Abstract
The amide linkage is a backbone of many organic molecules such as peptides, natural products, pharmaceutical agents, ligands and catalysts. Green synthesis of amides is a major challenge in the context of sustainable development. Here we designed transamidation catalysed by graphene oxide under concentrated solar radiation of various aromatic, aliphatic amides with amines under solvent-free conditions, in 52–98% yield. During the reaction, oxygenated groups such as carboxyl, hydroxyl, carbonyl and epoxy provide acidity to the graphene oxide catalyst. Concentrated solar irradiation is more efficient than conventional methods in terms of reaction time and energy consumption, with about 90% energy saved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently green chemistry has received considerable attention and its fruitful applications have led us to the development of environment-friendly and sustainable approaches for the synthesis of several target molecules. (Mekheimer et al. 2008) For several chemical transformations, the consumption of energy for heating is a major adverse effect on the environment. To overcome the problems associated with this, it is important to develop energy-efficient methods that use different sources to facilitate faster chemical transformations. (Ali and Khan 2017) In this context, the use of a non-conventional energy source like solar radiations for heating the reaction mixture can control the direct conversion of electricity into heat, (Weinstein et al. 2015) thus reducing the total energy consumption, allowing concentrated solar radiation catalysed organic reactions as a fast-growing area in the field of organic synthesis. (Tan et al. 2012)
Concentrated solar radiation causes a large reduction in reaction times, providing high to excellent yields, cheaper and available freely worldwide, which are complementary to green chemistry. Solar radiation has been used for many photochemical transformations like cycloadditions, (Gilbert and Heath 1987) Paterno-Buchi reaction, (Pohlmann et al. 1997) Diels–Alder reactions, (Amin et al. 2015) benzylic bromination, (Deshpande et al. 2015), oxidation of benzyl alcohols using task-specific ionic liquids, (Gadilohar et al. 2016), synthesis of Polyhydroquinolines via Hantzsch synthesis, (Mekheimer et al. 2008) and one-pot syntheses of 2,4,6-triaryl pyridine derivatives, (Kamble and Shankarling 2018) synthesis of symmetrical N, N′-disubstituted thiourea derivatives in water (Kumavat et al. 2013) using solar thermal energy and synthesis of pyrano[2,3-b]pyridine derivatives using the microwave or solar energy (Abdel Hameed 2018). Thus, concentrated solar radiation can be efficiently applied as an energy source for a heating number of chemical reactions that proceed at higher temperatures (> 60 °C) to attain required temperatures.
The amide bond is one of the most important functional groups in organic transformations, as it signifies the basic unit of all-natural peptides and protein linkage. (De Figueiredo et al. 2016) For the synthesis of a variety of natural products, bioactive polymers, and drug moieties; amides are proficient precursors. (Sabatini et al. 2017) Various synthetic procedures to access amides are well-documented in the literature, including the reactions of carboxylic acids (Lixue et al. 2017) and their derivatives (acid chlorides, Vaddula et al. 2013 alcohols, Xu et al. 2016) aldehydes, Kumari et al. 2014) esters, Gustafsson et al. 2008 and acid anhydrides, Upadhyay et al. 2010) with amines. A straightforward approach that reduces or eliminates the need for stoichiometric reagents for amidation is always desirable. Alternatively, transamidation has proved as an alternative and straightforward strategy to achieve amidation via the exchange of the constituents of two different amide groups. In this realm, several protocols involving metal catalyst such as Fe(NO3)3.9H2O, (Becerra-Figueroa et al. 2014) Cu(OAc)2, (Zhang et al. 2012) Cp2ZrCl2, (Atkinson et al. 2012) and metal-free catalysts such as K2S2O8, hypervalent iodine,(Vanjari et al. 2013) have been screened by many researchers under varied reaction conditions such as conventional heating, microwave-assisted reactions to achieve the desired transformation. Important to be mentioned that many of these methods require the use of harmful catalysts and large quantities of organic solvents. Lower energy efficiency is also one of the major shortcomings of such processes.
