Abstract
Sugarcane cultivars with a high (susceptible cultivars) and low (resistant cultivars) virus titer of Sugarcane yellow leaf virus were grown in the field. The carbohydrate composition in green leaf tops and in stems was determined. In RT-PCR of leaf extracts, susceptible cultivars had a high SCYLV-titer, whereas resistant cultivars had a very low titer. The cultivars differed in biomass yield, but these differences were not correlated with susceptibility. However, carbohydrate composition did have susceptibility-specific differences. Hexose levels were lower in green leaf tops and stalks of susceptible (strongly infected) cultivars than in those of resistant (weakly infected) cultivars. The stalks of susceptible cultivars also had less starch than those of resistant cultivars. Thus, the viral susceptibility (and infection) affected sugar metabolism. In addition, a positive correlation between hexose and starch in stems and between hexose and sucrose in green leaf tops was observed. The results from susceptible versus resistant cultivars were the opposite of those in the comparison between infected versus virus-free lines of the same cultivar. The breeding process apparently had unintentionally selected clones with modulated carbohydrate metabolism to avoid or compensate for the adverse effects of SCYLV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yellow leaf (YL) was noticed as a sugarcane disease in the 1990s when leaf yellowing and a yield decrease were reported from plantations in different regions of the world (Comstock et al. 1994; Schenck 1990). The polerovirus Sugarcane yellow leaf virus (SCYLV) was identified as the responsible pathogen (Vega et al. 1997). A survey of sugarcane cultivars in Hawaii revealed that some cultivars had a high SCYLV titer, whereas others had little or no virus (Schenck and Lehrer 2000). The cultivars with the high titer were considered as susceptible, the others as resistant. In studies of the pathogenicity, infected plants maintained the virus in stem pieces and rootstocks (Lehrer et al. 2007; Rassaby et al. 2003). Virus-free plants of susceptible cultivar H87-4094 were generated by meristem tip culture (Fitch et al. 2001), and these virus-free plants had 10–30% higher yield than infected plants (Lehrer et al. 2009). However, when different cultivars were compared, SCYLV-susceptible and -resistant cultivars (containing the “natural” SCYLV titer) did not differ significantly in growth or yield (Lehrer et al. 2009). The screening process for the breeding program was thus concluded to have selected for clones that had compensated for the adverse effects of SCYLV. In a carbohydrate analysis of infected plants, SCYLV impeded the export of assimilate from leaves (Lehrer et al. 2007; Yan et al. 2009). As in the previous comparison of infected and virus-free plants of the same cultivar, we undertook a similar analysis to compare susceptible and resistant cultivars (which contained, respectively, a naturally high and low virus titer) to reveal possible adaptations of the cultivars to cope with SCYLV infection.
Materials and methods
Growth and harvest of plants
Planting followed plantation practices. Stem cuttings 50 cm long and containing three nodes were incubated in water at 50°C for 30 min, then dipped in a propiconazole solution (Tilt, 25 g a.i./l, Novartis, Basel, Switzerland). Three field plots at the Experiment Station of Hawaii Agriculture Research Center (HARC) in Kunia were planted with 16 seed pieces of each cultivar in two parallel rows 1 m apart and 3 m long, with a drip irrigation tube in the centre covered with soil. The test plots were treated identically to commercial field practice.
The following cultivars were selected: susceptible cvs. H73-6110, H78-3606 and H87-4094, moderately susceptible cvs. H65-7052 and H78-3567, and resistant cvs. H78-4153, H78-7750, H87-4319 and H82-3569. All cultivars contained SCYLV; however, the titers were high in the susceptible and low in the resistant cultivars. A virus-free line of H87-4094 was generated by meristem tip culture (Fitch et al. 2001) and compared with the infected line of this cultivar.
The sugarcane was harvested after 16 months according to standards of the Hawaiian Breeding Program. All stems in 1.5 m of one double row, i.e., stems from eight stools, were cut at soil level. The green leaf tops were removed from the stalks, and tops and stems were weighed and chopped separately. One-kilogram samples of the chopped material were immediately frozen for later sugar extraction and carbohydrate analysis. Thus in each of the three plots, eight plants of each cultivar were harvested, weighed and chopped together. Sugar was determined in samples from that chopped, mixed material. Sugar was extracted from the chopped plant material as described by Payne (1968). One kilogram of chopped plant material was suspended in 2 l hot water for 15 min in a disintegrator, then the suspension was centrifuged, and the supernatant (=extract) was used for sugar determination.
