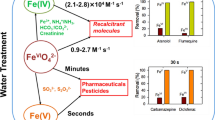
Abstract
Perfluoroalkyl and polyfluoroalkyl substances are occurring in consumer and industrial products. They have been found globally in the aquatic environment including drinking water sources and treated wastewater effluents, which has raised concern of potential human health effects because these substances may be bioaccumulative and extremely persistent. The saturated carbon–fluorine bonds of the substances make them resistant to degradation by physical, chemical, and biological processes. There is therefore a need for advanced remediation methods. Iron-based methods involving high-valent compounds are appealing to degrade these substances due to their high oxidation potentials and capability to generate environmentally friendly by-products. This article presents for the first time the oxidation ability of tetraoxy anions of iron(V) (FeVO4 3−, Fe(V)), and iron(IV) (FeIVO4 4−, Fe(IV)), commonly called ferrates, in neutral and alkaline solutions. Solid compounds of Fe(V) (K3FeO4) and Fe(IV) (Na4FeO4) were added directly into buffered solution containing perfluorooctansulfonate and perfluorooctanoic acid at pH 7.0 and 9.0, and mixed solutions were subjected to analysis for remaining fluoro compounds after 5 days. The analysis was performed by liquid chromatography–mass spectrometry/mass spectrometry technique. Fe(IV) showed the highest ability to oxidize the studied contaminants; the maximum removals were 34 % for perfluorooctansulfonate and 23 % for perfluorooctanoic acid. Both Fe(V) and Fe(IV) had slightly higher tendency to oxidize contaminants at alkaline pH than at neutral pH. Results were described by invoking reactions involved in oxidation of perfluorooctansulfonate and perfluorooctanoic acid by ferrates in aqueous solution. The results demonstrated potentials of Fe(V) and Fe(IV) to degrade perfluoroalkyl substances in contaminated water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Access to purified water is one of the greatest challenges in the twenty-first century due to the increasing global demand for water (United Nations Educational, Scientific, and Cultural Organization 2003). Any treatment method to purify water must be easy to implement and involve readily available materials. Iron is abundant on earth, and the application of iron-based technology is also attractive due to its environmentally benign products. Moreover, iron chemistry for water treatment is well understood, and iron-based materials are generally magnetic and can easily be removed after their applications in the treatment procedure thus rendering them more sustainable (Zboril et al. 2012). Iron has a unique range of valence states and polymorphs, which have found applications in environmental remediation and water treatment (Crane and Scott 2012; Sharma 2011). Examples include zero valent iron (ZVI) (Crane and Scott 2012; De la Cruz et al. 2012; Dhakshinamoorthy et al. 2012) and iron(II) sulfides in the treatment of contaminated groundwater (Butler et al. 2013) and ferrous (Fe(II)) and ferric (Fe(III)) ions as main constituents of Fenton and Fenton-like reactions to generate reactive •OH species in order to purify water. Salts of ferric ions are also very commonly used as coagulants in drinking water treatment.
In recent years, tetraoxy iron(VI) (FeVIO4 2−, Fe(VI)) referred to as ferrate(VI) has emerged as a novel compound to treat water because of its dual function as an oxidant and subsequent coagulant in the form of Fe(III) oxides/hydroxide (Jiang 2014; Lee et al. 2009). Several researchers have demonstrated the applications of Fe(VI) to detoxify pollutants and to disinfect viruses and bacteria as well as to remove toxic metals and micropollutants from water used as a drinking water source (Horst et al. 2013; Jiang 2007; Prucek et al. 2013; Sharma 2007; Sharma et al. 2012, 2013; Yang et al. 2012). Comparatively, little is known on the potentials of tetraoxy iron(IV) (FeIVO4 4−, Fe(IV)) and tetraoxy iron(V) (FeVO4 3−, Fe(V)) to treat contaminated water. The present paper aims for the first time to demonstrate the potential of Fe(IV) and Fe(V) to degrade recalcitrant compounds.
In this work, the selected recalcitrant compounds were the perfluoroalkyl compounds (PFCs), perfluorooctansulfonate, and perflouorooctanoic acid. These compounds have very high thermal and chemical stability and therefore are used in many industrial and consumer products such as shampoos, fume suppressants, paints, non-stick cookware, and floor polishes (Knepper and Lange 2012). Perfluorooctansulfonate and perfluorooctanoic acid have been detected in groundwater at concentrations higher than the provisional health advisory limits (Appleman et al. 2014). A remediation technique is required to remove perfluorooctansulfonate and perfluorooctanoic acid from groundwater, especially if that water is a potential source of drinking water. Treatment methods that have been applied to degrade perfluorooctansulfonate and perfluorooctanoic acid include incineration, coagulation, biofiltration, traditional oxidation techniques (chlorination, ozonation, and UV irradiation), sonolysis, and photocatalytic oxidation, some of which are energy intensive and expensive to implement (Appleman et al. 2014; Rahman et al. 2014). Advanced oxidation processes where the •OH radical is the reactive species have been found ineffective treating perfluorooctansulfonate and perfluorooctanoic acid (Appleman et al. 2014). However, some success with activated persulfate on the removal of perfluorooctansulfonate by the sulfate radical has been seen (Knepper and Lange 2012; Lee et al. 2013). A recent study showed the removal of perfluorooctanoic acid in the presence of superoxide and hydro peroxide reactive species (Mitchell et al. 2014). The present paper seeks to define the radical-behavior role of Fe(V) and Fe(IV) to degrade perfluorooctansulfonate and perfluorooctanoic acid, which behave like radicals. The objectives of the current paper are as follows: (1) to demonstrate the potential of high-valent iron species in treating selected recalcitrant contaminants, and (2) to determine the efficacy of Fe(IV) and Fe(V) species to treat selected contaminants at different pH.
