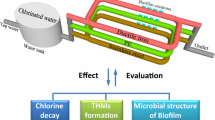
Abstract
Water research is usually done using simplified devices, e.g., beakers, in laboratory glassware, and thus may yield results that are not representative of real wastewater treatment plants that use complex pipe systems made of various materials. Therefore, here we studied for the first time the effect of different pipe materials on sulfamethazine chlorination and formation of disinfection by-products. We also studied the relationships between bacterial communities, pipe materials, sulfamethazine degradation, free chlorine decay and formation of disinfection by-products. Results show that the degradation of sulfamethazine was faster in pipes than in batch tests, due to the influences of sediment, iron and bacterial communities. Specifically, the total concentration of disinfection by-products after 22 h followed the order: 49.43 μg/L for stainless-steel pipe, 46.76 μg/L for ductile iron pipe, 39.12 μg/L for polyethylene pipe, and 19.22 μg/L for batch tests. 16S rRNA microbial analysis showed that ductile iron and stainless-steel pipes had the highest operational taxonomic unit (OTU) richness. Microbial communities between polyethylene and ductile iron pipe had a relatively higher similarity. Sphingomonas and Actinobacteria slowed down the decay of free chlorine in the stainless-steel pipe, which promoted the degradation of sulfamethazine. Mycobacterium and Pseudomonas are the dehalogenation-related bacteria, which reduced the formation of distribution by-products in polyethylene pipe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfamethazine is a member of the sulfonamides family, which has been widely used for human medicine and livestock husbandry due to their low cost and relatively high efficiency against many common bacterial infections (Dong et al. 2017). They are frequently detected in surface water and groundwater at concentration ranging from 1 to 1.9 ng/L (Thorsten et al. 2010). They are also detected in drinking water at concentration ranging from 0.1 to 60 ng/L (Padhye et al. 2014). Therefore, the continual occurrence of sulfonamides in the aquatic environment has drawn widespread attentions.
Nowadays, many investigations on the sulfamethazine chlorination have been conducted in a purified water matrix by chlorination. Sulfamethazine chlorination in water distribution systems has not been the subject of intensive researches. In addition, during chlorine disinfection process, chlorine can react with natural organic matters to form disinfection by-products (Lyon et al. 2014). Due to the potential toxicity and health risks, the formations of disinfection by-products have raised the public concern.
After disinfection, the remaining bacteria in drinking water may result in biofilms formation on the surface of pipes when the water goes through the water distribution system (Usher et al. 2014). Recently, 16S ribosomal ribonucleic acid (rRNA) gene, as a high-throughput and open method, has been applied to explore bacterial community and composition in drinking water (Li et al. 2017). The objectives of this study are to evaluate the formation of selected disinfection by-products during sulfamethazine chlorination in water distribution system under different pipe materials. Secondly, the effect of pipe materials on degradation of sulfamethazine was investigated by analyzing free chlorine decay and microbial community composition of the biofilm in different pipe material.
Experimental
Analytical methods
The detection of free residual chlorine was done by using DR2800 (HACH). The concentration of each sulfamethazine sample was monitored by high-performance liquid chromatography (Agilent 1200 Series, Agilent, Santa Clara, CA, U.S.). Liquid–liquid extraction (LLE) as a pretreatment technology was used to analyze the degradation by-products. The intermediate products of the sulfamethazine halogenation were analyzed by gas chromatography with electron capture detector (GC-450, Thermo Fisher).
To investigate the effect of bacterial on sulfamethazine degradation by chlorination in the water distribution system, polymerase chain reaction (PCR) amplification of the bacterial 16S rRNA genes V4–V5 region was performed using the forward primer 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and the reverse primer 907R (5′-CCGTCAATTCMTTTRAGTTT-3′). Sample-specific 7-bp barcodes were incorporated into the primers for multiplex sequencing.
Experimental procedures
Sodium hypochlorite as a disinfectant was used in the sulfamethazine experiments. Two parallel experiments were performed: One was conducted in dark condition under a thermostat water bath and the other was tested in a pilot-scale water distribution system. This water distribution system had three separate loops to test the effects of different materials, which were made of ductile iron, polyethylene, and stainless steel. The length and diameter of each loop were approximately 80 m and 150 mm, respectively.