Carbohydrate determination
Hexose determination Extract (0.2 ml) and ethanol (0.2 ml) were mixed and centrifuged for 10 min at 14,000 rpm, then 20 μl of this supernatant were added to 180 μl reaction buffer (100 mM triethanolamine–Cl pH 7.6, 3 mM NADP, 5 mM ATP, 10 mM MgSO4). The enzymatic reactions were started by addition of 0.3 U hexokinase, 0.15 U glucose-6-phosphate dehydrogenase, and 1 U phosphoglucose isomerase. The products were measured after a 30-min reaction in a microplate reader at 340 nm.
Sucrose determination Extract (20 μl) was treated with 1.38 ml KOH (7.5%) at 90°C for 10 min. Then 140 μl of this reaction product were incubated for 20 min with 1 ml anthron reagent (150 mg anthron + 76 ml sulfuric acid + 30 ml water) at 37°C. An aliquot (200 μl) of the sample was measured in a microplate reader at 630 nm.
Starch determination The pellet after sugar extraction was washed, suspended in 200 μl and heated for 5 min in hot water. Then 2.5 μl α-amylase solution (STA-Kit, Sigma-Aldrich, St. Louis, MO, USA) and 200 μl STA2 reagent (10 U amyloglucosidase) were added, and the mixture was incubated for 15 min at 60°C. Samples of 10–50 μl of each reaction mixture were assayed for hexoses as described.
In situ determination of starch
Pieces of leaves were taken from the top visible dew lap leaf, sampled shortly before sunrise, and frozen in liquid nitrogen. The tissue samples were dehydrated in an ethanol series, embedded in paraffin, cross-sectioned (10 μm thick) with a microtome and rehydrated in an ethanol series, exactly as used for in situ hybridization (Woo et al. 1999). The sections were fixed on microscope slides and incubated in iodine solution (1 g KI and 1 g iodine in 100 ml water) for 10 min, then stopped with water for 5 s. The water was wiped off, the slides were air-dried, and 200 μl of Aquatex mounting medium (Merck, Darmstadt, Germany) was added to the slides before covering with cover slips. The cross-sectional area of the stained starch granules and of the cells was determined with Image-Pro software (Media Cybernetics, Bethesda, MD, USA).
Grading of symptoms
Symptoms were visually recorded at 6–8-week intervals and graded from 0 (no symptoms) to 6 (very severe symptoms) as described in Lehrer and Komor (2008).
Determination of viral infection
Source leaves of different cultivars were homogenized, the RNA was purified from the soluble extract with phenol–chloroform and transcribed to cDNA (Comstock et al. 1998; Sambrook and Russell 2001). The cDNA was amplified by RT-PCR with gene-specific primers (forward: 5′-CTTTCAAGGTTCGCTCGTTC-3′, reverse: 5′-TGAGCTGGTTGACTGGAGTG-3′) creating a 165-bp fragment.
The presence of SCYLV was also tested by tissue blot immunoassay (TBIA) as described previously (Fitch et al. 2001).
Statistics
The significance of any differences was calculated by the program SigmaStat3.1 (Systat Software, Richmond, CA, USA), using a pairwise t-test, pairwise Mann–Whitney test, or a Kruskal–Wallis one-way analysis of variance on ranks for multigroup data. Regression lines and r 2 were calculated by the statistics function of the program SigmaPlot9.0 (Systat).
Results
Selection of susceptible and resistant cultivars
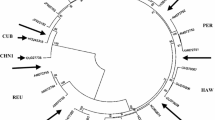
A previous survey using a TBIA had identified SCYLV-susceptible and SCYLV-resistant cultivars (Lehrer et al. 2009; Schenck and Lehrer 2000). Cultivars that expressed fluctuating levels of virus titer were called moderately susceptible. Tests for SCYLV by RT-PCR partly confirmed the different titers of virus in the different cultivars; however, the so-called resistant cultivars (H78-4153, H87-4319, H78-7750) that had appeared without SCYLV in TBIA, had SCYLV (Fig. 1), though at a much lower titer than, for example, H87-4094. H65-7052 appeared nearly virus-free, possibly the leaf had been sampled in a virus-poor phase. Nine cultivars were selected for a test of carbohydrate status of sugarcane plants at harvest time (16 months), the same cultivars that had been used previously in an extended yield test in different Hawaiian fields (Lehrer et al. 2009).
