Abstract
Orangutans are the only great ape in Asia. Since orangutan densities vary between habitat types within regions and within similar habitat types among regions, it is important to determine areas with high densities for their protection. In this paper we show that orangutan density in old-growth dryland forests in the Leuser Ecosystem, Sumatra is significantly related to the density of large strangling figs and topsoil pH. In addition, large fig density depends on topsoil pH. Provided that orangutans are present and no hunting or logging occur, topsoil pH seems a promising method for rapid assessment of potential orangutan density over large areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orangutans (Pongo sp.) are the only great apes found in Asia. During the Pleistocene they occurred throughout Southeast Asia from Southern China in the north to Java in the south (von Koeningswald 1982; Jablonski et al. 2000; Bacon and Long 2001). At present, their distribution is restricted to the islands of Sumatra and Borneo (Rijksen and Meijaard 1999). Current population estimates for Borneo are around 23,500 individuals (Rijksen and Meijaard 1999) and for Sumatra around 3,500 individuals (Wich et al. 2003). Orangutans are very vulnerable to extinction (Leighton et al. 1995) due to large-scale habitat destruction and hunting for the pet trade (Rijksen and Meijaard 1999), their large body size (Harvey et al. 1987) and long mean interbirth interval (Galdikas and Wood 1990). Adding to their vulnerability are the facts that orangutans live at fairly low densities (ranging from near 0 to 7 per km2 for Sumatra; van Schaik et al. 1995, 2001), require large home ranges and therefore large forest blocks (Singleton and van Schaik 2001) and are mainly restricted to lowland rainforest areas (Rijksen and Meijaard 1999).
It has been estimated that with current rates of forest loss all undisturbed lowland forest in Sumatra will have disappeared by 2005 (Jepson et al. 2001). Because Sumatran orangutans are very sensitive to the disturbance caused by selective logging, and are obviously unable to survive in areas where forest has been converted to other forms of land use, the Sumatran orangutan is classified as critically endangered by the World Conservation Union (Hilton-Taylor and Brackett 2000).
Orangutans are frugivorous (Galdikas 1978; Rodman 1988; Leighton 1993), and fruit plays an important role in their sociality. First, despite their semi-solitary life style (Galdikas 1978; Rijksen 1978; Wich et al. 1999; van Schaik 1999), they occasionally form feeding aggregations in large fruits trees such as strangling figs (Ficus spp.), as well as travel parties during periods of generally high fruit abundance (Rijksen 1978; Sugardjito et al. 1987; Utami et al. 1997; van Schaik 1999). Second, orangutan densities at different locations have been linked to availability of soft-pulp fruit (Djojosudharmo and van Schaik 1992; van Schaik et al. 1995; Buij et al. 2002). The proportion of all trees that produce soft-pulp or arillate fruit in an area explains most of the density variation (van Schaik et al. 1995). Although it is well established that large strangling figs are an important food source for Sumatran orangutans (Sugardjito et al. 1987; Utami et al. 1997), orangutan density was not correlated significantly with large fig density in the sample used by van Schaik et al. (1995), largely because swamp forests contain virtually no strangling figs but do contain high orangutan densities. Large strangling fig density, however, was found to correlate well with the topsoil pH (van Schaik 1996).
The purpose of this paper is to re-examine the relationship between orangutan density and fig density for dryland forests in the Leuser Ecosystem and also to examine whether topsoil pH and/or fig densities can be used to make even more rapid assessments of orangutan densities than counting nests.
Methods
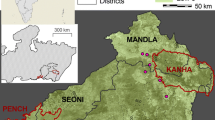
This study was conducted in the Leuser Ecosystem, Sumatra, Indonesia. The Leuser Ecosystem is a recently established conservation area of around 25,000 sq km that includes the Gunung Leuser National Park (for details on the area: van Schaik and Supriatna 1996; van Schaik et al. 2001), in northern Sumatra (Fig. 1).
