Abstract
After a cell wall protein fraction (CWP) of Pythium oligandrum was sprayed on sugar beet leaves, we screened leaves for induced expression of defence-related genes and for resistance against Cercospora leaf spot. In a western blot analysis, the CWP was primarily retained on the surface of leaves without degradation for at least 48 h after spraying. In northern blot analyses, four defence-related genes (β-1, 3-glucanase, acidic class III chitinase, 5-enol-pyruvylshikimate-phosphate synthase and oxalate oxidase-like germin) were expressed more rapidly in CWP-treated leaves compared to control leaves treated with distilled water (DW). When CWP was applied to a suspension of cultured cells of sugar beet, an oxidative burst was observed that did not occur after the DW treatment. In growth chamber trials after inoculation with Cercospora beticola, the severity of Cercospora leaf spot was significantly reduced in CWP-treated plants compared to the DW-treated controls. In a field experiment, CWP treatment was also effective against the disease. CWP did not reduce growth rate of the pathogen in plate tests. The results together suggest that the CWP from P. oligandrum can be retained on the leaf surface and induce expression of disease resistance genes, thereby reducing Cercospora leaf spot on sugar beet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pythium oligandrum, which is nonpathogenic to plants, is well known as an aggressive colonizer capable of attacking a wide range of fungal and oomycete plant pathogens (Benhamou et al. 1999; Lewis et al. 1989). Initial studies on the potential use of this species as a biocontrol agent focused particularly on its mycoparasitic characteristics. The biological control potential of P. oligandrum was demonstrated for damping-off and root diseases caused by fungal pathogens, with which P. oligandrum had been observed to act as a mycoparasite in dual culture tests (Al-Rawahi and Hancock 1998; Davanlou et al. 1999; Martin and Hancock 1987). P. oligandrum was also proposed to act as a biocontrol agent via production of antibiotics and hydrolytic enzymes (Benhamou et al. 1999; Lewis et al. 1989; Picard et al. 2000b) and competition for rhizosphere colonization against soilborne pathogens (Takenaka et al. 2008). Microscopic examination of the interaction between tomato and Fusarium oxysporum f. sp. radicis-lycopersici provided the first convincing evidence that P. oligandrum also has the potential to induce plant defence reactions (Benhamou et al. 1997). Later studies also demonstrated that P. oligandrum can induce plant defence reactions and resistance to bacterial and fungal pathogens, such as Ralstonia solanacearum in tomato (Hase et al. 2006, 2008; Masunaka et al. 2009; Takenaka et al. 2008), and Botrytis cinerea in tomato (Le Floch et al. 2003) and grapevine (Mohamed et al. 2007). In recent years, the ability of P. oligandrum to induce resistance has received considerable attention for developing control measures against different types of pathogen through the activation natural defences in plants.
The induction of a plant defence system may be mediated by elicitor molecules during the interaction between plants and microbes. Two types of elicitor proteins have been identified in P. oligandrum, and their biological and molecular characteristics determined. One type, oligandrin, is an extracellular elicitor protein with an elicitin domain and is secreted into the culture filtrates (Picard et al. 2000a). Elicitin is a family of low molecular weight proteins secreted by Phytophthora and some Pythium species and induces a hypersensitivity response in Nicotiana spp. (family Solanaceae) and in Brassica spp. and Raphanus spp. (family Brassicaceae) (Ponchet et al. 1999). The cell wall protein (CWP) contains another type of elicitor protein from P. oligandrum, the elicitin-like proteins, POD-1 and POD-2 (Takenaka et al. 2006). Molecular characterization of these proteins indicates that (i) POD-1 is 17.9 kDa, and POD-2 is 16.6 kDa in molecular mass; (ii) POD-1 and POD-2 have 82.9% amino acid homology; (iii) the amino acid sequences of POD-1 and POD-2 have 53.5% and 51.5% identity, respectively, to oligandrin in the elicitin domain; (iv) POD-1 and POD-2 have an O-glycosylated domain at their C-termini, which is not present in oligandrin; and (v) POD-1, POD-2 and oligandrin are phylogenetically distinct from previously defined elicitin and elicitin-like proteins (Takenaka et al. 2006).
Application of oligandrin to the apex of decapitated tomato plants induces the formation of structural and biochemical barriers in plants infected with Phytophthora parasitica (Picard et al. 2000a) or F. oxysporum f. sp. radicis-lycopersici (Benhamou et al. 2001). When oligandrin is placed on sectioned petioles of tobacco plants, phloem proteins accumulate in the mature sieve tubes of the leaves, and the level of phytoplasma infection is reduced (Lherminier et al. 2003). Recently, Mohamed et al. (2007) reported that oligandrin applied to grapevine roots could protect leaves against B. cinerea. Similarly, treatment of tomato leaves with CWP induces defence-related genes and increases the levels of ethylene, resulting in a decrease in disease severity after infection by Ralstonia solanacearum (Kawamura et al. 2009). When sugar beet roots were allowed to take up CWP from solution, the expression of defence-related genes and the enzymatic activities of phenylalanine ammonia-lyase and chitinase increased, and wall-bound phenolic compounds accumulated. As a consequence, the severity of seedling diseases caused by Rhizoctonia solani AG2-2 and Aphanomyces cochlioides was significantly reduced (Takenaka et al. 2003, 2006).
