Abstract
Root-knot nematodes (RKNs) are one of the most important biotic factors limiting crop productivity in many crop plants. The major RKN control strategies include development of resistant cultivars, application of nematicides and crop rotation, but each has its own limitations. In recent years, RNA interference (RNAi) has become a powerful approach for developing nematode resistance. The two housekeeping genes, splicing factor and integrase, of Meloidogyne incognita were targeted for engineering nematode resistance using a host-delivered RNAi (HD-RNAi) approach. Splicing factor and integrase genes are essential for nematode development as they are involved in RNA metabolism. Stable homozygous transgenic Arabidopsis lines expressing dsRNA for both genes were generated. In RNAi lines of splicing factor gene, the number of galls, females and egg masses was reduced by 71.4, 74.5 and 86.6%, respectively, as compared with the empty vector controls. Similarly, in RNAi lines of the integrase gene, the number of galls, females and egg masses was reduced up to 59.5, 66.8 and 63.4%, respectively, compared with the empty vector controls. Expression analysis revealed a reduction in mRNA abundance of both targeted genes in female nematodes feeding on transgenic plants expressing dsRNA constructs. The silencing of housekeeping genes in the nematodes through HD-RNAi significantly reduced root-knot nematode infectivity and suggests that they will be useful in developing RKN resistance in crop plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes (RKNs), classified in the genus Meloidogyne, are commonly considered sedentary parasites of the plants. Approximately 100 species of the Meloidogyne are distributed worldwide having a wide host range. The most widespread species are M. incognita, M. javanica, M. arenaria and M. hapla. RKNs are among the most destructive plant pathogens producing some of the most devastating symptoms that account for an estimated worldwide crop loss of hundreds of billions of dollars per year (Abad et al. 2008; Chitwood 2003; Elling 2013).
In the past few years, several strategies have been used to control RKN infection, including cultural, chemical and biological control. In tomato, the Mi-1 gene confers genetic resistance to three species of RKN (Vos et al. 1998), but this resistance breaks down under high temperatures (Williamson 1998). However, recently, resistance broken by short-term heat stress was reported to recover over time (Carvalho et al. 2015). Despite the effectiveness of the various strategies against nematodes, each has drawbacks; hence, a novel approach is required to control these parasites. RNA interference (RNAi) is a process in which dsRNA that corresponds to a particular gene is processed by Dicer to generate siRNAs, that in turn further activate the silencing complex (RISC) and recognize target mRNA and either degrade or prevent its translation into proteins. In 1998, Fire et al. reported silencing in Caenorhabditis elegans Maupas, using dsRNA and suggested it as a potential tool to study gene function by suppressing expression.
The RNAi mechanism can be used against nematode infection through a process called host-delivered RNAi (HD-RNAi); dsRNA corresponding to a specific nematode gene is expressed through host-plant mediated siRNA production. RNAi is triggered on the basis of homology between siRNA and the target mRNA upon nematode feeding.
The target gene can be chosen on the basis of its functional role in vital processes such as motility, feeding, development, mRNA metabolism, neurotransmission and different nematode effectors. Selection of the target gene is an important aspect of HD-RNAi that plays a key role in avoiding off-target effects. The criteria for effective target gene selection are that: (1) target gene sequences be discrete enough to ensure that the RNAi is nematode specific; and that (2) the target gene be conserved in various plant-damaging RKN species but absent in nontarget species such as chordates, plants, annelids, insect pollinators and mollusks (Danchin et al. 2013).
Numerous reports illustrate the success of RNAi against RKN infection (Bakhetia et al. 2008; Dinh et al. 2014a, b; Dong et al. 2016; Dutta et al. 2015; Fairbairn et al. 2007; Huang et al. 2006; Ibrahim et al. 2011; Jaouannet at al. 2013; Klink et al. 2009; Li et al. 2010; Lourenço-Tessutti et al. 2015; Niu et al. 2012, 2016; Papolu et al. 2013; Rosso et al. 2009; Sindhu et al. 2009; Steeves at al. 2006; Xue et al. 2013; Yadav et al. 2006). Varying levels of resistance have been demonstrated in different studies, and genes involved in nematode parasitism, development and reproduction have been targeted in a wide variety of plant systems including Arabidopsis, tobacco, tomato and soybean (Tamilarasan and Rajam 2013).