In this context, it is important to develop a metal-free, environmental-friendly, atom-economical, and green protocol for transamidation. Recently, great attention has been given to the development of carbocatalyst namely graphene, which is metal-free, green, and sustainable (Dreyer et al. 2010; Navalon et al. 2014; Mahajan and Gupta 2020). Graphene (the two-dimensional allotrope of carbon) (Jilani et al. 2018) and graphene oxide, have been fruitfully investigated for several organic transformations (Ohammadi and Golestanzadeh 2017). Graphene oxide has attracted much attention in the field of catalysis, due to its outstanding physical, chemical, and electrical characteristics and exceptionally high surface area (Dreyer et al. 2014; Cheng et al. 2015). The presence of oxygenated functionalities present on the surface imparts oxidizing properties and acidity (pH 4.5 at 1 mg/mL of water) to Graphene oxide (Szabó et al. 2006). These unique characteristics make the graphene oxide a promising carbocatalyst in synthetic organic chemistry (Table 1).
Previously reported methods for transamidation involve the usage of metal-based catalysts, oxidants, require high temperature and longer reaction time (Table 2 entries 1–7), Though microwave heating requires less reaction time providing higher yields but have certain limitations for large scale synthesis. Therefore, concentrated solar radiation for the transamidation (Table 2 entry 8) is more advantageous over other previously reported methods due to its cost-effectiveness; energy efficient, and environmental-friendly nature.
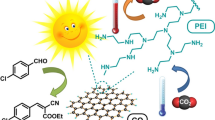
Here we present a green, energy-efficient and straightforward protocol to obtain diversely functionalized amides via transamidation employing graphene oxide as a catalyst under concentrated solar radiations in solvent-free reaction conditions (Fig. 1).
Experimental section
Graphene oxide catalysed transamidation by concentrated solar radiation method
All the reactions were carried out in Matunga, Mumbai, India (19° 01′18″ N 72° 51′53″ E/19.021632° N) under concentrated solar radiation. Carboxamide/Phthalimide (1 mmol), aliphatic/aromatic amine (1.2 mmol), and graphene oxide (20 wt%) were charged in a sealed tube (25 mL) at room temperature. The reaction mixture was stirred under concentrated solar radiation, maintaining the temperature in the range of 90–100 °C. After the reaction gets completed (monitored by thin-layer chromatography), the reaction mixture was cooled to room temperature and then dissolve in ethyl acetate (10 mL). The graphene oxide was removed from the reaction mass by filtration and reaction mass was then concentrated under vacuum to obtain the purified product directly. In some cases, the crude reaction mass was subjected to column chromatography to obtain a purified product (solvent system- Hexane: Ethyl acetate).
Results and discussion
Optimization of reaction parameters
To access the synthetic utility of graphene oxide for transamidation, Initially, benzamide (1a) and benzylamine (2a) were chosen as a model substrate (Table 3). The reaction was performed under conventional thermal heating at 130 °C for 20 h affording 97% yield of N-benzyl benzamide. (Table 2, entry 7). (Patel et al. 2019) Additionally, in search of energy-efficient protocol, we carried out reaction using concentrated solar radiation where it was completed within 2 h at 90–100 °C yielding 98% of product as concentrated solar radiation as an energy source is known to have a great impact on reducing time and temperature. (Table 3, entry 8). When Graphene oxide was absent, only 17% yield of product 3a was obtained (Table 3, entry 1). When the catalyst loading was increased to 5 wt% of 1a, the yield of 3a increased to 85% (Table 3, entry 2). Furthermore, we increased the catalyst loading to 15 wt%, 20 wt% and the product 3a was obtained with 91%, 95%, 98%, respectively (Table 3, entries 2–6). Further increase in the catalyst loading (25 wt%) does not improve the product yield or reduced reaction time. Moreover, different solvents were tried out to study the effect of solvents. In N, N-dimethyl formamide, the reaction gave the product yield of 10% (Table 3, entry 7), while in dimethyl sulphoxide the yield of product 3a was found to be 12% (Table 3, entry 8). For the reactions in other solvents such as Water, Acetonitrile, and Toluene the product 3a was not detected by TLC (Table 3, entries 9–11)

The time required for amide synthesis via transamidation using the conventional method was 20 h while concentrated solar radiation required only 2 h for completion of the reaction. The solar radiations contain Infra-red radiations which are responsible for the molecules to vibrate and rotate. The molecular species are heated only because of this vibrational and rotational motion. Concentrated solar radiation provides Infra-red radiation bombarded on the molecules which result in the radiation energy transformed into the heat energy with high efficiency, due to which at the normal pressure the superheating becomes possible. Due to hot spot bombardment in the reaction mass, the molecules vibrate and rotate faster which results in reaction become faster. The interaction between the reactant enhanced due to the collisions formed by the vibrations and rotation by concentrated solar radiation. This is the possible cause behind enhancing the rate of reaction. Concentrated solar radiation carries UV–Visible radiations along with Infra-red radiations which give synergetic effect for faster reaction (Ghorpade et al. 2015).