Sugar beets (Beta vulgaris L.) are cultivated only in the Hokkaido region of Japan, where Cercospora leaf spot caused by Cercospora beticola is one of the most important, widespread, and destructive foliar diseases affecting the crop. The disease is generally controlled via a field spray of fungicides three to five times per year (Watanabe et al. 2002). This approach, however, has raised concerns regarding the negative impact of the chemicals on the environment and the development of fungal resistance. One alternative approach to controlling the disease without the use of fungicides may be through the use of elicitors of plant defenses, which can be natural, safe and effective.
In this study, we sought to determine whether foliar spraying with CWP could trigger defence reactions in sugar beets similar to those observed after soaking roots in CWP solution (Takenaka et al. 2003, 2006). First, we determined the movement of CWP in sugar beet after its application, then we analyzed the induction of defence-related genes after direct spraying and the level of the oxidative burst in sugar beet suspension cultures. Next, we compared the relative effectiveness of CWP and fungicides in reducing C. beticola infection in the growth chamber and the field to determine whether a CWP spray is a viable approach for controlling Cercospora leaf spot.
Materials and methods
Fungal and plant materials
The P. oligandrum isolate NITE P-619 (MMR2: D-type based on the number of major proteins in CWP; Takenaka et al. 2003), isolated from soybean soil in Hokkaido Prefecture, was grown in V8 broth (Campbell’s V8 juice 100 ml/l, CaCO3 1.5 g/l) at 25°C for 3 weeks in the dark. Mycelial mats were collected by filtration, washed with distilled water (DW) and blotted dry with a paper towel; they were then used for the extraction of CWP. C. beticola isolate MAFF241661, obtained from infected sugar beet leaves from Hokkaido Prefecture, was grown in V8 broth agar containing 0.5% yeast extract at 23°C for 2–3 weeks with a 16-h photoperiod for spore production. Sugar beet plants (Beta vulgaris cv. Etopirika) were grown on quartz sand in glass tubes and received a daily supplement of 5 ml of a nutrient solution as described by Abe (1987) and 5 ml of a 500-fold dilution of Hyponex solution (Hyponex Japan, Osaka, Japan) at 1-week intervals for 21–23 days in a growth chamber at 23°C with a 16-h photoperiod (66.5 W/m2).
CWP extraction and application
CWP was extracted from P. oligandrum isolate MMR2 as described previously (Takenaka and Kawasaki 1994; Takenaka et al. 2006). Protein content was determined by the method of Lowry et al. (1951) using bovine serum albumin as the standard. Each seedling was sprayed with 2 ml CWP solution (100 µg/ml), and control seedlings were sprayed with the same volume of DW. Thereafter, sprayed seedlings were wrapped in a polyethylene bag to maintain humidity and were incubated in the growth chamber at 23°C with a 16-h photoperiod (66.5 W/m2). Leaf tissues were collected at indicated times after treatment, frozen in liquid nitrogen and stored at –80°C until used for protein isolation for western blot analysis and RNA isolation for northern blot analysis.
Western blot analysis
Sugar beet tissues for western blot analysis were ground in liquid nitrogen and dissolved in a 1:5 w/v ratio of electrophoresis sample buffer (65 mM Tris–HCl, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol). These homogenates were heated at 100°C for 5 min, centrifuged, and the supernatants collected. To detect CWP in the supernatants, we separated the solubilized proteins on 12.5% SDS-PAGE using the discontinuous buffer system described by Laemmli (1970) and transferred them onto a nitrocellulose membrane. Western blot analysis was performed with a polyclonal antiserum against purified POD-1; antibody binding was detected using the WesternBreeze immunodetection kit (Invitrogen, Carlsbad, CA, USA) and the manufacturer’s instructions. To remove nonspecific reactions against the numerous sugar beet proteins, we pretreated the antiserum with sugar beet seedling homogenates by dilution at 1:1000 in leaf homogenate prepared with TBST (20 mM Tris–HCl, 0.15 M NaCl, 0.05% NaN3, 0.05% Tween 20, pH 7.5) containing 0.2% bovine serum albumin.