Yadav et al. (2006) generated tobacco transgenic lines expressing dsRNA for the splicing factor and integrase genes of M. incognita that showed great potential as RNAi targets. Splicing factor and integrase genes target mRNA metabolism and impair the development of M. incognita. Gall formation and the number of females feeding on transgenic plants expressing dsRNA were significantly reduced. However, these two genes have not been evaluated in Arabidopsis plants, which is the major model plant for evaluating nematode resistance using the HD-RNAi approach. The use of Arabidopsis in the majority of the studies so far prompted us to evaluate these genes, which are very effective in inducing resistance in tobacco. Therefore, the present study was designed to generate and evaluate the stable transgenic Arabidopsis lines expressing dsRNA of splicing factor and integrase genes in terms of RKN resistance.
Materials and methods
Designing the RNAi vector
The splicing factor and integrase gene constructs were used (Yadav et al. 2006). For the expression of dsRNA in transgenic Arabidopsis plants, a 349 and 624 bp fragment of the splicing factor and integrase gene, respectively, were amplified in sense and anti-sense direction using gene-specific primers and cloned in pBC06 vector. The pBC06 was driven by the CaMV35S promoter, placed upstream of the intron of the Arabidopsis MADS-box gene (Y12776) flanked by two multiple cloning sites.
Maintenance of pure culture of root-knot nematode in tomato
A pure culture of root-knot nematode, M. incognita (Kofoid and White) Chitwood race 1, maintained on tomato plants, was obtained from Dr. Anil Sirohi, Division of Nematology, Indian Agricultural Research Institute (IARI), New Delhi in March 2008 and maintained on tomato (Solanum lycopersicum L.) plants in a greenhouse of IARI, New Delhi. Originally, the nematodes were collected from the brinjal field of the Division of Vegetable Science, ICAR-IARI, Pusa Campus, New Delhi during August 2006. For assessing culture purity, a single egg mass and its corresponding female were harvested and gently washed with tap water. The nematode was identified using sequence-characterized amplified region (SCAR) primers (Zijlstra et al. 2000) and the perineal pattern on the female (Eisenback et al. 1985). After confirmation of the species as M. incognita, a single egg mass was allowed to hatch, and second-stage juveniles (J2s) were used to generate a pure culture on tomato plants. Two-week-old tomato seedlings, grown in cocopeat, vermiculite and sand (1:1:1), were infected with a pure culture of M. incognita (Atamian et al. 2012). After 6–7 weeks, egg masses were hand-picked and allowed to hatch at 28 °C in petri plates containing 10–15 mL of sterile water.
Development of transgenic plants containing splicing factor and integrase genes
Arabidopsis thaliana (Col-0) plants were transformed with splicing factor and integrase RNAi constructs using the floral dip method (Clough and Bent 1998). T1 seeds were screened on a plate of MS medium containing kanamycin (50 µg/mL). Kanamycin-resistant plants were transferred to autoclaved soilrite and grown in a greenhouse at 22 °C with 16 h light/8 h dark. T2 seeds were used to raise T3 homozygous lines of transgenic plants. RNAi transgenic plant roots, shoots and leaves were compared with the wild type to check for any phenotypic variations that might incidentally modify the infection of nematodes.
Nematode infection assay of transgenic Arabidopsis lines
Arabidopsis thaliana seeds were surface-sterilized (2 min in 70% ethanol and 7 min in 0.1% mercuric chloride + 0.1% SDS) and washed three times with sterile distilled water to remove residual solutions and vernalized for 72 h at 4 °C before germination on Gamborg’s B-5 medium. Plates were maintained at 22 °C under a 16 h light/8 h dark. After 18 days of growth, wild-type and transgenic plants were uprooted and transferred to a 24-well tray, with one plant per well, of the vermiculite, cocopeat and sand mix. The trays were sealed with cling film and allowed to grow in a growth chamber with 16 h light/8 h dark. After 1 week, the roots of each plant were inoculated with freshly hatched 1000 second-stage juveniles (J2s) of M. incognita (Atamian et al. 2012). After 6–7 weeks post inoculation, the plants were uprooted and washed with tap water to remove any growth mixture. The galls, females and egg masses on each plant root were counted and used as a measure of nematode vulnerability; means were calculated from 10 to 15 replicates per RNAi line. Images were captured using a Nikon DS-Fi2 camera attached to a microscope (Eclipse 80i and stereomicroscope SMZ1000, Tokyo, Japan), and the area and diameter of nematode females were recorded using microscope software.