Further, we carried out the reaction at RT under visible light emitted from fluorescence lamp (60 W) but no product formation was seen even after keeping for long reaction time (48 h). This confirmed the reaction proceeds via thermal and not a photochemical transformation.
Derivative study
With optimized reaction conditions, we further examined the applicability of graphene oxide-catalysed transamidation of primary aromatic, heteroaromatic, and aliphatic amides with various aliphatic, aromatic amines as shown in Electronic Supporting Information, Table S1. All the reactions offered excellent yields of the respected products. It is observed that the aliphatic amides are more reactive than aromatic amides, however, all amines (aliphatic, aromatic, cyclic) worked effectively under these reaction conditions. For comparison, all the reactions were performed under both conventional as well as concentrated solar radiation heating. Although the yields of the product were almost similar under both methods, the reaction time was substantially reduced to 120–240 min under concentrated solar radiation as compared to conventional heating which required 8–30 h for completion of the reaction (Patel et al. 2019).
Conclusion
In conclusion, we have developed an energy-efficient and comparatively green synthetic protocol for the transamidation of carboxamide and phthalimide with various amines using graphene oxide. The current work offers good-to-excellent yield of corresponding products in less reaction time. The use of Concentrated solar radiation for the transamidation by environment-friendly, metal-free, and inexpensive graphene oxide makes the method safer, cleaner, and energy saving. We envisioned that metal-free, low-cost, non-toxic, and environmentally friendly graphene oxide could constitute a highly effective catalyst for transamidation using a clean energy source. A further improvement in the process would open up the new pathway for the incorporation of solar energy in the organic reactions thereby decreasing the energy load. Incorporation of renewable energy sources, minimization of raw materials makes the process not only green but energy efficient also.