RT-PCR of RNA-derived cDNA from source leaves of different cultivars of sugarcane. RNA from leaves of six representative cultivars was extracted, transcribed to cDNA and amplified by RT-PCR. H73-6110 and H87-4094 are susceptible, H65-7052 is moderately susceptible, and H78-4153, H87-4319 and H78-7750 are resistant cultivars. The virus-free clone of H87-4094 was used as a negative control. The amplified Sugarcane yellow leaf virus (SCYLV) was 165 bp long, the size standard was a 50-bp DNA ladder (left)
Biomass of green leaf tops and stalks
The cultivars were grown in three plots in the field station of HARC and harvested after 16 months. The plants were cut by hand, and green leaf tops were separated from their stems. The stalk biomass of the different cultivars ranged between 100 and 200 t/ha, depending on cultivar and plot. The biomass of green leaf tops ranged between 15 and 45 t/ha. Differences showed up between some cultivars, but there was no correlation with SCYLV susceptibility (Fig. 2a, b). Severely symptomatic SCYLV-infected plants had bushy leaf tops, and differences might have been expected in the ratio of stalks to leaf tops. Visual inspections during the 4 months before harvest indeed showed that the high-titer, susceptible cultivars had mild to moderately severe symptoms in contrast to the low-titer, resistant cultivars (Table 1). However the stalk to leaf top ratio was not correlated with SCYLV susceptibility (Fig. 2c).
Biomass (fresh mass) of stalks (a) and green leaf tops (b), and the ratio of stalk to green leaf tops (c) of Sugarcane yellow leaf virus (SCYLV)-resistant and -susceptible cultivars infected by SCYLV. SCYLV-resistant cultivars (white), moderately susceptible cultivars (grey), susceptible cultivars (dark grey). Mean ± SD
Carbohydrate content of green leaf tops and stalks of SCYLV-susceptible and SCYLV-resistant cultivars
In situ localization and determination of starch in source leaves, harvested shortly before sunrise, showed that the susceptible cultivars had more starch in the bundle sheath cells than did the resistant cultivars, but even the resistant cultivar had higher starch levels than in the leaves of the virus-free plant (Fig. 3). The ratio of starch to cytoplasm area was significantly lower (P < 0.05) in the virus-free compared to the resistant and the susceptible cultivars (Table 2). The susceptible cultivar even had small starch grains in the mesophyll, a feature that was absent in the virus-free and the resistant cultivars.
Starch in sugarcane leaves from Sugarcane yellow leaf virus (SCYLV)-free, SCYLV-resistant and a SCYLV-susceptible (high titer) cultivar. Leaf samples from SCYLV-free and SCYLV-infected plant of cv. H87-4094, from the SCYLV-resistant cv. H78-4153, and from the susceptible (infected) cv. H73-6110 were collected in the hour shortly before sunrise, fixed, sectioned and stained with iodine
When the plants were harvested during the day, the carbohydrates of complete green leaf tops consisted mostly of sucrose, which was 4-fold higher (when based on hexose units) than the hexose content and 100-fold higher than the starch content (Table 3). The sucrose and the hexose levels were not significantly different between resistant and susceptible cultivars, starch had a slight trend toward lower content in the susceptible cultivars (P = 0.19, Table 3). The stalks contained (as expected) mostly sucrose. The sucrose content was 40-fold higher (based on hexose units) than the content of hexoses and 200-fold higher than starch (Table 3). Susceptible cultivars had significantly lower hexoses and starch in the stalks than did the resistant cultivars (Table 3). The moderately susceptible cultivar H65-7052 with strongly fluctuating SCYLV-titer was grouped with the susceptible cultivars, because it contained a 100-fold higher SCYLV-titer than in the other moderately susceptible cultivar H87-4319 (J. Zhu et al., unpublished manuscript). In contrast H87-4319 was grouped with the resistant cultivars based on its virus titer.