Location of surveyed areas. The numbers on the map refer to those in Table 1
To determine orangutan densities in a variety of habitats in the Leuser Ecosystem we conducted nest counts (e.g. van Schaik et al. 1995; Buij et al. 2003) along 24 line transects (Fig. 1), each at least 1,500 m long, in locations that varied from primary lowland forests to submontane forests. Hunting and logging did not occur in any of the sampled locations and are therefore not important in setting orangutan density in these areas. Two experienced observers estimated the perpendicular distances of all identified nests from transect lines. Swamp forests were excluded in the analyses presented here since there are no or almost no fig species in these forests (van Schaik, unpublished data). Only old-growth forest (dryland) sites were selected that were sufficiently far from swamp forests not to be influenced by these.
Nest counts were converted into orangutan nest densities by the method developed by van Schaik et al. (1995) and refined and explained in detail by Buij et al. (2003). This method only requires a single survey and produces reasonably accurate estimates as has been shown for several sites on Sumatra for which densities were known (van Schaik et al. 1995; Buij et al. 2003). The method is based on the line transect (Brockelman and Ali 1987). Orangutan densities can be estimated from nest counts using the following formula:
in which d = orangutan density (animals/km2), N = number of nests observed along the transect, L = length of the transect covered (km), w = estimated width of the strip of habitat actually covered (km), p = proportion of nest builders in the population, r = rate at which nests are produced (n/day/ind), and t = time during which a nest remains visible (in days).
The values for p and r were obtained from earlier studies, and show little variation for sites on Sumatra for which these have been measured (van Schaik et al. 1995). The value for t was found by using the positive relationship between topsoil pH and nest decay (Buij et al. 2003). The rationale behind this relationship is that nest decay is thought to be partly dependent on the hardness of the wood of the nest trees and the hardness of wood is thought to be higher with a decrease in topsoil pH (Buij et al. 2003). The perpendicular distance of nests to the transect line was recorded to estimate w. To increase the accuracy of the estimation of w, transects with similar distributions of perpendicular distances were pooled. The distribution of perpendicular distances of nests was compared for all transects with Kolmogorov-Smirnov tests. Four groups of transects with similar perpendicular distances could be identified (see w values in Table 1). Their w value was calculated using the computer programme “Distance 4.0.” in which several models can be used to estimate w (Thomas et al. 2001). In accordance with recommendations of Buckland et al. (1993), perpendicular distance data were truncated to at most 10% of the largest perpendicular values to minimise the influence of outliers. Detection intervals were varied to avoid possible heaping at favoured distances; histograms were analysed with cut-off points at 4-, 5-, 6-, 7-, 8- and 9-m intervals. To determine the distribution that best fitted the perpendicular distance data, five recommended models were selected to fit the distance histograms (Buckland et al. 1993 for details): uniform with cosine expansions, half-normal with cosine or hermite expansions, and hazard rate with either cosine or simple polynomial expansions. As suggested by Buckland et al. (1993), model selection was based on a quantitative method for model selection that uses Akaike’s information criterion (AIC) values to evaluate the fit of the model to the data; models that gave the lowest AIC value were used to estimate w (according to recommendations by Buckland et al. 1993). However, when the model with the lowest AIC value gave significant goodness-of-fit values between the expected and observed detection histogram (made from the perpendicular distance data), the model with the second lowest AIC value was given priority, since significant goodness-of-fit statistics may indicate that the wrong model is being fitted to the detection histogram (Buckland et al. 1993).
The pH of the topsoil was measured every 250 m along each transect using a Hellige pH meter (cf. van Schaik and Miranto 1985), which had been demonstrated to be accurate (van Noordwijk and Hairiah 1986). For two areas in which no direct pH measurements were made values were extracted from a regional physical planning programme for transmigration (RePPProT) report (1988). Values obtained by the Hellige pH meter and those extracted from the RePPProT show good correspondence; thus the values from the literature for the two sites at which no direct pH measurements were made were thought to be reliable.
Strangling figs were also counted with the line-transect method; one observer recorded strangling fig sightings by walking slowly along the transect trail. Only large, free-standing stranglers were included, i.e. those that had fully encompassed their host and had a full crown at the tree’s canopy level. Procedures for estimating strip width for fig trees were similar to those used by Buij et al. (2003) for nests.
Results
A summary of the survey data is presented in Table 1. Orangutan density varies between 0.23 and 6.0 ind/km2 with an average of 1.5 and a standard deviation of 1.4. Orangutan density showed a positive correlation with strangling fig tree density (Fig. 2, R 2 =0.29, P =0.006, N =24) and with topsoil pH (Fig. 3, R 2 =0.41, P =0.002, N =24).
