Abstract
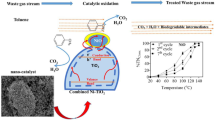
Air pollution by volatile organic compounds is a major health issue due to increasing industrialization and urbanization, notably in the developing countries. Cleaning organic pollutants by catalytic combustion is a potential solution, but actual methods require relatively high temperatures, thus increasing remediation costs. There is therefore a need for methods that operate at mild temperatures. Here we prepared a novel catalyst made of Pd nanoparticles entrapped in TiO2 nanotubes by vacuum-assisted impregnation. Then, we tested this catalyst for butane combustion. The catalyst was characterized by N2 adsorption–desorption isotherms, transmission electronic microscopy, energy-dispersive X-ray analysis coupled with a scanning transmission electron microscope, X-ray photoelectron spectroscopy and temperature programmed oxidation. Results show a complete combustion of butane at 130 °C, which is about 20 °C lower than temperatures required by actual catalysts made of Pd nanoparticles deposited on the exterior surface of TiO2 nanotubes. Structure characterization suggests that this higher performance at lower temperature is explained by the confinement of TiO2 nanotubes. Such a confinement could hinder the metal sintering and, in turn, facilitate the formation of PdO during oxidation on the entrapped Pd nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic gas waste pollution has recently become the world’s highest environmental issue due to the rapid development of industrial and urbanization, especially for the developing countries, such as China and India (Krishnaraj 2015; Yu et al. 2014; Sanchez et al. 2016). Catalytic combustion is one of the promising alternative technologies for the organic gas abatement, such as alkane and arenes, because theses gaseous waste can be completely oxidized to CO2 and H2O over catalysts at much lower temperatures than those of thermal oxidation. The widely used catalysts for catalytic combustion include the supported noble metal, such as platinum, palladium and ruthenium (Deng et al. 2015; Centi 2001; Silva et al. 2007), or metal oxides including MnO2, CeO2, TiO2 (Chlala et al. 2016; Amini et al. 2014) and so on. Among these reported catalysts, the palladium (Pd)-based catalysts are the preferred ones due to their high catalytic activity toward most part of the organic gas at relatively low reaction temperature. Thus, so far scientists (Centi 2001; Cargnello et al. 2012; Deng and Nevell 1999; Lovón-Quintana et al. 2016) have developed many high-performances Pd-based catalysts applied in various organic gas abatement. Among these reported literatures, it is found that the recent study of VOC combustion generally occurred at relatively high temperature, which is far behind the target that eliminates the VOC at mild temperature. Therefore, there is an urgent need to build a high performance catalyst that can lower the complete combustion temperature as low as possible.
Entrapping the active components in a confined space has been proved to be an efficient strategy to improve the catalyst’s performance since the pioneer work by Bao’s group (Chen et al. 2008). They have declared that the confinement effect of carbon nanotube on the entrapped active component can hinder the metal sintering process, facilitate the electron transfer and significantly enhance the activity and stability of the catalysts. In our group, we have also reported a series of novel highly dispersed noble metal nanoparticles entrapped in TiO2 nanotubes catalysts and verified the confinement effect of TiO2 nanotubes on the entrapped metal nanoparticles (Yang et al. 2014, 2015) as well. For instance, the Pd nanoparticles confining in the inner pores of TiO2 nanotubes demonstrated enhanced activity toward the hydrogenation of cinnamaldehyde.
Inspired by the above observation and experiment results, entrapping Pd nanoparticles in TiO2 nanotubes would be an effective way to improve its catalytic combustion performance as well. To the best of our knowledge, the research on confinement effect toward the catalytic combustion has not been reported yet. Indeed, conducting such investigation can broaden the scope of knowledge and utilization of the TiO2 nanotubes derived confined catalysts and help to explore the intrinsic mechanism of synergy effect between the host and entrapped components. Therefore, herein we attempt to prepare a Pd nanoparticles entrapped in TiO2 nanotubes catalyst and investigate its performance toward the catalytic combustion of butane, with the purpose of exploring the confinement effect of TiO2 nanotubes on the confined Pd nanoparticles over its oxidation activity.