In water distribution system experiments, the initial dosage of sulfamethazine was 200 μg/L (0.8 μM), the temperature was 20 °C, flow velocity was 1.0 m/s, pH was 7.5, and 1.4 mg/L of sodium hypochlorite was added. Samples of 1 mL were collected at 0, 0.5, 1, 2, 5 min, and samples of 100-mL were collected for high-performance liquid chromatography, samples of 5 L were collected at 0, 12, 24 h for PCR, and 60-mL samples were used to detect disinfection by-products. Excessive sodium thiosulfate was added to each sample to quench further reactions. The batch tests were carried out in a 1000-mL glass beaker; other conditions were consistent with the water distribution system experiments.
Results and discussion
The degradation of sulfamethazine
Figure 1 shows the degradation kinetic of sulfamethazine chlorination in different pipe materials. The results showed that the degradation of sulfamethazine in different pipe materials followed the order: stainless-steel pipe > polyethylene pipe > ductile iron pipe > batch tests. According to the concentration of free chlorine in stainless-steel, polyethylene, ductile iron, and batch tests after 30-min reaction, the amount of free chlorine available for sulfamethazine degradation was higher in the stainless-steel (0.73 mg/L) pipe than in the polyethylene (0.69 mg/L) and ductile iron (0.63 mg/L) pipes. A linear relationship was found between the first-order rate constant (ka) for the degradation of sulfamethazine and the initial free chlorine concentration in all experiments. Therefore, the reaction between sulfamethazine and free chlorine could be described by a pseudo-first-order kinetic model:
where ka is the rate constant of sulfamethazine degradation, [SMZ]t is the concentration of sulfamethazine at time t (min), and [SMZ]0 is the initial concentration of sulfamethazine (200 ug/L, 1.0 umol/L). The pseudo-first-order rate constants (ka) of the sulfamethazine degradation in stainless-steel, polyethylene, ductile iron, and batch tests were 0.806, 0.687, 0.681, and 0.619, respectively, which remained linear (R2 > 0.90) in all cases.
Effect of different pipe materials on degradation of sulfamethazine in water distribution system (T = 20 °C, pH = 7.5, initial free chlorine = 1.4 mg/L and initial sulfamethazine = 1 μmol/L). [SMZ] is the concentration of sulfamethazine at time t (min), and [SMZ]0 is the initial concentration of sulfamethazine. Due to the influence by sediment, iron and bacterial community in the water distribution system, the degradation of sulfamethazine in different pipe materials followed the order: stainless-steel pipe > polyethylene pipe > ductile iron pipe > batch tests
Through the visible pipe section, more iron and sediment were observed in the stainless-steel pipe than in the polyethylene pipe and ductile iron pipe. The sediment in the water distribution system included NOM, metal ions, corroded metal, and solid metal oxides (Lytle and Liggett 2016). Especially for iron, Fe(II) can be oxidized to Fe(III) by free chlorine, which could advance oxidation (Pérez-Moya et al. 2010). In addition, the concentrations of total organic carbon (TOC) were detected in stainless-steel (8.16 mg/L), polyethylene (9.39 mg/L), and ductile iron (9.87 mg/L) pipes, which showed polyethylene pipe had more natural organic matters and stainless-steel pipe had least. The more natural organic matters in pipe would consume more free chlorine, which was consistent with the decay of free chlorine. In a word, the degradation of sulfamethazine was affected by sediment, iron, and bacterial community in the water distribution system.
The formation of disinfection by-products
The yields of disinfection by-products during sulfamethazine chlorination at different pipe materials are shown in Fig. 2. In the whole, the total concentration of disinfection by-products after 22 h followed the order: stainless-steel (49.43 μg/L) > ductile iron (46.76 μg/L) > polyethylene pipe (39.12 μg/L) > batch tests (19.22 μg/L). According to Mao’s research, the amount of total organic carbon leached to the water when exposed to polyethylene pipe, higher than in water exposed to glass controls (Mao et al. 2017). Compared with the batch tests, the bacterial and natural organic matter in ductile iron, polyethylene, and stainless-steel pipe contributed to 143, 104, and 157% for disinfection by-products formation, respectively. What is more, the formation of disinfection by-products was increased by 47% in ductile iron, 46% in polyethylene, and 18% in stainless-steel pipe with the reaction time increasing. However, the concentration of total disinfection by-products was almost unchanged in batch tests after 3 h, which indicated the formation of disinfection by-products in sulfamethazine chlorination was stopped in 3 h.