These correlations may reflect three possible causal pathways. First, pH affects strangling fig density, which in turn affects nest density (in which case, strangling figs determine orangutan density and pH is merely a correlate). Second, pH and strangling fig density both affect orangutan density independently. Third, pH affects strangling fig density, but then both still affect orangutan density. In the latter two cases, both pH and strangling figs have an independent effect on orangutan density. Partial correlations support the second or third interpretation: the orangutan density remained correlated to both topsoil pH (Fig. 3, holding fig density constant: R 2 =0.26, P =0.014, N =21) and strangling fig density (holding pH constant: R 2 =0.17, P =0.048, N =21). Indeed, the third model is supported because topsoil pH is correlated to fig density (R 2 =0.16, P =0.054, N =24).
Discussion
The results presented in this paper indicate that both the pH and strangling fig density affect orangutan density. Together, pH and strangling fig density explain 47% of the variation in estimated orangutan density in this sample. The relationship is not an artifact of the usage of pH in estimating the duration of nest visibility (t), because it is almost as strong when nest densities themselves are used as the dependent variable (R 2 =0.45, P =0.002, N =24). Because orangutan densities were estimated using nest surveys, a procedure creating larger error than when based on long-term studies, it is possible that this relationship is still underestimated. At least for Sumatra, we believe that the refined nest survey method (Buij et al. 2003) produces unbiased estimates of orangutan density as suggested by the correspondence with real densities and the impressions at additional sites.
We will now explore the nature of the causal effects of topsoil pH and strangling fig density. In this region, soil pH is related to several forest variables in addition to the density of free-standing strangling figs. It is linked to overall litter production (van Schaik and Miranto 1985), probably because it is an expression of plant-accessible phosphate concentrations (van Noordwijk and Hairiah 1986). Thus, pH may reflect the general fruit productivity of the forest from the perspective of a consumer. This relationship may not hold elsewhere, however (Proctor et al. 1983; Scott et al. 1992).
The effect of strangling fig density may be both direct and indirect. At Ketambe, strangling figs are the dominant component of the orangutan’s diet (Rijksen 1978; Utami et al. 1997). They attract many orangutans (and other frugivores) and have been observed with more than eight individual adult orangutans with several dependent offspring at times when they contain a maximum number of fruits (personal observation). The production of strangling fig fruits is relatively less seasonal than that of other fruits (van Schaik 1996), thus making up for a fairly steady fruit supply that might be involved in setting the lower density limit for orangutans in a Sumatran dryland forest. Accordingly, the number of large strangling figs visited per month is rather constant (Sugardjito et al. 1987). This is further supported by the observation that orangutans shift their diet towards non-fig fruits when non-fig fruit trees have their availability peak (Sugardjito et al. 1987). In addition, fruit availability shows masting (Wich and van Schaik 2000) and in lean periods figs probably are an important food source. All these observations suggest that strangling figs provide orangutans with a major staple food, and that their density is causally linked to orangutan densities.
A relationship between fruit tree density and ape densities is not unique for orangutans and has also been reported for chimpanzees (Pan troglodytes) in the Kibale National Park, Uganda (Balcomb et al. 2000). Balcomb et al. (2000) show that chimpanzee density shows a positive relationship with the density of large, fleshy-fruit trees and suggest that chimpanzee densities can be quickly assessed by counting the trees.
In conclusion, these results show that orangutan density can be predicted by strangling fig density and soil pH. This suggests that once the presence of orangutans in an area is established, a rough estimate of their density can be obtained by estimating topsoil pH. This is easy, cheap, and requires no special skills. However, we should also stress the limitations of this method. First, in swamp areas, several other tree species show a similar pattern of high abundance and aseasonal fruiting. Indeed, the highest orangutan densities so far have been found in swamp areas with virtually no strangling figs (van Schaik et al. 1995). Our results are therefore only valid for non-swamp areas. Second, use of this measure assumes no hunting or logging. While the presence of hunting would make density predictions based on ecological features impossible, future efforts should incorporate forests affected by selective logging. Third, it remains to be established whether the same relationship also holds in Borneo. Recent surveys by one of us (S.A. Wich) in another area on Sumatra show that pH and strangling fig density are reasonable indicators of orangutan density and thus provide support for the practical application of this method in other areas in Sumatra (Wich and Geurts, unpublished data). We also suggest, therefore, that existing conservation programmes such as the Leuser Management Unit use such methods to determine orangutan density estimates in areas of the Leuser Ecosystem that have not been surveyed as yet.