Northern blot analysis
Total RNA was isolated from CWP- and DW-treated leaf tissues using an RNeasy Plant Mini Kit (Qiagen, Mississauga, ON, Canada) and the manufacturer’s instructions. RNA (5 μg) was separated by electrophoresis on denaturing formaldehyde agarose gels and transferred to nylon membranes (Hybond-N+, Amersham Bioscience, Little Chalfont, U.K.) via capillary transfer, as described by Sambrook and Russell (2001). Expression of the genes encoding β-1, 3-glucanase 2 (Glu2), acidic class III chitinase (SE2), 5-enol-pyruvylshikimate-phosphate synthase (EPSPS) and oxalate oxidase-like germin (OxOLG) was analyzed. The genes for EPSPS and OxOLG proteins were previously identified and cloned as CWP-induced genes in the root and hypocotyl tissues of sugar beet using PCR-based cDNA library subtraction (Takenaka et al. 2006). Glu2 and SE2 were found to accumulate in necrotic tissue and in the vicinity of necrotic lesions in sugar beet infected with C. beticola (Gottschalk et al. 1998; Nielsen et al. 1993). To detect the expression of Glu2 (X75946) and SE2 (S66038), we amplified both fragments of approximately 500 bp with RT-PCR using the primers 5′-CAACTTACCTTCCGAGGAAG-3′ and 5′-GCATACTTGGGGGTGCCAATG-3′ for Glu2, and the primers 5′-ATTGTCATATACTGGGGCC-3′ and 5′-ATGGCAGTGCTTAAGCTGGC-3′ for SE2. Total RNA from CWP-treated sugar beet tissues was used as the template, and RT-PCR was performed using One Shot Superscript One-Step RT-PCR with Platinum Taq (Invitrogen). The following PCR conditions were used: 1 cycle at 55°C for 30 min; 40 cycles at 94°C for 15 s, 55°C for 30 s, and 68°C for 30 s for PCR; and 1 cycle at 68°C for 5 min for final extension. The PCR product was cloned into the PCR 2.1-TOPO Vector and used to transform E. coli TOPO10 (Invitrogen). To confirm the identity of the cloned DNA, we determined the nucleotide sequence of the insert using an ABI PRISM 3100-Avant Genetic Analyzer (Applied Bioystems, Foster City, CA, USA) with the BigDye Terminator Cycle Sequencing FS Ready Reaction Kit (Applied Biosystems) according to the manufacturer’s instructions. The cloned DNA fragments were labeled with a digoxigenin (DIG) northern starter kit (Roche Molecular Biochemicals, Mannheim, Germany). Northern hybridization with DIG-Easy Hyb Granules and immunological detection using alkaline phosphatase-conjugated anti-digoxigenin antibody and the chemiluminescent substrate CDP-Star were performed following the manufacturer’s instructions (Roche Molecular Biochemicals).
Sugar beet cell cultures and oxidative burst measurement
The sugar beet line NK-219 mm-O was used to generate suspensions of cultured cells because of its comparatively high frequency of generating calli from leaf blades (H. Tamagake, unpublished data). This line was provided by the Biomass Research Team of National Agricultural Research Center of Hokkaido Region. In vitro plants raised by germinating sterilized seeds were first grown in Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with sucrose (30 g/l) and agarose (8 g/l), pH 5.8, in a growth chamber at 23°C with a 16-h photoperiod (66.5 W/m2). For callus induction, leaf samples from plants growing in the tissue culture were placed on the culture medium described supplemented with 6-benzylaminopurine (BAP) (0.25 g/l), and incubated in the dark at 25°C. When friable callus developed, it was transferred into liquid medium (the same tissue culture medium supplemented with BAP as described except without agarose) and incubated in a rotary shaker at 120 rpm, 25°C, in the dark, and freshly reinoculated every week. Three- to six-day-old cultured cells were collected by filtration, washed with the assay buffer (5 mM MES buffer containing 175 mM mannitol, 0.5 mM CaCl2 and 0.5 mM K2SO4, pH 6), and then resuspended at 0.1 g /ml in the assay buffer and equilibrated at 25°C at 120 rpm in the dark. After at least a 2-h equilibration, 200 μl of CWP solution (920 μg/ml) or DW (as control) was added to 1 ml of the cell suspension, which was then reincubated at 25°C and 120 rpm in the dark. The production of active oxygen species (AOS) was measured by chemiluminescence using ferricyanide-catalyzed oxidation of luminol as described by Piedras et al. (1998). Briefly, at specific intervals, 50 μl of the cell supernatant was removed and mixed with 150 μl of 50 mM potassium phosphate buffer, pH 7.9, and 25 μl of 1.1 mM luminol. The reaction was initiated by addition of 25 μl of 14 mM ferricyanide and after 5 s the emission of light was determined over a 20 s interval using a Luminescencer-JNRII (ATTO Co., Tokyo, Japan).