RNA isolation and gene expression analysis of RNAi females
Expression of M. incognita genes were analyzed using semi-qRT-PCR. Total RNA was isolated using the RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The RNA integrity was checked by formaldehyde gel electrophoresis with ethidium bromide dye. A Nanodrop Spectrophotometer 8000 (Thermo Scientific, Wilmington, DE, USA) was used to calculate purity ratios and quantify total RNA. Roughly 600 ng RNA was used to synthesize cDNA with a Protoscript M-MuLV first strand synthesis kit (NEB, Massachusetts, USA) and oligo d (T)23VN primers. cDNA was normalized and re-quantified before semi-qRT-PCR. RT primers were designed from the cDNA sequence of selected genes using the online Primer3 portal (http://frodo.wi.mit.edu/primer3/) (Rozen and Skaletsky 2000). PCR was carried out on a G-Storm, GS1 Thermal Cycler (Gene Technologies, Somerset, UK) with the following cycling conditions: initial melting temperature of 94 °C for 2 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 40 s and 72 °C for 1 min; and a final extension of 10 min at 72 °C. The PCR products were electrophoretically separated in an agarose gel and visualized using the G:Box gel documentation system (Syngene, UK).
Results
Infection assay of dsRNA-expressing transgenic Arabidopsis line
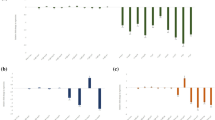
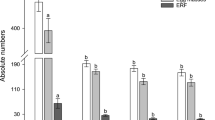
For the nematode infection assay, three independent, homozygous T3 Arabidopsis RNAi lines (integrase: E1, E2, E3; splicing factor: SF-E1, SF-E2 and SF-E3) were inoculated with 1000 J2s of M. incognita per plant. For each transgenic event, 10 replicates were used for the analysis, and each experiment was repeated twice. The response of these lines against the RKN was evaluated at 45 days post inoculation (dpi) by determining the number of gall, female and egg mass and compared with control Arabidopsis plants harboring the empty vector (Fig. 1). In the case of splicing factor RNAi lines (SF-E1, SF-E2 and SF-E3), significant reduction was observed in the number of gall (71.6–74.5%), female (68.1–71.4%) and egg mass (81.8–86.6%) compared with the empty vector control (Fig. 2a). The integrase gene RNAi lines had 63.3–66.8%, fewer galls, 55.5–59.5% fewer females and 49.2–63.4% fewer egg mass compared with the vector control (Fig. 2b). Altogether, transgenic lines of both genes had significantly reduced number of galls, females and egg masses, indicating a deleterious effect of silencing of these target genes on the growth and development of nematodes.
Root-knot nematode infection assay in roots of Arabidopsis plants. a Empty-vector-transformed plant (control) showing numerous galls compared with the b transgenic line expressing splicing factor dsRNA (SF-RNAi) and c transgenic line expressing integrase dsRNA (Int-RNAi). d–f Enlargement of control, SF-RNAi and Int-RNAi plants. Arrowheads indicate galls
RNAi lines with a reduction in number of galls, females and egg masses relative to the control. Three independent lines were evaluated for infection. a Splicing factor RNAi lines (SF-E1, SF-E2 and SF-E3). b Integrase RNAi lines (integrase-E1, integrase-E2 and integrase-E3). Each bar represents the mean ± SE (n = 10). An asterisk indicates statistically significant differences in one-way ANOVA and Tukey test (p ≤ 0.05)
Morphological analysis of nematode female feeding on dsRNA-expressing transgenic Arabidopsis plants
For assessing the effect of host-delivered RNAi (HD-RNAi) on RKN, females were isolated from transgenic plants expressing dsRNA of splicing factor and integrase genes and stained with acid fuchsin. Females feeding on transgenic lines expressing splicing factor dsRNA or expressing integrase dsRNA were very small and distorted in shape compared with females isolated from control plants (Fig. 3a–c). Females isolated from both the RNAi lines also had smaller surface area and diameter than did females feeding on control plants (Table 1).