The carbohydrate species had some correlation among each other. In the green leaf tops, hexoses and sucrose were positively correlated, hexose and starch were negatively correlated (Fig. 4a). In stalks, hexoses were correlated positively with starch and slightly negatively with sucrose (Fig. 4b).
Relationship between the hexoses, sucrose and starch content per gram fresh mass of sugarcane green leaf tops and stems at harvest. The plants were harvested at noon at the age of 16 months. Open symbols susceptible (high titer) cultivars, grey symbols moderately susceptible (moderately high titer) cultivars, black symbols Sugarcane yellow leaf virus-resistant (infected but with low titer) cultivars. Data are the mean for eight plants in three replicates. The regressions of the plots for green leaf tops, upper graph r 2 = 0.88, lower graph r 2 = 0.93, for stems (right) upper graph r 2 = 0.99, and lower graph r 2 = 0.82
Discussion
Infection of sugarcane plants by SCYLV causes 10–30% yield reduction even when the plants are asymptomatic. However, the yields of naturally infected (the strain was probably the BRA-PER type; A. ElSayed, personal observation) cultivars of different SCYLV-susceptibilities, did not differ in tests in 10 Hawaiian fields (Lehrer et al. 2009). In general, this picture also emerged in the study here, where biomass of susceptible and resistant cultivars was compared in more detail, namely, of green leaf tops and of stalks, grown in a field of the Experiment Station. There were some differences in biomass between the cultivars on this particular field, but obviously, the difference was not along SCYLV-susceptibility.
The differences in carbohydrate composition indicated that SCYLV susceptibility had an impact on carbohydrate physiology of the plants. In a comparison of carbohydrate partitioning in SCYLV-infected and virus-free plants of cv. H87-4094, sucrose export from the leaves was reduced in the infected plants (Lehrer et al. 2007), resulting in a backup of sucrose, changes in chloroplast ultrastructure and finally degradation of chlorophyll (Yan et al. 2009). The present study showed that leaves of a susceptible cultivar harvested at the end of the night had stronger starch staining than did the resistant cultivar (Fig. 3), similar to the results of Yan et al. (2009). However the green leaf tops of susceptible cultivars when harvested during daytime revealed a trend toward less starch (Table 2). This discrepancy can be explained in two ways. The higher starch content in infected leaves is only evident at the end of the night when normally all transitory starch has been degraded and mobilized, whereas during day the starch levels rise strongly, and differences in starch are obscured. In addition, the green leaf top contains not only green source leaves, but also rolled-in sink leaves and the apical division zone internodes. Thus, the carbohydrate composition of the green leaf top is dominated by the young internodes and sink leaves, in which the resistant cultivars apparently contain more starch than the susceptible cultivars. Hexose in the leaves originates from transitory starch breakdown, which may explain rising hexose at the cost of starch.
No differences were observed in sucrose content of the green leaf tops, in contrast to SCYLV-infected plants of the cultivar H87-4094, where the sucrose concentration in the green leaf tops was 1.93-fold (±0.32, SD) higher than in virus-free plants. Hexose is positively correlated to sucrose. An increase in hexoses may result in more sucrose when sucrose is in equilibrium with hexose via sucrose synthase.
The stems of susceptible cultivars had less hexoses and less starch than resistant cultivars (Table 3), which was similarly observed in infected versus virus-free plants of cv. H87-4094 (Lehrer et al. 2007). In general, lower hexose and starch content indicates a more mature state of sugarcane internodes. So it appears that stem internodes of SCYLV-susceptible cultivars were faster in ripening than resistant cultivars, a feature that is observed to the extreme when sugarcane plants became symptomatic (Lehrer et al. 2007). Sucrose is delivered to the stem, and hexose is produced by invertase; thus high hexose content favors starch synthesis.