Plant assay for suppression of Cercospora leaf spot by CWP
Sugar beet plants (cv. Etopirica, a cultivar susceptible to Cercospora leaf spot disease) for the growth chamber tests were grown in plastic pots (1.5 l, 12 cm diameter, and 15 cm deep) containing artificial horticultural soil (N 374 mg/kg, P2O5 1485 mg/kg, K2O 242 mg/kg and MgO 165 mg/kg; Engei-baido, Hokkai Sankyo Co. Ltd., Kitahiroshima, Japan) at 25°C (day)/20°C (night) with a 16-h photoperiod (66.5 W/m2). The plants were given 500-fold diluted Hyponex solution (Hyponex Japan) once a week. Plants used for artificial inoculation with C. beticola were 55–57 days old, when there were 10–12 fully expanded leaves. The experiment was designed to test the efficacy of CWP application before inoculation with C. beticola. Four treatments were carried out using a hand sprayer at the rate of 10 ml per plant, 24 h before inoculation with C. beticola: (i) DW control, (ii) CWP at 10 μg/ml, (iii) CWP at 100 μg/ml and (iv) difenoconazole, a fungicide with sterol demethylation inhibitory characteristics, at 83.3 μg a.i. (activity ingredient)/ml. The C. beticola inoculum was prepared by harvesting spores from V8 broth agar containing 0.5% of yeast extract using water and a paintbrush. Spore concentration was determined with a hemacytometer, and adjusted to 3,000 spores/ml. The spore suspension was applied with a hand sprayer to sugar beet leaves at the rate of 10 ml per plant. Plants were covered with transparent polyethylene bags to maintain humidity in the growth chamber for 5–7 days, then the polyethylene bags were removed and the incubation was continued until disease assessment. Disease severity in each treatment group was estimated 2 and 3 weeks after inoculation using a scale of 0–5: 0 = no symptoms on fully expanded leaves; 1 = small lesions on <50% of fully expanded leaves; 2 = small lesions on >50% of fully expanded leaves; 3 = necrosis on <50% of fully expanded leaves; 4 = necrosis on >50% of fully expanded leaves; 5 = leaf and leaf stalk dead. Each treatment group consisted of nine plants, and the experiment was repeated three times. The effect of CWP on disease suppression was determined by an analysis of variance with experimental treatments as blocks. Dunnett’s one-sided t test was used to determine significant differences (P < 0.05).
A field trial was conducted in Memuro, Hokkaido, northern Japan in 2008. Experimental field plots of 6.5 m × 12 m, incorporating 17 sugar beet rows, were arranged in a randomized block design. The experiment consisted of five different treatments with four replicate plots per treatment. Each plant received 10 ml from a hand sprayer: (i) control (untreated); (ii) two CWP applications at 10 μg/ml each on 10 and 26 July 2008; (iii) three CWP applications at 10 μg/ml each on 10 and 26 July, and 8 August 2008; (iv) four CWP applications at 10 μg/ml each on 10 and 26 July, and 8 and 22 August 2008; (v) two mancozeb applications at 1.5 mg a.i./ml each on 26 July and 22 August 2008 and one difenoconazole application at 100 μg a.i./ml on 8 August 2008. C. beticola inoculum was prepared by mixing a powder from dried infected leaf tissues with five times the mass of the soil. The mixture was applied on 10 July 2008 to the soil surface around the plant at 13.6 g per plant. Disease severity was assessed in 25–30 plants per replicate plot on 8 September 2008, and the effect of CWP on disease suppression was determined using the same disease index and statistical analysis described.
Plate test for reduction of pathogen growth
To investigate the ability of CWP to reduce the hyphal growth of C. beticola, we applied CWP solution (5 μg in 50 μl), difenoconazole (16.7 μg a.i. in 50 μl), and sterilized DW (50 μl) to paper disks near the growing margin of a fresh culture of C. beticola grown in PDA plates at 25°C for 10 days, and fungal growth rates were compared 3 days after treatment.
Results
Western blot analysis of CWP-treated sugar beet tissues
The presence of POD-1 and POD-2 in sugar beet leaves after spraying with CWP was investigated using western blot analysis with a polyclonal antiserum to POD-1. Representative western blots from one of the three independent experiments are shown in Fig. 1. POD-1 was detected from CWP-treated leaves from 4 to 48 h after treatment without degradation, but not in DW control. From CWP-treated leaves, POD-2 was also detected at a lower level from 4 to 48 h after treatment. Because POD-1 and POD-2 have an 83% homology in their amino acids, POD-2 has a lower reactivity to the POD-1 antiserum. When the surfaces of CWP-treated leaves had been washed with tap water at the time of sampling, the intensities of detected bands of POD-1 were significantly reduced and POD-2 was not detected (Fig. 1). The result suggests that POD-1 and POD-2 are primarily retained on the surface of sugar beet leaves for at least 48 h and do not migrate into the leaves after spraying with a CWP solution.
Western blot analysis using an antiserum to POD-1 of leaf extracts from sugar beet plants treated with cell wall protein fraction (CWP), which contains POD-1 and POD-2, or distilled water (DW). Each sugar beet seedling was sprayed with 2 ml of CWP solution (100 μg/ml) or 2 ml of DW. Leaf tissues were collected 0, 4, 12, 24 and 48 h after treatment
Change in expression patterns of defence-related genes in sugar beet leaf tissues
To investigate whether CWP application to sugar beet leaves induced defence-related genes, we screened leaves for the expression of the genes for basic β-1, 3-glucanase (Glu2) and acidic class III chitinase (SE2) at 0, 4, 12 and 24 h after spraying with CWP or DW using northern blot analysis; these enzymes were previously reported to accumulate in leaves of sugar beet during infection with C. beticola (Gottschalk et al. 1998; Nielsen et al 1993). In addition, we examined the pattern of expression of the genes for 5-enol-pyruvylshikimate-phosphate synthase (EPSPS) and oxalate oxidase-like germin (OxOLG); these genes were previously identified as highly expressed in sugar beet roots that had been dipped into a CWP solution (Takenaka et al. 2006). Representative northern blots from three or four independent experiments are shown in Fig. 2. At 0 h after spraying, basal levels of these four gene transcripts were low to nondetectable (Fig. 2). SE2 and Glu2 transcripts were strongly induced 4 h after spraying with CWP, but thereafter declined to lower levels. By contrast, in DW-treated leaves transcript levels retained low to nondetectable. Although the level of the EPSPS and OxOLG transcripts in sugar beet leaves increased over time after treatment with DW; however, CWP treatment caused a rapid increase in gene expression within 4 h of treatment.