Experiment
Preparation of catalyst
TiO2 nanotubes were synthesized by a previously described method (Sun and Li 2003). TiO2 nanotubes supported palladium catalysts were prepared by the following procedures (Yang et al. 2015). The Pd nanoparticles deposited in the inner pores of TiO2 nanotubes (Pd-in/TiO2-nanotube) was prepared by vacuum-assisted impregnation route using an aqueous solution containing a calculated amount of PdCl2 under pressure of <10−2 pa. The mixture was then vacuum dried overnight at 40 °C, followed by reduction in H2 at 300 °C for 2 h. A referenced Pd catalyst, with Pd nanoparticles dispersed on the exterior surface of TiO2 nanotubes, was prepared by a typical wet-impregnation method and denoted as Pd-out/TiO2-nanotube.
Catalyst evaluation
The performance for the catalytic oxidation of butane over the catalysts was investigated in a fixed-bed quartz flow reactor at atmospheric pressure, equipped with mass-flow meter to control the mass velocity of inlet gas. Before the combustion, the catalysts were pre-activated by calcination in the air at 300 °C for 3 h. The gas mixture containing butane of 2 vol%, O2 of 50 vol% and N2 balanced were fixed at total flow rate of 50 cm3 min−1, corresponding to a gas hourly space velocities of 20,000 h−1. Agilent 7890A was used to detect butane, CO and CO2 in tail gas. The operating parameters were as follows: temperature of the detector, 180 °C; temperature of the column, 50 °C; carrier gas, helium 30 cm3 min−1.
Catalyst characterization
N2 adsorption–desorption isotherms were measured with a Tristar 3010 isothermal nitrogen sorption analyzer (Micromeritics). Transmission electronic microscopy (TEM) was carried out on a JEM-2010 microscope using an accelerating voltage of 200 kV. An energy-dispersive X-ray spectroscope (EDX) analyzer attached to Zeiss Sigma scanning transmission electron microscope (STEM) operating at 15 kV was used to analyze the chemical components. X-ray photoelectron spectroscopy (XPS) was performed with an AXIS Ultra DLD (Kratos, Britain) to examine the catalysts’ electronic properties. Temperature programmed oxidation (TPO) test was carried out in a Micrometrics Pulse ChemiSorb 2705 apparatus.
Results and discussion
Catalytic activity of the TiO2 nanotube supported Pd catalysts toward butane combustion
Figure 1a shows the butane conversion as a function of the reaction temperature over the Pd catalysts. Over the Pd-out/TiO2-nanotube, the oxidation of butane started at ca. 130 °C followed by the steep increase in conversion up to nearly 100% at 150 °C, while over the Pd-in/TiO2-nanotube catalyst, it started at 100 °C and 100% of propane conversion was achieved at temperature of 130 °C. It demonstrates that compared with the Pd nanoparticles deposited on outer pores of TiO2 nanotubes, the entrapped Pd nanoparticles exhibit enhanced catalytic activity toward the combustion of butane.
a Effect of temperature on butane conversion and b time on stream butane conversion over Pd-out/TiO2-nanotube and Pd-in/TiO2-nanotube catalysts. The Pd-in/TiO2-nanotube, with Pd nanoparticles confined within inner pores of TiO2 nanotubes, showed complete combustion at lower temperature than that of the Pd-out/TiO2-nanotube with Pd nanoparticles deposited on exterior surface of TiO2 nanotubes. These indicate the enhanced oxidation activity and stability of entrapped Pd nanoparticles as result of confinement of effect of TiO2 nanotubes
To demonstrate the stability of the Pd-in/TiO2-nanotube catalysts, we measured butane conversion as a function of time on stream at constant temperatures of 180 °C, as shown in Fig. 1b. Both Pd-in/TiO2-nanotube and Pd-out/TiO2-nanotube showed complete butane conversion at the initial reaction stage (~5 h). Then the Pd-out/TiO2-nanotube exhibited some decline, approximately 8% upon the time evolved. Negligible conversion decline was observed for the Pd-in/TiO2-nanotube catalyst during the stability test. The deactivation of Pd catalyst in combustion is related to the metal sintering/leaching, coke or water-induced inhibition (Gholami et al. 2015). These results demonstrate well that the Pd-in/TiO2-nanotube has higher activity and stability than the Pd-out/TiO2-nanotube toward butane combustion.
Structure characterization of the TiO2 nanotube supported Pd catalysts
The specifications of used catalysts were characterized by nitrogen sorption analyzer. The porosity of both samples, including surface area, pore size and pore volume, maintained well after combustion reaction (seeing Table 1), suggesting the good thermal stability of TiO2 nanotubes.