Experimental methods
All chemicals used in the study were reagent grade (>95 % purity, Fisher Scientific) unless otherwise noted and were used without further purification. A solid potassium salt of Fe(VI) (K2FeO4) was prepared by the wet chemical method (Luo et al. 2011) and the purity was >95 %. Sodium salt of Fe(IV) (Na4FeO4) and potassium salt of Fe(V) (K3FeO4) were synthesized using thermal methods (Dedushenko et al. 2010; Jeannot et al. 2002). The purity of the salts was confirmed by the Mossbauer technique. Purities of the samples were ≥80 %. All solutions were prepared in water (18 MΩ cm−1), which was obtained by passing double-distilled deionized water to the Milli-Q water purification system. A solution of Fe(VI) was prepared by adding 198 mg of solid K2FeO4 to 1 L buffer solution at pH 9.0, where the solution is most stable. Concentration of Fe(VI) in solution was measured by absorbance at 510 nm (ε510 = 1,150 M−1 cm−1). A UV–Vis spectrophotometer (Agilent 8453) was used for measuring absorbance.
In order to avoid adsorption of perfluorooctansulfonate and perfluorooctanoic acid to treatment bottle walls, all experiments, except buffers, were performed using 1-L HDPE bottles. Buffers were prepared in 4-L glass volumetric flasks. Experiments were carried out either at pH 9.0 (0.001 M borate/0.005 M Na2HPO4 buffer) or at pH 7.0 (0.03 M KH2PO4/0.05 M Na2HPO4 buffer). Stock solutions of perfluorooctansulfonate (Alfa Aesar) and perfluorooctanoic acid (Strem Chemicals) were prepared by adding 50 mg of each compound to 1-L HDPE bottles and then diluting to 1 L with the appropriate buffer. Each treatment bottle was prepared in duplicate, and a same procedure for control bottles (not added Fe(IV) or Fe(V)) was applied. The initial concentrations of perfluorooctansulfonate and perfluorooctanoic acid were 0.779–1.252 and 4.515–7.022 mgL−1, respectively. For the testing, pre-weighed amounts of Fe(IV), Fe(V), and Fe(VI) were added to separate, empty 1-L HDPE bottles. Fe(IV) and Fe(V) were added to bottles in amounts, equal to 50 times the stoichiometric amount to fully oxidize the PFC. The addition was done in glove box to avoid contact of humidity with Fe(IV) and Fe(V), which can degrade their salts rapidly. After addition of the ferrate compounds, the bottles were capped and wrapped with parafilm, removed from the glove box. Each testing bottle was opened individually and the PFC solution was immediately poured into the ferrate-containing bottle. The bottles were then quickly capped and inverted fifteen times to ensure complete mixing.
A liquid chromatography–mass spectrometry/mass spectrometry (LC–MS/MS) method was used to analyze perfluorooctansulfonate and perfluorooctanoic acid. Standards in acetonitrile were prepared by diluting with an equal amount of water. The range of standards was 1–500 μgL−1. Concentrated samples were diluted to this range. The internal standard used was 13C, which was spiked at a consistent level for all standards and samples. Percent removal was calculated based on the pre- and post-treatment concentrations of each compound. Samples were allowed to react for 5 days in the treatment bottle before analyzing for perfluorooctansulfonate and perfluorooctanoic acid.
Results and discussion
In initial experiments, a solid salt of Fe(IV) was added into buffered solution to determine the interaction of Fe(IV) ion with water at pH 9.0. The solution instantaneously turned the pink-violet color characteristics of Fe(VI) ion and then quickly reduced to Fe(III) evidenced by a rust-brown color. Interestingly, Fe(IV) converted into higher valent Fe(VI) ion, which can be described by Eq. (1).
The formation of Fe(VI) and Fe(III) from the decomposition of Fe(IV) due to water present in air has been confirmed by Mossbauer spectroscopy (Jeannot et al. 2002). Similar experiments were also performed by adding solid salt of Fe(V) into buffered solution at pH 9.0. In this case, the buffered solution also changed to the distinct color of Fe(VI) ion. Spectra collected immediately (initial) and after 2 and 22 h are shown in Fig. 1. The initial spectra had a characteristic peak of Fe(VI) at 510 nm and thus confirmed the decomposition of Fe(V) to Fe(VI). The instantaneous formation of Fe(VI) was surprising as previous pulse radiolysis studies in aqueous solution (Rush and Bielski 1989) have shown the release of Fe(III) ion and hydrogen peroxide from the decomposition of Fe(V) ion in alkaline medium illustrated in Eq. (2).