Size and shape of females of root-knot nematode feeding on Arabidopsis control plants and transgenic lines expressing dsRNA at 45 dpi. a Females from control plants were large with a normal shape compared with b small females with an aberrant phenotype from the dsRNA::splicing factor transgenic line and c dsRNA::integrase transgenic line
Transcript downregulation in M. incognita females feeding on RNAi plants
At 45 dpi, females were isolated from the dsRNA expressing RNAi line of splicing factor and integrase genes. We isolated three batches (each contained 20 female nematodes) of nematodes for each gene construct along with their corresponding control females. Females were frozen in liquid nitrogen and used for RNA isolation. Semi-qRT-PCR was used to quantify the transcript level in the females feeding on the RNAi lines and control plants. Actin was used as an internal control gene to normalize the samples. For each of the RNAi lines, the expression of the respective gene was significantly downregulated in females extracted from transgenic plants compared with the control (Fig. 4a, b).
Semi-quantitative RT-PCR analysis of targeted genes in root-knot nematode females feeding on control and RNAi plants. a Expression of integrase gene. b Expression of splicing factor gene. E1, E2 and E3 are independent transgenic lines of integrase and splicing factor RNAi lines, and C1, C2 and C3 are control plants containing empty vector. Actin was used as a housekeeping gene
Discussion
Root-knot nematodes infect nearly all vascular plants and is a major problem in a wide variety of crops. In the decade since work on developing resistance against RKN using RNAi began, RNAi has become a potent tool for controlling infection by plant parasitic nematodes and implemented successfully in C. elegans to analyze the function of important genes (Fire et al. 1998; Kamath et al. 2003). There have also been several reports in different crops on the successful reduction of nematode infection, up to 90% in certain cases, using housekeeping genes, such as secretory genes, kinases, transcription factor, neuropeptides, peptides and genes involved in metabolism. In this study splicing factor and integrase genes, involved in nematode metabolism and development, were targeted using HD-RNAi approach.
Although our RNAi lines targeting splicing factor and integrase genes of nematode significantly reduced (49–86%) the number of galls, females and egg masses compared with the empty vector controls, Yadav et al. (2006) reported up to 90% reduction for M. incognita using the same genes in transgenic tobacco RNAi lines. Clearly, the resistance of tobacco and Arabidopsis vary greatly, underlying the uniqueness of each plant system and variation in their responses. However, it also brings forth the point that good candidate genes for RNAi, once identified, will be an immensely useful resource and the judicious use of the genes singly or in combination hold a key to generating nematode-resistant crops.
The root-knot nematode females isolated from the dsRNA expressing Arabidopsis lines of splicing factor and integrase genes were also smaller and their morphology distorted compared with the controls, similar to the elongated, transparent and developmentally weak females from the transgenic tobacco lines (Yadav et al. 2006). The use of these genes to control nematodes in agricultural crops appears to be a viable approach, and pyramiding of both genes may improve field control of nematodes. However, the host plant is a dynamic system, and each will behave in a unique manner in terms of resistance. Numerous candidate genes need to be evaluated with extensive molecular and infection analysis and select the best to develop nematode resistance in crop plants.