Northern blot analysis of four defence-related gene transcripts (Glu2 basic β-1, 3-glucanase, SE2 acidic class III chitinase, EPSPS 5-enol-pyruvylshikimate-phosphate synthase, OxOLG oxalate oxidase-like germin, EtBr ethidium bromide) in leaf tissues of sugar beet plants each sprayed with either 2 ml cell wall protein fraction (CWP, 100 μg/ml) or 2 ml distilled water (DW). Total RNA isolated at 4, 12 and 24 h after treatment
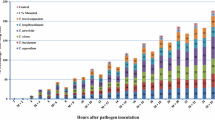
Induction of oxidative burst in sugar beet cells
Defence reactions can include a rapid and intense production of highly reactive and toxic oxygen species generated through the sequential one-electron reduction of oxygen (Garcia-Brugger et al. 2006). To determine whether the CWP treatment of sugar beet influenced the rate of production of active oxygen species (AOS), we screened the cultured sugar beet cell line NK-219 mm-O using a chemiluminescence assay. The pattern of AOS generation in one of the three independent experiments is shown in Fig. 3. Cultured cells treated with DW had low levels of AOS generation, and there were no significant changes throughout the assay from 0 to 6 h after treatment. In contrast, the cultured cells treated with CWP had an increase in AOS generation within 1 h and reached a maximum level at 5 h after treatment (Fig. 3). CWP treatment caused an approximately 3.2-fold increase in the maximum level of AOS generation compared with DW treatment. The level of AOS generation induced by the CWP treatment decreased after 5 h. The other two experiments had slightly different accumulation patterns: the maximum level of AOS generation was observed at 2 and 3 h, and the relative increase after CWP treatment was 2.7 fold and 4.3 fold, respectively (data not shown). Nevertheless, a significant induction of AOS generation was observed from 0 to 6 h after CWP treatment.
Active oxygen species (AOS) in sugar beet cells (line NK-219 mm-0) treated with cell wall protein fraction (CWP) or distilled water (DW) were determined by a chemiluminescence assay. Cells were treated by adding either 200 μl of CWP solution (920 μg/ml) or distilled water (DW) to 1 ml of cell suspension, then incubated for the indicated times
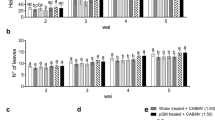
Cercospora leaf spot suppression
We examined the ability of CWP to reduce Cercospora leaf spot in sugar beet in a growth chamber, by comparing CWP-, fungicide- (difenoconazole) and DW-treated plants that had been challenged with C. beticola 24 h after treatment. The fungicide treatment was most effective in controlling the disease. However, disease severity at 2 weeks after inoculation was reduced by 50.7 and 52.0% after treatment with 10 μg/ml and 100 μg/ml of CWP, respectively, compared with DW (Fig. 4); these reductions in disease severity were statistically significant. At 3 weeks after inoculation, the severity of the disease increased in plants in all treatment groups, however, the rate of increase was significantly reduced by 25.9% after 10 μg/ml of CWP and by 46.0% after 100 μg/ml of CWP as compared with the DW control.
Effect of pretreatment with cell wall protein fraction (CWP) on Cercospora leaf spot disease severity on sugar beet plants in growth chambers. Plants were challenge inoculated with Cercospora beticola (3 × 104 spores per plant) 24 h after treatment with 10 ml per plant of distilled water (DW), CWP at 10 μg/ml, CWP at 100 μg/ml or difenoconazole at 83.3 μg a.i./ml. Disease severity was estimated 2 and 3 weeks after inoculation using an index on a 0 -5 scale as described in the Materials and methods. Error bars indicate standard errors of three independent experiments and asterisks indicate significant differences (P < 0.05) according to Dunnett’s test between CWP and DW treatments at the same sampling interval
The efficacy of CWP in controlling Cercospora leaf spot was also examined in a field experiment in 2008. A heavy Cercospora leaf spot attack developed in untreated control plots as a result of the artificial inoculation and climate conditions favorable for disease (Table 1). In heavily attacked plants, two to three CWP spray applications did not significantly affect disease progression compared with untreated controls. However, four CWP spray applications significantly reduced disease severity as compared with the DW control as the fungicidal treatment (mancozeb + difenoconazole + mancozeb) did.
When difenoconazole (16.7 μg a.i. in 50 μl) or a CWP solution (5 μg in 50 μl) was applied to paper disks located near the growing margin of a fresh culture of C. beticola in a PDA plate, fungal growth was significantly reduced around the difenoconazole treated-paper disk but not around the CWP-treated disk (Fig. 5). These results demonstrate that CWP does not act as an antibiotic, unlike difenoconazole, despite having significant activity in reducing Cercospora leaf spot development in growth chamber and field experiments.