The entrapment of Pd nanoparticles within inner pores of TiO2 nanotubes has been verified in our previous report (Yang et al. 2015). Figure 2 shows the scanning transmission microscope (STEM) images of the spend catalysts after 20 h of oxidation reaction. The tubular morphology of TiO2 nanotubes was preserved well (Fig. 2a, e), which can explain the reason for the high surface area of spend catalysts. The entrapped Pd nanoparticles and non-entrapped Pd nanoparticles can be seen as well. In the elementary mapping, for the Pd-in/TiO2-nanotube, the Pd element dispersed uniformly on the TiO2 nanotubes (Fig. 2h), while the Pd-out/TiO2-nanotube exhibits somehow aggregation (Fig. 2d), indicating that the Pd-out/TiO2-nanotube is more susceptible to aggregate than the Pd-in/TiO2-nanotube during oxidation. The average particle size of Pd nanoparticles calculated from the STEM images is 5.4 and 3.1 nm, respectively. The corresponding energy-dispersive X-ray spectroscope-derived results (see Table 1) verified the coexistence of the Pd, Ti and O and revealed the Pd/Ti atomic ratio of 0.05 and 0.04 for Pd-out/TiO2-nanotube and Pd-in/TiO2-nanotube, respectively.
Scanning transmission electron microscope (STEM) images and the corresponding elementary mapping from the selected part in STEM image of used Pd-out/TiO2-nanotube (a–d) and Pd-in/TiO2-nanotube (e–h) catalysts. The Pd-in/TiO2-nanotube holds uniformly dispersed Pd nanoparticles on the TiO2 nanotubes, while the Pd-out/TiO2-nanotube exhibits somehow aggregation, indicating that the Pd-out/TiO2-nanotube is more susceptible to aggregate than the Pd-in/TiO2-nanotube during oxidation
Figure 3a is the temperature programmed oxidation (TPO) curves of used Pd catalysts. Of greater relevance to activity is the feature in temperature range of 300–400 °C, which has been attributed to the oxidation of Pd to PdO (Miller and Malatpure 2015). The Pd-in/TiO2-nanotube shows an oxidation peak at 300 °C, which is lower than that of Pd-out/TiO2-nanotube. It indicates the easier formation of PdO for the entrapped Pd nanoparticles, demonstrating that the confinement effect of TiO2 nanotubes could change the oxidation capacity of entrapped Pd nanoparticles. Studies (Gélin and Primet 2002; Bychkov et al. 2016) have evidenced that PdO phase is the activity site for the Pd-based catalyst rather than the metallic Pd phase. It demonstrates that entrapped Pd nanoparticles could possess better oxidation activity than the “non-entrapped” Pd nanoparticles.
Temperature programmed oxidation (TPO) curves (a) and detailed X-ray photoelectron spectra (XPS) of Pd (b) for Pd-out/TiO2-nanotube and Pd-in/TiO2-nanotube catalysts. The Pd-in/TiO2-nanotube presents low temperature of Pd oxidation at TPO plot and high content of PdO phase in the XPS spectra relative to the Pd-out/TiO2-nanotube, indicating the easier formation of PdO for the entrapped Pd nanoparticles as result of confinement effect of TiO2 nanotubes
X-ray photoelectron spectra (XPS) are used to provide surface chemistry information of the two catalysts after 20 h of butane combustion. As shown in Fig. 3b, the observed binding energy value range of 336.7–336.8 and 334.8–335.1 eV is attributable to the Pd2+ and Pd0 species (Choudhury et al. 2016), respectively. Both the Pd catalysts show dominant Pd2+ state, such as PdO, with a small quantity of metallic Pd (Pd0). After deconvolution of the Pd 3d5/2 spectrum, compositions of Pd0/Pd2+ were estimated to be 0.08 and 0.05 for the Pd-out/TiO2-nanotube and Pd-in/TiO2-nanotube, respectively. Correspondingly, the Pd0/Pd2+ values of the fresh catalysts were 0.72 and 0.61 (Yang et al. 2015), respectively. This implies that most part of the metallic Pd was oxidized to PdO phase during butane combustion, especially for the Pd-in/TiO2-nanotube. The mixed Pd/PdO state determines the rate of methane oxidation (Hoffmann et al. 2015), and thus this composition change in the Pd phase during oxidation reaction would reflect the various oxidation capabilities of the Pd species between the two TiO2 nanotube loading Pd catalysts.