References
Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Ségurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, Schiex T, Smant G, Weissenbach J, Wincker P (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909–915
Atamian HS, Roberts PA, Kaloshian I (2012) High and low throughput screens with root-knot nematodes Meloidogyne spp. J Vis Exp 61:e39
Bakhetia M, Urwin PE, Atkinson HJ (2008) Characterisation by RNAi of pioneer genes expressed in the dorsal pharyngeal gland cell of Heterodera glycines and the effects of combinatorial RNAi. Int J Parasitol 38:1589–1597
Carvalho LM, Benda ND, Vaughan MM, Cabrera AR, Hung K, Cox T, Abdo Z, Allen LH, Teal PE (2015) Mi-1-mediated nematode resistance in tomatoes is broken by short-term heat stress but recovers over time. J Nematol 47:133–140
Chitwood DJ (2003) Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Manag Sci 59:748–753
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Danchin EG, Arguel MJ, Campan-Fournier A, Perfus-Barbeoch L, Magliano M, Rosso MN, Da Rocha M, Da Silva C, Nottet N, Labadie K, Guy J, Artiguenave F, Abad P (2013) Identification of novel target genes for safer and more specific control of root-knot nematodes from a pan-genome mining. PLoS Pathog 9:e1003745
Dinh PTY, Brown CR, Elling AA (2014a) RNA interference of effector gene Mc16D10L confers resistance against Meloidogyne chitwoodi in Arabidopsis and potato. Phytopathology 104:1098–1106
Dinh PTY, Zhang L, Brown CR, Elling AA (2014b) Plant-mediated RNA interference of effector gene Mc16D10L confers resistance against Meloidogyne chitwoodi in diverse genetic backgrounds of potato and reduces pathogenicity of nematode offspring. Nematology 16:669–682
Dong L, Xu J, Chen S, Li X, Zuo Y (2016) Mi-flp-18 and Mi-mpk-1 genes are potential targets for Meloidogyne incognita control. J Parasitol 102:208–213
Dutta TK, Papolu PK, Banakar P, Chaudhary D, Sirohi A, Rao U (2015) Tomato transgenic plants expressing hairpin construct of a nematode protease gene conferred enhanced resistance to root-knot nematodes. Front Microbiol 6:260
Eisenback JD, Sasser JN, and CC (1985) Diagnostic characters useful in the identification of the four most common species of root-knot nematodes (Meloidogyne spp.) In: Sasser JN, Carter CC (eds.) An advanced treatise on Meloidogyne 1: Biology and control, North Carolina State University Graphics, Raleigh, pp 95–112
Elling AA (2013) Major emerging problems with minor Meloidogyne species. Phytopathology 103:1092–1102
Fairbairn DJ, Cavallaro AS, Bernard M, Mahalinga-Iyer J, Graham MW, Botella JR (2007) Host-delivered RNAi: an effective strategy to silence genes in plant parasitic nematodes. Planta 226:1525–1533
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Huang G, Allen R, Davis EL, Baum TJ, Hussey RS (2006) Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci USA 103:14302–14306
Ibrahim HMM, Alkharouf NW, Meyer SLF, Aly MA, Gamal El-Din Ael K, Hussein EH, Matthews BF (2011) Post-transcriptional gene silencing of root-knot nematode in transformed soybean roots. Exp Parasitol 127:90–99
Jaouannet M, Magliano M, Arguel MJ, Gourgues M, Evangelisti E, Abad P, Rosso MN (2013) The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant Microbe Interact 26:97–105
Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:231–237
Klink VP, Kim KH, Martins V, Macdonald MH, Beard HS, Alkharouf NW, Lee SK, Park SC, Matthews BF (2009) A correlation between host-mediated expression of parasite genes as tandem inverted repeats and abrogation of development of female Heterodera glycines cyst formation during infection of Glycine max. Planta 230:53–71
Li J, Todd TC, Oakley TR, Lee J, Trick HN (2010) Host-derived suppression of nematode reproductive and fitness genes decreases fecundity of Heterodera glycines Ichinohe. Planta 232:775–785
Lourenço-Tessutti IT, Souza Junior JDA, Martins-de-Sa D, Viana AA, Carneiro RM, Togawa RC, de Almeida-Engler J, Batista JA, Silva MC, Fragoso RR, Grossi-de-Sa MF (2015) Knock-down of heat-shock protein 90 and isocitrate lyase gene expression reduced root-knot nematode reproduction. Phytopathology 105:628–637
Niu J, Jian H, Xu J, Chen C, Guo Q, Liu Q, Guo Y (2012) RNAi silencing of the Meloidogyne incognita Rpn7 gene reduces nematode parasitic success. Eur J Plant Pathol 134:131–144
Niu J, Liu P, Liu Q, Chen C, Guo Q, Yin J, Yang G, Jian H (2016) Msp40 effector of root-knot nematode manipulates plant immunity to facilitate parasitism. Sci Rep 6:19443