Future work should be aimed at removing these limitations. Successful management of the last orangutan populations and proper assessment of habitat suitability for reintroduction efforts require the presence of a simple method to predict orangutan densities in all unhunted forests within its geographic range.
References
Bacon AM, Long VT (2001) The first discovery of a complete skeleton of a fossil orang-utan in a cave of the Hao Binh province, Vietnam. J Hum Evol 41:227–242
Balcomb SR, Chapman CA, Wrangham RW (2000) Relationship between chimpanzee (Pan troglodytes) density and large, fleshy-fruit tree density: conservation implications. Am J Primatol 51:197–203
Brockelman WY, Ali R (1987) Methods of surveying and sampling forest primate populations. In: Marsh CW, Mittermeier RA (eds) Primate conservation in the tropical rain forest. Liss, New York, pp 23–62
Buckland ST, Anderson DR, Burnham KP, Laake JL (1993) Distance sampling: estimating abundance of biological populations. Chapman and Hall, London
Buij R, Wich SA, Lubis AH, Sterck EHM (2002) Seasonal movements in the Sumatran orangutan (Pongo pygmaeus abelii) and consequences for conservation. Biol Conserv 107:83–87
Buij R, Singleton I, Krakauer E, van Schaik CP (2003) Rapid assessment of orangutan density. Biol Conserv 114:103–113
Djojosudharmo S, van Schaik CP (1992) Why are orangutans so rare in the highlands. Trop Biodivers 1:11–22
Galdikas BMF (1978) Orangutan adaptation at Tanjung Puting Reserve, Central Borneo. PhD Dissertation, University of California, Los Angeles
Galdikas BMF, Wood J (1990) Great ape and human birth intervals. Am J Phys Anthropol 83:185–192
Harvey PH, Martin RD, Clutton-Brock TH (1987) Life histories in comparative perspective. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 181–196
Hilton-Taylor C, Brackett D (2000) 2000 IUCN red list of threatened species. IUCN, Gland, Switzerland
Jablonski NG, Whitfort MJ, Roberth-Smith N, Qinqi Xu (2000) The influence of life history and diet on the distribution of catarrhine primates during the Pleistocene in eastern Asia. J Hum Evol 39:131–157
Jepson P, Jarvie JK, MacKinnon K, Monk KA (2001) The end for Indonesia’s lowland forests? Science 292:859–861
Koeningswald GHR von (1982) Distribution and evolution of the orangutan, Pongo pygmaeus (Hoppius). In: Boer LEM de (ed) The orangutan. Its biology and conservation. Junk, The Hague, pp 1–16
Leighton M (1993) Modeling dietary selectivity by Bornean orangutans: evidence for integration of multiple criteria in fruit selection. Int J Primatol 14:257–314
Leighton M, Seal US, Soemarna K, Adjisasmito M, Wijaya, Mitra Setia T, Shapiro G, Perkins L, Traylor-Holzer K, Tilson R (1995) Orangutan life-history and VORTEX analysis. In: Nadler RD, Galdikas BMF, Sheeran LK, Rosen N (eds) The neglected ape. Plenum, New York, pp 97–109
MacKinnon J (1974) The ecology and behaviour of wild orangutans (Pongo pygmaeus). Anim Behav 22:3–74
Noordwijk M van, Hairiah K (1986) Mycorrhizal infection in relation to soil pH and soil phosphorus content in a rain forest of northern Sumatra. Plant Soil 96:299–302
Proctor J, Anderson JM, Vallack HW (1983) Comparative studies on soil and litter fall in forests at a range of altitudes on Gunung Mulu, Sarawak. Malay For 46:60–76
RePPProT (1988) Review of Phase I, Sumatra. Report of the Government of the Republic of Indonesia, Ministry of Transmigration
Rijksen HD (1978) A field study of Sumatran orangutans (Pongo pygmaeus abelii Lesson 1827). Veenman and Zonen, Wageningen
Rijksen HD, Meijaard E (1999) Our vanishing relative: the status of wild orangutans at the close of the twentieth century. Kluwer, Dordrecht