A plate assay of growth inhibition of Cercospora beticola. Sterilized distilled water (SDW, 50 μl), cell wall protein fraction (CWP, 5 μg in 50 μl) for difenoconazole (DI, 16.7 μg a.i. in 50 μl) was applied to paper disks located near the growing margin of a fresh culture of C. beticola in a PDA plate and incubated at 25°C for 3 days
Discussion
In this study we examined the ability of the CWP isolated from the biocontrol agent P. oligandrum to induce sugar beet defence reactions and resistance against Cercospora leaf spot when directly sprayed onto leaves. First, we investigated the movement of CWP using western blot analysis after spraying. POD-1 and POD-2 were clearly detected from leaves without degradation until 48 h after spraying, but they diminished on the surface of the leaves that were washed with tap water at the time of sampling (Fig. 1). This result suggests that the CWP persists primarily at the site of application although migration into leaves cannot be completely ruled out. Cryptogein, an elicitin secreted by Phytophthora cryptogea, can migrate over substantial distances, probably in its native form, when applied to cut root tissues of tobacco plants (Devergne et al. 1992; Zanetti et al. 1992). Similarly, oligandrin, an extracellular elicitin-like protein of P. oligandrum, is transported through the vascular system in tomato after application to a decapitated apex or a wounded petiole (Picard et al. 2000a). On the contrary, the cell wall protein CBEL, isolated from Phytophthora parasitica var. nicotianae, binds to plant cell walls and elicits necrosis and defence gene expression in tobacco (Villalba Mateos et al. 1997). CBEL has two cellulose binding domains (CDBs) that belong to the carbohydrate module 1 family (Gaulin et al. 2006). The CBDs of CBEL are essential and sufficient to stimulate defence responses in tobacco and Arabidopsis thaliana leaf infiltration assays (Gaulin et al. 2006). They have been defined as novel pathogen-associated molecular patterns (PAMPs) in oomycetes that might act through interaction with the plant cell wall. The amino acid sequences of POD-1 (AB217820) and POD-2 (AB217821) do not have this type of CBD, but they also have the ability to bind to fibrous cellulose in a dose-dependent manner (S. Takenaka, unpublished data). The CDBs of POD-1 and -2 to cellulose will be investigated in future work.
The data presented here indicate that foliar spraying with CWP caused a rapid induction of the defence-related genes Glu2, SE2, EPSPS and OxOLG (Fig. 2). Transient inductions of Glu2 and SE2 during the early stages of infection with C. beticola are much stronger in tolerant plants than in susceptible plants (Nielsen et al. 1993, 1994). EPSPS and OxOLG genes were identified by PCR-based cDNA library subtraction as highly expressed after application of CWP to sugar beet roots (Takenaka et al. 2006). EPSPS is an enzyme active in one of the initial steps in the shikimate pathway, and OxOLG catalyses the oxidation of oxalate, yielding CO2 and H2O2. Our chemiluminescence assay of cultured sugar beet cells showed that a significantly higher production of AOS was observed 1–6 h after treatment with CWP (Fig. 3). AOS production is a representative early signaling event following elicitor perception in plants (Garcia-Brugger et al. 2006). In a wide range of incompatible interactions between plants and pathogen such as bacteria, fungi or viruses, biphasic production of AOS has been observed with the first phase peaking after 20 min, and the second phase occurring 4–6 h later. This second phase has been correlated with plant resistance (Garcia-Brugger et al. 2006). Although a first phase peak was not observed in this study because we did not examine the response within 1 h of treatment, the second phase was observed at the anticipated time after treatment, indicating that AOS production caused by CWP treatment is correlated with plant resistance. These results suggested that spraying CWP on sugar beet leaves triggers the basal defense system with the production of AOS, antimicrobial metabolites and pathogenesis-related proteins. Recently, our group undertook a more detailed analysis of the molecular mechanisms of CWP-induced resistance, such as signaling pathways, in laboratory-grown miniature tomatoes cv. Micro-Tom. A cDNA array analysis of gene expression in tomato roots at 4 h after dipping roots in CWP solution showed that 144 genes were upregulated, including defence-related genes (e.g., PR protein P14 and class II chitinase genes), oxidative stress-related genes (e. g., glutathione S-transferase and superoxide dismutase genes), jasmonic acid (JA)- and ethylene-related genes, and genes encoding enzymes involved in phytoalexin production (Takahashi et al. 2006). Hase et al. (2006) reported that the level of ethylene in CWP-treated plants is transiently elevated six- to 11-fold at 4 to 8 h after CWP treatment, followed by high expression of three basic ethylene-inducible defence-related genes, PR-2b, PR-3b and PR-5b.