Confinement effect of TiO2 nanotubes on the entrapped Pd nanoparticles toward combustion reactivity
The catalytic performance results verify that the confinement effect of TiO2 nanotubes can enhance the activity of entrapped Pd nanoparticles toward butane combustion. The fresh Pd-in/TiO2-nanotube holds smaller particle size than that the Pd-out/TiO2-nanotube does, which contribute to the more of active sites. Moreover, the constrained effect induced by the tubular cavity hindered the particles aggregation for the Pd-in/TiO2-nanotube, as justified by the STEM images of Fig. 2. It implies the confinement effect of TiO2 nanotubes could hinder the metal sintering of entrapped Pd nanoparticles during gas oxidation process and maintain catalytic stability, a similar phenomenon envisioned in the case of liquid hydrogenation by Pd-in/TiO2-nanotube (Yang et al. 2015).
Another important respect on the confinement effect toward entrapped Pd nanoparticles’ oxidation activity is the redox state or electronic properties. Theory calculations and experiments (Xin et al. 2014) have revealed that the dissociative adsorption of hydrocarbon molecule on the PdO (101) facet is the rate-determining step in the catalytic combustion process. In the earlier work of others’ group (Gélin and Primet 2002) and (Bychkov et al. 2016), it has verified that palladium in the oxidized state, instead of the metallic one, has much high activity in alkane combustion. Therefore, the surface composition and physic-chemical properties of the PdO phase are the crucial factors influencing the catalytic activity. The composition of Pd species detected by the XPS has revealed that the Pd0/Pd2+ value varied dependent on the confinement effect. Namely, the Pd-in/TiO2-nanotube holds more PdO phase than that of Pd-out/TiO2-nanotube does. This can be ascribed to the easy oxidized properties of entrapped Pd nanoparticles, as shown in TPO of Fig. 3a. It suggests that the electronic transfer induced by the confinement effect could make the entrapped Pd nanoparticles more susceptible to be oxidized.
Conclusion
The catalytic performance of Pd-in/TiO2-nanotube, with Pd nanoparticles entrapped in TiO2 nanotubes, toward butane combustion was investigated for the first time. Pd-in/TiO2-nanotube catalyst showed higher oxidation activity and lower totally combustion temperature than Pd-out/TiO2-nanotube. The enhanced activity can be ascribed to the confinement effects of TiO2 nanotubes on the entrapped Pd nanoparticles which improve the dispersion of Pd nanoparticles and facilitate formation of PdO. This new and efficient method entrapped the active metal nanoparticles within the TiO2 nanotubes, to lower the temperature for combustion of VOC, which will has potential applications in catalytic combustion of organic gas at mild temperature.
References
Amini M, Naslhajian H, Farnia SMF (2014) V-doped titanium mixed oxides as efficient catalysts for oxidation of alcohols and olefins. New J Chem 38:1581–1586. doi:10.1039/C4NJ00066H
Bychkov VY, Tulenin YP, Slinko MM, Khudorozhkov AK, Bukhtiyarov VI, Sokolov S, Korchak VN (2016) Self-oscillations during methane oxidation over Pd/Al2O3: variations of Pd oxidation state and their effect on Pd catalytic activity. Appl Catal A 522:40–44. doi:10.1016/j.apcata.2016.04.024
Cargnello M, Delgado Jaen JJ, Hernandez Garrido JC, Bakhmutsky K, Montini T, Calvino Gamez JJ, Gorte RJ, Fornasiero P (2012) Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337:713–717. doi:10.1126/science.1222887
Centi G (2001) Supported palladium catalysts in environmental catalytic technologies for gaseous emissions. J Mol Catal A 173:287–312. doi:10.1016/S1381-1169(01)00155-8
Chen W, Fan Z, Pan X, Bao X (2008) Effect of confinement in carbon nanotubes on the activity of Fischer—Tropsch iron catalyst. J Am Chem Soc 130:9414–9419. doi:10.1021/ja8008192