Concentration of disinfection by-products formation in batch and three pipes during the sulfamethazine degradation (1,1-DCP 1,1-dichloro-2-propanone, TCA trichloroaldehyde, TBM bromoform, BDCM bromodichloromethane, DBCM dibromochloromethane, TCM chloroform, DCAN dichloroacetonitrile, BCAN bromodichloroacetonitrile). The total concentration of disinfection by-products after 22 h followed the order: stainless-steel (49.43 μg/L) > ductile iron (46.76 μg/L) > polyethylene pipe (39.12 μg/L) > batch tests (19.22 μg/L). The yields of disinfection by-products in stainless-steel, ductile iron and polyethylene pipes followed the order: chloroform (23–35%) > trichloroaldehyde (14–30%) > dibromochloromethane (18–25%) > bromodichloromethane (13–22%). The yields of disinfection by-products in batch tests followed the order: trichloroaldehyde (58.3%) > chloroform (15.1%) > dibromochloromethane (9.3%). Bromoform was formed less than 0.5 μg/L, due to the low concentration of Br−
In addition, the yields of carbonenous disinfection by-products during sulfamethazine chlorination in water distribution system followed the order: chloroform (23–35%) > trichloroaldehyde (14–30%) > dibromochloromethane (18–25%) > bromodichloromethane (13–22%). However, the yields of carbonenous disinfection by-products during sulfamethazine chlorination in batch tests followed the order: trichloroaldehyde (58.3%) > chloroform (15.1%) > dibromochloromethane (9.3%). Nitrogenous disinfection by-products detected in samples were mainly bromochloroacetonitrile and dichloroacetonitrile. The concentration of dichloroacetonitrile was much higher in chlorination in stainless-steel pipe than in other materials after 22 h. Compared with the water distribution systems, the batch tests formed less bromochloroacetonitrile and dichloroacetonitrile due to less natural organic matters and simpler conditions. The total nitrogenous disinfection by-products formation during sulfamethazine chlorination in stainless-steel, polyethylene, ductile iron, and batch tests were 4.27, 2.96, 1.43, and 0.87 μg/L, respectively. Besides, the concentration of nitrogenous disinfection by-products was almost unchanged after 10 h. Previous studies found that the C–C and C–N bond in sulfamethazine could be divided by chlorination in the first 10 h, which was in agreement with the front result.
Bacterial community
Sequence diversity analysis
Different pipe materials affected the development of biofilms on pipe surfaces. Based on the clustering of these sequences at 97% gene similarity, 2371, 1958, and 2762 OTUs were found in the biofilms after 24 h from ductile iron, polyethylene, and stainless-steel pipe, respectively. The results showed ductile iron and stainless-steel pipes had the highest OTU richness than polyethylene pipe, which was expressed by ACE and Chao1 indices (Table 1). In addition, the ductile iron biofilm had the highest bacterial diversity as expressed by Shannon index of 8.99, while polyethylene biofilm showed the lowest level of bacterial diversity with a Shannon index of 7.73. Beta diversity analysis principal component analysis was used to show the similarity between different samples, which illustrated the microbial communities between polyethylene and ductile iron pipe had a relatively higher similarity.
According to the results of Venn diagram, 2208 OTUs were shared by all samples, while 770, 660, and 1341 OTUs were specific for the pipe walls of ductile iron, polyethylene, and stainless-steel pipes, respectively. It was reported that iron or steel pipes had a much higher biofilm formation potential than polyethylene pipe, due to the rough surface of stainless-steel or ductile iron pipes, which could provide more suitable conditions for biofilm formation (Yu et al. 2010). Previous studies had confirmed the bacterial quantity was higher in stainless-steel or ductile iron pipe than that in polyethylene pipe (Zhang et al. 2018).
The operational taxonomic units (OTU): determined with a 3% width. The Chao1 and abundance-based coverage (ACE) estimators reflected the richness of the community, whereas the Shannon diversity index and the Simpson index reflected the community uniformity. DI, PE, SS: samples were collected at 0, 12, and 24 h with sulfamethazine chlorination from ductile iron, polyethylene, and stainless-steel pipes, respectively. These results showed that the difference of OTU richness and the diversity in different pipe materials. Ductile iron and stainless-steel pipes had the highest OTU richness than polyethylene pipe, polyethylene, and ductile iron pipe had a relatively higher similarity.