References
Abdel Hameed AM (2018) Efficient synthesis of pyrano[2,3-b]pyridine derivatives using microwave or solar energy. Environ Chem Lett 16:1423–1427. https://doi.org/10.1007/s10311-018-0744-5
Ali H, Khan E (2017) Environmental chemistry in the twenty-first century. Environ Chem Lett 15:329–346. https://doi.org/10.1007/s10311-016-0601-3
Amin S, Barnes A, Buckner C et al (2015) Diels-alder reaction using a solar irradiation heat source designed for undergraduate Organic Chemistry Laboratories. J Chem Educ 92:767–770. https://doi.org/10.1021/ed500850c
Ao S, Chandra Mohan D, Adimurthy S (2014) Chitosan: an efficient recyclable catalyst for transamidation of carboxamides with amines under neat conditions. Green Chem 16:4122–4126. https://doi.org/10.1039/C4GC01402B
Atkinson BN, Chhatwal AR, Lomax HV et al (2012) Transamidation of primary amides with amines catalyzed by zirconocene dichloride. Chem Commun 48:11626–11628. https://doi.org/10.1039/C2CC37427G
Becerra-Figueroa L, Ojeda-Porras A, Gamba-Sánchez D (2014) Transamidation of carboxamides catalyzed by Fe(III) and water. J Org Chem 79:4544–4552. https://doi.org/10.1021/jo500562w
Cheng Y, Fan Y, Pei Y, Qiao M (2015) Graphene-supported metal/metal oxide nanohybrids: synthesis and applications in heterogeneous catalysis. Catal Sci Technol 5:3903–3916. https://doi.org/10.1039/C5CY00630A
De Figueiredo RM, Suppo JS, Campagne JM (2016) Nonclassical routes for amide bond formation. Chem Rev 116:12029–12122
Deshpande S, Gadilohar B, Shinde Y et al (2015) Energy efficient, clean and solvent free photochemical benzylic bromination using NBS in concentrated solar radiation (CSR). Solar Energy 113:332–339. https://doi.org/10.1016/j.solener.2015.01.008
Dreyer DR, Park S, Bielawski W, Ruoff RS (2010) The chemistry of graphene oxide. https://doi.org/10.1039/b917103g
Dreyer DR, Todd AD, Bielawski CW (2014) Harnessing the chemistry of graphene oxide. Chem Soc Rev 43:5288–5301. https://doi.org/10.1039/C4CS00060A
Gadilohar BL, Deshpande SS, Pinjari DV, Shankarling GS (2016) Concentrated solar radiation aided energy efficient protocol for oxidation of alcohol using biodegradable task specific ionic liquid-choline peroxydisulfate. Solar Energy 139:328–336. https://doi.org/10.1016/j.solener.2016.09.044
Ghorpade P, Gadilohar B, Pinjari D et al (2015) Concentrated solar radiation enhanced one pot synthesis of DES and N-Phenyl phthalimide. Solar Energy 122:1354–1361
Gilbert A, Heath P (1987) Specific ortho photocycloaddition of enol ethers to 2-substituted anisoles: facile synthesis of bicyclo[4.2.0]octa-2,7-dienes in sunlight. Tetrahedron Lett 28:5909–5912. https://doi.org/10.1016/S0040-4039(01)81088-1
Gustafsson T, Ponten F, Seeberger PH (2008) Trimethylaluminium mediated amide bond formation in a continuous flow microreactor as key to the synthesis of rimonabant and efaproxiral. Chem Commun. https://doi.org/10.1039/B719603B
Jilani A, Othman MHD, Ansari MO et al (2018) Graphene and its derivatives: synthesis, modifications, and applications in wastewater treatment. Environ Chem Lett 16:1301–1323. https://doi.org/10.1007/s10311-018-0755-2
Kamble SS, Shankarling SG (2018) Amalgamation of CSR and DES: an energy efficient protocol for the one-pot synthesis of 2,4,6- triaryl pyridine derivatives. ChemistrySelect 3:10464–10467. https://doi.org/10.1002/slct.201801690
Kumari S, Shekhar A, Mungse HP et al (2014) Metal-free one-pot synthesis of amides using graphene oxide as an efficient catalyst. RSC Adv 4:41690–41695. https://doi.org/10.1039/C4RA07589G
Kumavat PP, Jangale AD, Patil DR et al (2013) Green synthesis of symmetrical N, N′-disubstituted thiourea derivatives in water using solar energy. Environ Chem Lett 11:177–182. https://doi.org/10.1007/s10311-012-0394-y