Our growth chamber test indicated that CWP, even at 10 μg/ml, was effective at reducing Cercospora leaf spot, one of the most important foliar diseases that farmers need to control with fungicidal sprays (Fig. 4). A field test in 2008 showed that two to three CWP applications (10 μg/ml each) were ineffective, but that four CWP spray applications significantly reduced disease severity under high inoculum pressure and favorable conditions for disease development (Table 1). These reductions are probably due to the ability of CWP to induce resistance in sugar beet because CWP had no fungicidal activity against C. beticola in plate tests (Fig. 5). The ultimate aim of our research with CWP is to control disease in practical, commercial situations. One problem of protein elicitor use in the field is the difficulty of preparing large quantities. However, CWP is not too laborious to prepare; the average amount of CWP extracted from mycelial mats of P. oligandrum was 2.68 mg (dry mass)/g (fresh mass), indicating a remarkably high protein fraction in P. oligandrum. Another problem is insufficient disease control compared to fungicides. In the field, expression of induced resistance caused by elicitors is likely to be influenced by several factors such as the environment, plant genotype and nutrition. As Walters et al. (2005) pointed out, we need to pay attention to factors that are likely to influence the effectiveness of biocontrols in the field.
References
Abe H (1987) Studies on the ecology and control of Polymyxa betae Keskin, as a fungal vector of the causal virus (beet necrotic yellow vein virus) of rhizomania disease of sugar beet. Hokkaido Central Agricultural Experiment Station Report #60, Naganuma, Hokkaido, Japan
Al-Rawahi AK, Hancock JG (1998) Parasitism and biological control of Verticillium dahliae by Pythium oligandrum. Plant Dis 82:1100–1106
Benhamou N, Rey P, Chérif M, Hockenhull J, Tirilly Y (1997) Treatment with the mycoparasite Pythium oligandrum triggers induction of defense-related reactions in tomato roots when challenged with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 87:108–122
Benhamou N, Rey P, Picard K, Picard K, Tirilly Y (1999) Ultrastructural and cytochemical aspects of the interaction between the mycoparasite Pythium oligandrum and soilborne plant pathogens. Phytopathology 89:506–517
Benhamou N, Bélanger RR, Rey P, Tirilly Y (2001) Oligandrin, the elicitin-like protein produced by the mycoparasite Pythium oligandrum, induces systemic resistance to Fusarium crown and root rot in tomato plants. Plant Physiol Biochem 39:681–696
Davanlou M, Madsen AM, Madsen CH, Hockenhull J (1999) Parasitism of macroconidia, chlamydospores and hyphae of Fusarium culmorum by mycoparasitic Pythium species. Plant Pathol 48:352–359
Devergne J-C, Bonnet P, Panabières F, Blein J-P, Ricci P (1992) Migration of the fungal protein Cryptogein within tobacco plants. Plant Physiol 99:843–847
Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecouriex D, Poinssot B, Wendehenne D, Pugin A (2006) Early signaling events induced by elicitors of plant defenses. Mol Plant-Microbe Interact 19:711–724
Gaulin E, Dramé N, Laffitte C, Torto-Alalibo T, Martinez Y, Ameline-Torregrosa C, Khatib M, Mazarguil H, Villalba-Mateos F, Kamoun S, Mazars C, Dumas B, Bottin A, Esquerré-Tugayé MT, Rickauer M (2006) Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen-associated molecular patterns. Plant Cell 18:1766–1777
Gottschalk TE, Mikkelsen JD, Nielsen JE, Nielsen KK, Brunstedt J (1998) Immunolocalization and characterization of a β-1, 3-glucanase from sugar beet, deduction of its primary structure and nucleotide sequence by cDNA and genomic cloning. Plant Sci 132:153–167
Hase S, Shimizu A, Nakaho K, Takenaka S, Takahashi H (2006) Induction of transient ethylene and reduction in severity of tomato bacterial wilt by Pythium oligandrum. Plant Pathol 55:537–543
Hase S, Takahashi S, Takenaka S, Nakaho K, Arie T, Seo S, Ohashi Y, Takahashi H (2008) Involvement of jasmonic acid signaling in bacterial wilt disease resistance induced by biocontrol agent Pythium oligandrum in tomato. Plant Pathol 57:870–876
Kawamura Y, Hase S, Takenaka S, Kanayama Y, Yoshikawa H, Takahashi H (2009) INF1 elicitin activates jasmonic acid- and ethylene-mediated signaling pathways and induces resistance to bacterial wilt disease in tomato. J Phytopathol 157:287–297
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685
Le Floch G, Rey P, Déniel F, Benhamou N, Picard K, Tirilly Y (2003) Enhancement of development and induction of resistance in tomato plants by antagonist, Pythium oligandrum. Agronomie 23:455–460
Lewis K, Whipps JM, Cooke RC (1989) Mechanisms of biological disease control with special reference to the case study of Pythium oligandrum as an antagonist. In: Whipps JM, Lumdsen RD (eds) Biotechnology of fungi for improving plant growth. Cambridge University Press, Cambridge, pp 191–217
Lherminier J, Benhamou N, Larrue J, Milat M-L, Boundon-Padieu E, Nicole M, Blein J-P (2003) Cytological characterization of elicitin-induced protection in tobacco plants infected by Phytophtora parasitica or Phytoplasma. Phytopathology 93:1308–1319