Bacterial community and composition
The structure of the bacterial community in biofilms on polyethylene, stainless-steel, and ductile iron pipes were analyzed by Illumina MiSeq high-throughput sequencing. Figure 3a shows the relative abundance of the most common OTUs at the class level among live bacteria during the degradation reaction in different pipe materials. Phylum Proteobacteria was the most abundant in bacterial microbiota, more than 50%. The dominant bacteria in all samples of classes were Alphaproteobacteria (14–18%), Betaproteobacteria (8–12%), Gammaproteobacteria (2–6%). Another dominant phyla were Actinobacteria (6–12%), which was consistent with the results of previous studies (Williams et al. 2004). Furthermore, the bacterial community structures were compared at the genus level in Fig. 3b. It could be observed that the most representative genera were Sphingomonas (11–14%), Bacillus (3.2–4.1%), and Gemmatimonas (3.1–3.5%).
Relative abundance of different bacteria in different pipe materials (a was at the class level; b was at the genus level). Phylum Proteobacteria was the most abundant in bacterial microbiota, more than 50%. The dominant bacteria in all samples of classes were Alphaproteobacteria (14–18%), Betaproteobacteria (8–12%), Gammaproteobacteria (2–6%). Sphingomonas was high abundance in stainless-steel pipe (14.5%). The relative abundance of the dehalogenation-related bacteria (Mycobacterium and Pseudomonas) were high abundance in polyethylene pipe
This research investigated the effect of different pipe materials in sulfamethazine degradation, disinfection by-products, and bacterial community structures in a pilot-scale water distribution system. Compared with three different pipe materials, chlorine decay in ductile iron pipe was fastest, while chlorine decay in stainless-steel pipe was slower than polyethylene pipe. As shown in Fig. 3b, Sphingomonas, known as chlorine-resistant bacteria, was high abundance in stainless-steel pipe (14.5%), which was in accordance with the results that chlorine was slower in the stainless-steel pipe than in the polyethylene (11.3%) and ductile iron pipe (11.9%). In addition, Actinobacteria was resistant to the residual free chlorine, more than twice as abundant in the stainless-steel pipe (11.9%) than in polyethylene (7.9%) and ductile iron pipes (6.7%), which promoted the degradation of a variety of antibiotics. This result was also resistant to the residual of free chlorine in water distribution system. What is more, the relative abundance of Betaproteobacteria was higher in polyethylene and ductile iron pipe, which could oxidize methyl compounds to improve the degradation of sulfamethazine in polyethylene and ductile iron pipe (Jenkins 1987). Some researchers have confirmed that Mycobacterium and Pseudomonas are the dehalogenation-related bacteria, which can reduce the disinfection by-products in some conditions (Chuang and Tung 2012). The relative abundance of the dehalogenation-related bacteria in ductile iron, polyethylene, and stainless-steel pipes was 0.4, 3.6, and 1.4%, respectively. Therefore, the dehalogenation-related bacteria in polyethylene pipe affected the formation of disinfection by-products.
Conclusions
The aim of this study demonstrated the influence of pipe materials on sulfamethazine degradation, free chlorine decay and disinfection by-products formation with community analysis by Illumina MiSeq high-throughput sequencing. The degradation rates of sulfamethazine in different pipe materials followed the order: stainless-steel pipe > polyethylene pipe > ductile iron pipe > batch tests. Meanwhile, the total concentration of disinfection by-products after 22 h followed the order: stainless-steel (49.43 μg/L) > ductile iron (46.76 μg/L) > polyethylene pipe (39.12 μg/L) > batch tests (19.22 μg/L). Compared with the batch tests, the bacterial and natural organic matter in ductile iron, polyethylene, and stainless-steel pipe contributed to 143, 104, and 157% for disinfection by-products formation, respectively. According to the results analyzed by 16S rRNA in three different pipe materials, ductile iron, and stainless-steel pipes had the highest OTU richness, and the microbial communities between polyethylene and ductile iron pipe had a relatively higher similarity. Sphingomonas and Actinobacteria slowed down the decay of free chlorine in the stainless-steel pipe. Mycobacterium and Pseudomonas could reduce the formation of distribution by-products in polyethylene pipe. These results are helpful for the treatment of microproducts and disinfection by-products formation in water distribution system with different pipe materials.