Lixue J, Jing Y, Fanfan N et al (2017) A high-efficient method for the amidation of carboxylic acids promoted by triphenylphosphine oxide and oxalyl chloride. Heteroat Chem 28:e21364. https://doi.org/10.1002/hc.21364
Mahajan A, Gupta P (2020) Carbon-based solid acids: a review. Environ Chem Lett 18:299–314. https://doi.org/10.1007/s10311-019-00940-7
Mekheimer RA, Hameed AA, Sadek KU (2008) Solar thermochemical reactions: four-component synthesis of polyhydroquinoline derivatives induced by solar thermal energy. Green Chem 10:592–593. https://doi.org/10.1039/B715126H
Navalon S, Dhakshinamoorthy A, Alvaro M, Garcia H (2014) Carbocatalysis by graphene-based materials. Chem Rev 114:6179–6212. https://doi.org/10.1021/cr4007347
Ohammadi O, Golestanzadeh M (2017) Recent advances in organic reactions catalyzed by graphene oxide and sulfonated graphene as heterogeneous nanocatalysts: a review. New J Chem 41:11471–11497. https://doi.org/10.1039/C7NJ02515G
Patel KP, Gayakwad EM, Patil VV, Shankarling GS (2019) Graphene oxide: a metal-free carbocatalyst for the synthesis of diverse amides under solvent-free conditions. Adv Synth Catal 361:2107–2116. https://doi.org/10.1002/adsc.201801673
Pohlmann B, Scharf H-D, Jarolimek U, Mauermann P (1997) Photochemical production of fine chemicals with concentrated sunlight. Solar Energy 61:159–168. https://doi.org/10.1016/S0038-092X(97)00043-1
Sabatini MT, Boulton LT, Sheppard TD (2017) Borate esters: simple catalysts for the sustainable synthesis of complex amides. Sci Adv. https://doi.org/10.1126/sciadv.1701028
Srinivas M, Hudwekar AD, Venkateswarlu V et al (2015) A metal-free approach for transamidation of amides with amines in aqueous media. Tetrahedron Lett 56:4775–4779. https://doi.org/10.1016/j.tetlet.2015.06.052
Szabó T, Tombácz E, Illés E, Dékány I (2006) Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 44:537–545. https://doi.org/10.1016/j.carbon.2005.08.005
Tan CW, Tan KH, Ong YT et al (2012) Energy and environmental applications of carbon nanotubes. Environ Chem Lett 10:265–273. https://doi.org/10.1007/s10311-012-0356-4
Upadhyay SK, Pingali SRK, Jursic BS (2010) Comparison of microwave-assisted and conventional preparations of cyclic imides. Tetrahedron Lett 51:2215–2217. https://doi.org/10.1016/j.tetlet.2010.02.092
Vaddula BR, Varma RS, Leazer J (2013) Mixing with microwaves: solvent-free and catalyst-free synthesis of pyrazoles and diazepines. Tetrahedron Lett 54:1538–1541. https://doi.org/10.1016/j.tetlet.2013.01.029
Vanjari R, Kumar Allam B, Nand Singh K (2013) Hypervalent iodine catalyzed transamidation of carboxamides with amines. RSC Adv 3:1691–1694. https://doi.org/10.1039/C2RA22459C
Weinstein LA, Loomis J, Bhatia B et al (2015) Concentrating solar power. Chem Rev 115:12797–12838. https://doi.org/10.1021/acs.chemrev.5b00397
Xu Q, Xie H, Zhang E-L et al (2016) Selective catalytic Hofmann N-alkylation of poor nucleophilic amines and amides with catalytic amounts of alkyl halides. Green Chem 18:3940–3944. https://doi.org/10.1039/C6GC00938G
Zhang M, Imm S, Bähn S et al (2012) Efficient copper(II)-catalyzed transamidation of non-activated primary carboxamides and ureas with amines. Angew Chem Int Ed 51:3905–3909. https://doi.org/10.1002/anie.201108599
Acknowledgements
The authors are greatly thankful to the University Grant commission- UGC-CAS for providing financial assistance.
Funding
The Funded was provided by University Grants Commission (F.25-1/2014(BSR) No.5-65/2007(BSR)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, K.P., Kamble, S.S., Boraste, D.R. et al. Green transamidation catalysed by graphene oxide under concentrated solar irradiation. Environ Chem Lett 18, 1731–1735 (2020). https://doi.org/10.1007/s10311-020-01034-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01034-5