In conclusion, the SCYLV-susceptible cultivars differed from the resistant cultivars in carbohydrate composition, indicating an impact of SCYLV on sugar physiology. However, some results, for example, the biomass ratio of stalk to green leaf tops and the amounts of sucrose and starch in green leaf tops were opposite of the results obtained for cv. H87-4094 when comparing infected to virus-free plants (Lehrer et al. 2007). That discrepancy may lie in the process by which commercial cultivars are selected. The breeding program selected cultivars for maximum yield under the Hawaiian field conditions in the presence of SCYLV, so that lower-yielding clones of the progeny were eliminated, irrespective of a latent SCYLV infection. Therefore SCYLV-susceptible clones had to compensate by other means for the virus-caused sucrose backup, possibly by increasing sucrose transport activities or decreasing sucrose retrieval along the translocation pathway. The SCYLV test by RT-PCR showed that all cultivars contained the virus but at very different levels; the virus was barely detectable in the resistant varieties. Quantitative RT-PCR revealed that the SCYLV titer differed by 2–3 orders of magnitude (J. Zhu et al., submitted).
This study underlines the ability of plants, exemplified here by different cultivars, to modulate their carbohydrate metabolism to avoid or compensate for the adverse metabolic effects caused by viral infection.
References
Comstock JC, Irvine JE, Miller JD (1994) Yellow leaf syndrome appears on the United States mainland. Sugar J 56:33–35
Comstock JC, Irey MS, Lockhart BEL, Wang ZK (1998) Incidence of yellow leaf syndrome in CP cultivars based on polymerase chain reaction and serological techniques. Sugar Cane 4:21–24
Fitch MMM, Lehrer AT, Komor E, Moore PH (2001) Elimination of Sugarcane yellow leaf virus from infected sugarcane plants by meristem tip culture visualized by tissue blot immunoassay. Plant Pathol 50:676–680
Lehrer AT, Komor E (2008) Symptom expression of yellow leaf disease in sugarcane cultivars with different degrees of infection by Sugarcane yellow leaf virus. Plant Pathol 57:178–189
Lehrer AT, Moore PH, Komor E (2007) Impact of Sugarcane yellow leaf virus (ScYLV) on the carbohydrate status of sugarcane: comparison of virus-free plants with symptomatic and asymptomatic virus-infected plants. Physiol Mol Plant Pathol 70:180–188 (addition 71: 260)
Lehrer AT, Wu KK, Komor E (2009) Impact of Sugarcane yellow leaf virus on growth and sugar yield of sugarcane. J Gen Plant Pathol 75:288–296
Payne JH (ed) (1968) Sugar cane factory analytical control: the official methods of the Hawaiian Sugar Technologist. Elsevier, Amsterdam
Rassaby L, Girard J-C, Letourmy P, Chaume J, Irey MS, Lockhart BEL, Kodja H, Rott P (2003) Impact of Sugarcane yellow leaf virus on sugarcane yield and juice quality in Réunion Island. Eur J Plant Pathol 109:459–466
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
Schenck S (1990) Yellow leaf syndrome—a new sugarcane disease. Annual report no. 38, Hawaiian Sugar Planters Association, Aiea
Schenck S, Lehrer AT (2000) Factors affecting the transmission and spread of Sugarcane yellow leaf virus. Plant Dis 84:1085–1088
Vega J, Scagliusi SMM, Ulian EC (1997) Sugarcane yellow leaf disease in Brazil: evidence of association with a luteovirus. Plant Dis 81:21–26
Woo H-H, Orbach MJ, Hirsch AM, Hawes MC (1999) Meristem-localized inducible expression of a UDP-glycosyltransferase gene is essential for growth and development in pea and alfalfa. Plant Cell 11:2303–2315
Yan S-L, Lehrer AT, Hajirezaei MR, Springer A, Komor E (2009) Modulation of carbohydrate metabolism and chloroplast structure in sugarcane leaves which were infected by Sugarcane yellow leaf virus (SCYLV). Physiol Mol Plant Pathol 73:78–87
Acknowledgments
The authors are grateful to the field workers of the Hawaii Agriculture Research Center for their help and to the staff of the analytical lab for standard sugar determinations. Thanks to Alberto Bertolini, University Udine, who participated in the in situ starch determination project. This work was funded by Deutsche Forschungsgemeinschaft and by Deutscher Akademischer Austauchdienst, which supported Yan Shih-Long, Alberto Bertolini and Blanca Fontaniella.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehrer, A., Yan, SL., Fontaniella, B. et al. Carbohydrate composition of sugarcane cultivars that are resistant or susceptible to Sugarcane yellow leaf virus . J Gen Plant Pathol 76, 62–68 (2010). https://doi.org/10.1007/s10327-009-0210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-009-0210-0