atlantic Forest. Primates 52, 155–161 (2011). https://doi.org/10.1007/s10329-011-0235-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-011-0235-9