Rodman PS (1988) Diversity and consistency in ecology and behaviour. In: Schwartz JH (ed) Orangutan biology. Oxford University Press, New York, pp 31–51
Schaik CP van (1996) Strangling figs: their role in the forest. In: Schaik CP van, Supriatna J (eds) Leuser, a Sumatran sanctuary. Yayasan Bina Sains Hayati, Jakarta, pp 111–119
Schaik CP van (1999) The socioecology of fission–fusion sociality in orangutans. Primates 40:69–86
Schaik CP van, Miranto E (1985) Spatial variation in the structure and litterfall of a Sumatran rain forest. Biotropica 17:196–205
Schaik CP van, Supriatna J (1996) Leuser, a Sumatran sanctuary. Yayasan Bina Sains Hayati, Jakarta
Schaik van CP, Azwar, Priatna D (1995) Population estimates and habitat preferences of orangutans based on line transects of nests. In: Nadler RD, Galdikas BMF, Sheeran LK, Rosen N (eds) The neglected ape. Plenum, New York, pp 129–147
Schaik CP van, Monk KA, Robertson JMY (2001) Dramatic decline in orang-utan numbers in the Leuser Ecosystem, Northern Sumatra. Oryx 35:14–25
Scott DA, Proctor J, Thompson J (1992) Ecological studies on a lowland evergreen rain forest on Maraca Island, Roraima, Brasil. II. Litter and nutrient cycling. J Ecol 80:705–717
Singleton I, van Schaik CP (2001) Orangutan home range size and its determinants in a Sumatran swamp forest. Int J Primatol 22:877–911
Sugardjito J, te Boekhorst IJA, van Hooff JARAM (1987) Ecological constraints on the grouping of wild-orangutans (Pongo pygmaeus) in the Gunung Leuser National Park, Sumatra, Indonesia. Int J Primatol 8:17–41
Thomas L, Laake JL, Strindberg S, Marques FFC, Borchers DL, Buckland ST, Anderson DR, Burnham KP, Hedley SL, Pollard JH (2001) Distance 4.0. Beta 3. Research Unit for Wildlife Population Assessment, University of St. Andrews, St Andrews
Utami SS, Wich SA, Sterck EHM, van Hooff JARAM (1997) Food competition between wild orangutans in large fig trees. Int J Primatol 18:909–927
Wich SA, van Schaik CP (2000) The impact of El Niño on mast fruiting in Sumatra and elsewhere in Malesia. J Trop Ecol 16:563–577
Wich SA, Sterck EHM, Utami SS (1999) Are orangutan females as solitary as chimpanzee females? Folia Primatol 70:23–28
Wich SA, Singleton I, Utami-Atmoko SS, Geurts ML, Rijksen HD, van Schaik CP (2003) The status of the Sumatran orangutan Pongo abelii: an update (Pongo abelii). Oryx 1:49–54
Acknowledgements
We gratefully acknowledge the co-operation and support of the Indonesian Institute of Science (LIPI, Jakarta), the Indonesian Nature Conservation Service (PHPA) in Jakarta, Medan, and Kutacane (Gunung Leuser National Park Office), Universitas National (UNAS, Jakarta), and the Leuser Development Programme (LDP, Medan), and especially M. Griffiths and Dr. K. Monk. We thank Sulaiman, Rahimin, and Matplin for helping with data collection. Financial support was generously provided by the Netherlands Foundation for the Advancement of Tropical Research (WOTRO), the Wildlife Conservation Society (WCS) of New York, and the Lucie Burgers Foundation for Comparative Behaviour Research. Jan van Hooff and Liesbeth Sterck are acknowledged for support throughout the project. We thank the European Commission and the Government of Indonesia as the funding agencies for the Leuser Development Programme.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wich, S., Buij, R. & van Schaik, C. Determinants of orangutan density in the dryland forests of the Leuser Ecosystem. Primates 45, 177–182 (2004). https://doi.org/10.1007/s10329-004-0080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-004-0080-1