Papolu PK, Gantasala NP, Kamaraju D, Banakar P, Sreevathsa R, Rao U (2013) Utility of host delivered RNAi of two FMRF amide like peptides, flp-14 and flp-18, for the management of root knot nematode, Meloidogyne incognita. PLoS One 8:e80603
Rosso MN, Jones JT, Abad P (2009) RNAi and functional genomics in plant parasitic nematodes. Annu Rev Phytopathol 47:207–232
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Sindhu AS, Maier TR, Mitchum MG, Hussey RS, Davis EL, Baum TJ (2009) Effective and specific in planta RNAi in cyst nematodes: expression interference of four parasitism genes reduces parasitic success. J Exp Bot 60:315–324
Steeves RM, Todd TC, Essig JS, Trick HN (2006) Transgenic soybeans expressing siRNAs specific to a major sperm protein gene suppress Heterodera glycines reproduction. Funct Plant Biol 33:991–999
Tamilarasan S, Rajam MV (2013) Engineering crop plants for nematode resistance through host-herived RNA interference. Cell Dev Biol 2:114
Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, Hogers R, Frijters A, Groenendijk J, Diergaarde P, Reijans M, Fierens-Onstenk J, de Both M, Peleman J, Liharska T, Hontelez J, Zabeau M (1998) The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat Biotechnol 16:1365–1369
Williamson VM (1998) Root-knot nematode resistance genes in tomato and their potential for future use. Annu Rev Phytopathol 36:277–293
Xue B, Hamamouch N, Li C, Huang G, Hussey RS, Baum TJ, Davis EL (2013) The 8D05 parasitism gene of Meloidogyne incognita is required for successful infection of host roots. Phytopathology 103:175–181
Yadav BC, Veluthambi K, Subramaniam K (2006) Host-generated double stranded RNA induces RNAi in plant-parasitic nematodes and protects the host from infection. Mol Biochem Parasitol 148:219–222
Zijlstra C, Donkers-Venne DTHM, Fargette M (2000) Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2:847–853
Acknowledgements
We gratefully acknowledge the financial support from National Agricultural Innovative Project (NAIP) and NASF (National Agricultural Science Fund) previously called the National Fund for Basic Strategic and Frontier Application Research in Agriculture (NFBSFARA), Indian Council of Agricultural Research (ICAR), New Delhi, India. The authors thank Dr. K. V. Prabhu, Dr. Rajendra Singh and the staff of National Phytotron Facility (NPF), IARI, New Delhi, India for providing space in the greenhouse to maintain nematode culture on tomato plants and Arabidopsis thaliana in the growth chambers.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

10327_2017_701_MOESM1_ESM.jpg
Supplementary Fig. 1 PCR-based confirmation of the presence of splicing factor and integrase genes in control and transgenic Arabidopsis plants expressing dsRNA of splicing factor and integrase genes. a) Amplification of splicing factor gene from DNA samples isolated from control and representative transgenic plants of three independent lines (SF-E1, SF-E2, SF-E3 and control samples were loaded in lanes 1, 2, 3 and 4, respectively). b) Amplification of integrase gene from DNA samples isolated from control and representative transgenic plants of three independent lines (integrase-E1, integrase-E2, integrase-E3 and control samples were loaded in lanes 1, 2, 3 and 4 respectively). Lane M: 100 bp ladder marker. (JPG 109 KB)

10327_2017_701_MOESM2_ESM.jpg
Supplementary Fig. 2 RT-PCR based expression analysis of splicing factor and integrase genes in control and transgenic Arabidopsis plants expressing dsRNA of splicing factor and integrase genes. a) Expression of splicing factor gene from the RNA samples isolated from control and representative transgenic plants of three independent lines (SF-E1, SF-E2, SF-E3 and control samples were loaded in lanes 1, 2, 3 and 4, respectively). b) Expression of integrase gene from RNA samples isolated from control and representative transgenic plants of three independent lines (integrase-E1, integrase-E2, integrase-E3 and control samples were loaded in lanes 1, 2, 3 and 4, respectively). Lane M: 100 bp ladder marker. (JPG 86 KB)
Rights and permissions
About this article
Cite this article
Kumar, A., Kakrana, A., Sirohi, A. et al. Host-delivered RNAi-mediated root-knot nematode resistance in Arabidopsis by targeting splicing factor and integrase genes. J Gen Plant Pathol 83, 91–97 (2017). https://doi.org/10.1007/s10327-017-0701-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-017-0701-3