Chlala D, Giraudon JM, Nuns N, Lancelot C, Vannier R-N, Labaki M, Lamonier JF (2016) Active Mn species well dispersed on Ca2+ enriched apatite for total oxidation of toluene. Appl Catal B 184:87–95. doi:10.1016/j.apcatb.2015.11.020
Choudhury S, Betty CA, Bhattacharyya K, Saxena V, Bhattacharya D (2016) Nanostructured PdO thin film from Langmuir–Blodgett precursor for room-temperature H2 gas sensing. ACS Appl Mater Interf 8:16997–17003. doi:10.1021/acsami.6b04120
Deng Y, Nevell TG (1999) Oscillations of methane combustion over alumina-supported palladium catalysts under oxygen-deficient conditions. J Mol Catal A 142:51–60. doi:10.1016/S1381-1169(98)00286-6
Deng C, Yang W, Zhou J, Liu Z, Wang Y, Cen K (2015) Catalytic combustion of methane, methanol, and ethanol in microscale combustors with Pt/ZSM-5 packed beds. Fuel 150:339–346. doi:10.1016/j.fuel.2015.02.018
Gélin P, Primet M (2002) Complete oxidation of methane at low temperature over noble metal based catalysts: a review. Appl Catal B 39:1–37. doi:10.1016/S0926-3373(02)00076-0
Gholami R, Alyani M, Smith KJ (2015) Deactivation of Pd catalysts by water during low temperature methane oxidation relevant to natural gas vehicle converters. Catalysts 5:561–594. doi:10.3390/catal5020561
Hoffmann M, Kreft S, Georgi G, Fulda G, Pohl M-M, Seeburg D, Berger-Karin C, Kondratenko EV, Wohlrab S (2015) Improved catalytic methane combustion of Pd/CeO2 catalysts via porous glass integration. Appl Catal B 179:313–320. doi:10.1016/j.apcatb.2015.05.028
Krishnaraj R (2015) Control of pollution emitted by foundries. Environ Chem Lett. doi:10.1007/s10311-015-0500-z
Lovón-Quintana JJ, Santos JBO, Lovón ASP, La-Salvia N, Valença GP (2016) Low-temperature oxidation of methane on Pd-Sn/ZrO2 catalysts. J Mol Catal A 411:117–127. doi:10.1016/j.molcata.2015.08.001
Miller JB, Malatpure M (2015) Pd catalysts for total oxidation of methane: support effects. Appl Catal A 495:54–62. doi:10.1016/j.apcata.2015.01.044
Sanchez A, Artola A, Font X, Gea T, Barrena R, Gabriel D, Sanchez-Monedero M, Roig A, Cayuela M, Mondini C (2016) Greenhouse gas emissions from organic waste composing. Environ Chem Lett 13:223–238. doi:10.1007/s10311-015-0507-5
Silva M, Burrows H, Formosinho S, Alves L, Godinho A, Antunes M, Ferreira D (2007) Photocatalytic degradtion of chlorophenols using Ru(bpy) 2+3 S2O 2-8 . Environ Chem Lett 5:143–149. doi:10.1007/s10311-007-0096-z
Sun X, Li Y (2003) Synthesis and characterization of ion-exchangeable titanate nanotubes. Chem Eur J 9:2229–2238. doi:10.1002/chem.200204394
Xin Y, Wang H, Law CK (2014) Kinetics of catalytic oxidation of methane, ethane and propane over palladium oxide. Combust Flame 161:1048–1054. doi:10.1016/j.combustflame.2013.10.023
Yang X, Yu X, Long L, Wang T, Ma L, Wu L, Bai Y, Li X, Liao S (2014) Pt nanoparticles entrapped in titanate nanotubes (TNT) for phenol hydrogenation: the confinement effect of TNT. Chem Commun 50:2794–2796. doi:10.1039/C3CC49331H
Yang X, Wu L, Ma L, Li X, Wang T, Liao S (2015) Pd nano-particles (NPs) confined in titanate nanotubes (TNTs) for hydrogenation of cinnamaldehyde. Cata Commun 59:184–188. doi:10.1016/j.catcom.2014.10.031
Yu S, Zhang Q, Yan R, Wang S, Li P, Chen B, Liu W, Zhang X (2014) Origin of air pollution during a weekly heavy haze episode in Hangzhou, China. Environ Chem Lett 12:543–550. doi:10.1007/s10311-014-0483-1
Acknowledgements
This work was supported by the National Scientific Foundation of China (Project No. 51661145022 and 21303210) and the Science & Technology Plan Project of Guangdong Project of Guangdong Province, China (No. 2013B050800002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Lu, X., Wu, L. et al. Pd nanoparticles entrapped in TiO2 nanotubes for complete butane catalytic combustion at 130 °C. Environ Chem Lett 15, 421–426 (2017). https://doi.org/10.1007/s10311-017-0608-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-017-0608-4