References
Chuang YH, Tung HH (2012) Isolation of HAA degrading bacteria from drinking water using complex media. Sustain Environ Res 22(5):287–294
Dong F, Li C, He G, Chen X, Mao X (2017) Kinetics and degradation pathway of sulfamethazine chlorination in pilot-scale water distribution systems. Chem Eng J 321:521–532. https://doi.org/10.1016/j.cej.2017.03.130
Jenkins O (1987) Methylophilus: a new genus of methanol-utilizing bacteria. Int J Syst Bacteriol 37:446–448. https://doi.org/10.1099/00207713-37-4-446
Li C, Dong F, Feng L, Zhao J, Zhang T, Cizmas L, Sharma VK (2017) Bacterial community structure and microorganism inactivation following water treatment with ferrate(VI) or chlorine. Environ Chem Lett 15(3):525–530. https://doi.org/10.1007/s10311-017-0623-5
Lyon BA, Cory RM, Weinberg HS (2014) Changes in dissolved organic matter fluorescence and disinfection byproduct formation from UV and subsequent chlorination/chloramination. J Hazard Mater 264(2):411. https://doi.org/10.1016/j.jhazmat.2013.10.065
Lytle DA, Liggett J (2016) Impact of water quality on chlorine demand of corroding copper. Water Res 92:11–21. https://doi.org/10.1016/j.watres.2016.01.032
Mao G, Wang Y, Hammes F (2017) Short-term organic carbon migration from polymeric materials in contact with chlorinated drinking water. Sci Total Environ 613–614:1220–1227. https://doi.org/10.1016/j.scitotenv.2017.09.166
Padhye LP, Yao H, Kung’U FT, Huang CH (2014) Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res 51(6):266. https://doi.org/10.1016/j.watres.2013.10.070
Pérez-Moya M, Graells M, Castells G, Amigó J, Ortega E, Buhigas G, Pérez LM, Mansilla HD (2010) Characterization of the degradation performance of the sulfamethazine antibiotic by photo-Fenton process. Water Res 44(8):2533–2540. https://doi.org/10.1016/j.watres.2010.01.032
Thorsten C, Schneider RJ, Färber HA, Dirk S, Meyer MT, Goldbach HE (2010) Determination of antibiotic residues in manure, soil, and surface waters. CLEAN Soil Air Water 31(1):36–44. https://doi.org/10.1002/aheh.200390014
Usher KM, Kaksonen AH, Cole I, Marney D (2014) Critical review: microbially influenced corrosion of buried carbon steel pipes. Int Biodeterior Biodegrad 93(93):84–106. https://doi.org/10.1016/j.ibiod.2014.05.007
Williams MM, Domingo JW, Meckes MC, Kelty CA, Rochon HS (2004) Phylogenetic diversity of drinking water bacteria in a distribution system simulator. J Appl Microbiol 96(5):954–964. https://doi.org/10.1111/j.1365-2672.2004.02229.x
Yu J, Kim D, Lee T (2010) Microbial diversity in biofilms on water distribution pipes of different materials. Water Sci Technol 61(1):163. https://doi.org/10.2166/wst.2010.813
Zhang K, Cao C, Zhou X, Zheng F, Sun Y, Cai Z, Fu J (2018) Pilot investigation on formation of 2,4,6-trichloroanisole via microbial O -methylation of 2,4,6-trichlorophenol in drinking water distribution system: an insight into microbial mechanism. Water Res 131:11–21. https://doi.org/10.1016/j.watres.2017.12.013
Acknowledgements
This work was kindly supported by the National Natural Science Foundation of China (Nos. 51578487, 51778565), the National Major Projects for Water Pollution Control and Treatment (Grant No. 2017ZX07201003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, F., Li, C., Lin, Q. et al. Effect of pipe materials on disinfection by-products and bacterial communities during sulfamethazine chlorination in a pilot-scale water distribution system. Environ Chem Lett 17, 1039–1044 (2019). https://doi.org/10.1007/s10311-018-00823-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-00823-3