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Martin FN, Hancock JG (1987) The use of Pythium oligandrum for biological control of preemergence damping-off caused by P. ultimum. Phytopathology 77:1013–1020
Masunaka A, Nakaho K, Sakai M, Takahashi H, Takenaka S (2009) Visualization of Ralstonia solanacearum cells during biocontrol of bacterial wilt disease in tomato with Pythium oligandrum. J Gen Plant Pathol 75:281–287
Mohamed N, Lherminier J, Farmer M-J, Fromentin J, Béno N, Houot V, Milat M-L, Blein J-P (2007) Defense responses in grapevine leaves against Botrytis cinerea induced by application of a Pythium oligandrum strain or its elicitin, oligandrin, to roots. Phytopathology 97:611–620
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nielsen KK, Mikkelsen JD, Kragh KM, Bojsen K (1993) An acid class III chitinase in sugar beet: induction by Cercospora beticola, characterization, and expression in transgenic tobacco plants. Mol Plant-Microbe Interact 6:495–506
Nielsen KK, Bojsen K, Collinge DB, Mikkelsen JD (1994) Induced resistance in sugar beet against Cercospora beticola: induction by dichloroisonicotinic acid is independent of chitinase and β-1, 3-glucanase transcript accumulation. Physiol Mol Plant Pathol 45:89–99
Picard K, Ponchet M, Blein J-P, Rey P, Tirilly Y, Benhamou N (2000a) Oligandrin. A proteinaceous molecule produced by the mycoparasite Pythium oligandrum induces resistance to Phytophthora parasitica infection in tomato plants. Plant Physiol 124:379–395
Picard K, Tirilly Y, Benhamou N (2000b) Cytological effects of cellulases in the parasitism of Phytophthora parasitica by Pythium oligandrum. Appl Environ Microbiol 66:4305–4314
Piedras P, Hammond-Kosack KE, Harrison K, Jones JDG (1998) Rapid, Cf-9- and Avr9-dependent production of active oxygen species in tobacco suspension cultures. Mol Plant-Microbe Interact 11:1155–1166
Ponchet M, Panabières F, Milat M-L, Mikes V, Montillet J-L, Suty L, Triantaphylides C, Tirilly Y, Blein J-P (1999) Are elicitins cryptograms in plant-Oomycete communications? Cellular Mol Life Sci 56:1020–1047
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor, NY
Takahashi H, Ishihara T, Hase S, Chiba A, Nakaho K, Arie T, Teraoka T, Iwata M, Tugane T, Shibata D, Takenaka S (2006) Beta-cyanoalanine synthase as a molecular marker for induced resistance by fungal glycoprotein elicitor and commercial plant activators. Phytopathology 96:908–916
Takenaka S, Kawasaki S (1994) Characterization of alanine-rich, hydroxyproline-containing cell wall proteins and their application for identifying Pythium species. Physiol Mol Plant Pathol 45:249–261
Takenaka S, Nishio Z, Nakamura Y (2003) Induction of defense reactions in sugar beet and wheat by treatment with cell wall protein fractions from the mycoparasite Pythium oligandrum. Phytopathology 93:1228–1232
Takenaka S, Nakamura Y, Kono T, Sekiguchi H, Masunaka A, Takahashi H (2006) Novel elicitin-like proteins isolated from the cell wall of the biocontrol agent Pythium oligandrum induce defence-related genes in sugar beet. Mol Plant Pathol 7:325–339
Takenaka S, Sekiguchi H, Nakaho K, Tojo M, Masunaka A, Takahashi H (2008) Colonization of Pythium oligandrum in the tomato rhizosphere for biological control of bacterial wilt disease analyzed by real-time PCR and confocal laser-scanning microscopy. Phytopathology 98:187–195
Villalba Mateos F, Rickauer M, Esquerré-Tugayé MT (1997) Cloning and characterization of a cDNA encoding an elicitor of Phytophthora parasitica var. nicotianae that shows cellulose-binding and lectin-like activities. Mol Plant-Microbe Interact 10:1045–1053
Walters D, Walsh D, Newton A, Lyon G (2005) Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 95:1368–1373
Watanabe H, Hayasaka M, Funahashi S, Kawashima T, Nagashima S (2002) Relationship between spraying schedule of fungicides and occurrence of Cercospora leaf spot of sugar beet in 2000. Proc Jpn Soc Sugar Beet Tech 43:78–85
Zanetti A, Beauvais F, Huet J-C, Pernollet J-C (1992) Movement of elicitins, necrosis-inducing proteins secreted by Phytophthora sp., in tobacco. Planta 187:163–170
Acknowledgments
This research was supported by a Grant-in-Aid (Development of new biorational techniques for sustainable agriculture) from the Ministry of Agriculture, Forestry and Fisheries, Japan. We thank the members of Biomass Research Team of National Agricultural Research Center of Hokkaido Region for generous gift of sugar beet line NK-219 mm-0 and for support of the field test. We also thank Atsuko Muraki and Miyuki Yamaguchi for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takenaka, S., Tamagake, H. Foliar spray of a cell wall protein fraction from the biocontrol agent Pythium oligandrum induces defence-related genes and increases resistance against Cercospora leaf spot in sugar beet. J Gen Plant Pathol 75, 340–348 (2009). https://doi.org/10.1007/s10327-009-0186-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-009-0186-9