A more recent study on the decomposition of solid Fe(V) in air exposure using Mossbauer spectroscopy showed the formation of Fe(VI) and Fe(III) (Dedushenko et al. 1999), which may be expressed as Eq. (3).
The Fe(VI) ion formed from the solid was ultimately converted into Fe(III) ion, which was also observed in the pulse radiolysis studies.
In the next set of experiments, solids of Fe(V) and Fe(IV) were added individually into buffered solution containing perfluorooctansulfonate at pH 9.0. Each of the mixed solutions was allowed to react with perfluorooctansulfonate for 5 days at which point the solution turned rust-brown indicating reduction of Fe(III). Solutions were then filtered and analyzed for perfluorooctansulfonate. The results are given in Fig. 2. Perfluorooctansulfonate was resistant to Fe(VI), while both Fe(V) and Fe(IV) were able to oxidize perfluorooctansulfonate. Fe(IV) had the highest ability to oxidize perfluorooctansulfonate with removal percentage of 34 %. The removal percentage by Fe(V) was 21 %. This order of reactivity of removing perfluorooctansulfonate at pH 9.0 is different from the earlier reactivity of ferrates with cyanide at pH 12.2 (Fe(V) > Fe(IV) > Fe(VI)) (Sharma et al. 2001). Similar experiments were conducted for perfluorooctanoic acid. As shown in Fig. 2, the removal percentages for perfluorooctanoic acid were lower, and there was no difference between Fe(V) and Fe(IV) (17 % removal for both). Generally, perfluorooctansulfonate is more reactive than perfluorooctanoic acid with ferrates (Fig. 2).
Removal percentages of perfluorooctylsulfonate and perfluorooctanoic acid by different high-valent iron species at pH 9.0. Fe(VI) is almost unreactive and shows very little removals. Fe(IV) shows the maximum removal. The removal percentage for perfluorooctanesulfonate by Fe(V) is between Fe(VI) and Fe(IV). Comparatively, perfluorooctanoic acid removal by Fe(V) and Fe(IV) is similar
Finally, experiments were conducted at a neutral pH. The removal percentages of perfluorooctansulfonate and perfluorooctanoic acid are presented in Fig. 3. The removal percentages were generally lower at pH 7.0 than those at pH 9.0. For example, removal of perfluorooctansulfonate decreased to 28 % at pH 7.0 from 34 % at pH 9.0. The pH dependence of removal can be explained by considering the rates of competing reactions occurring in the reaction systems, which are (1) ferrates with themselves (Fe(IV) + Fe(IV), Fe(V) + Fe(V), and Fe(VI) + Fe(VI)), (2) ferrates with water (Fe(IV) + H2O, Fe(V) + H2O, and Fe(VI) + H2O), and (3) ferrates with compounds (Fe(IV) + perfluorooctansulfonate/perfluorooctanoic acid, Fe(V) + perfluorooctansulfonate/perfluorooctanoic acid, and Fe(VI) + perfluorooctansulfonate/perfluorooctanoic acid). All the three possible reactions for each ferrate species are pH dependent, and the pH dependence behavior is different depending on both ferrate and compound moiety (Lee and von Gunten 2012; Noorhasan et al. 2010). The results seen in Figs. 2 and 3 represent commutative effectiveness of different ferrate species. It seems that reactions of Fe(IV) with either perfluorooctansulfonate or perfluorooctanoic acid dominate the other possible (self-)reaction and water to cause degradation of the parent compounds. Based on the chemistry of Fe(VI), the oxidation by Fe(IV) was expected to increase in decreasing the pH from 9.0 to 7.0, but lower efficiency at pH 7.0 than that at pH 9.0 was seen. This suggests that the reactions (1) and (2) may compete much more with the reaction (3) at a neutral condition than under alkaline pH. Fe(V) is expected to have much higher oxidation ability than Fe(IV), but lower removal percentage by Fe(V) further indicates the other possible reactions besides the reaction of Fe(V) with perfluorooctansulfonate/perfluorooctanoic acid. It appears that the decomposition of Fe(V) by water and another Fe(V) has higher slope of pH dependence than that of the dependence of the reaction with perfluorooctansulfonate/perfluorooctanoic acid, resulting in lower effectiveness of Fe(VI) to oxidize both compounds.
Removal percentages of perfluorooctylsulfonate and perfluorooctanoic acid by different high-valent iron species at pH 7.0. Removal of perfluorooctylsulfonate by ferrates follows the order Fe(IV) > Fe(V) > Fe(VI) similar to the order at pH 9.0. Removal of perfluorooctanoic acid by Fe(IV) is the highest among different ferrate species. Both Fe(VI) and Fe(V) shows very little removal (~10 %) of perfluorooctanoic acid at pH 7.0
Testing for F− ion was also performed to learn the complete mineralization of degraded parent perfluorooctansulfonate and perfluorooctanoic acid compounds by Fe(V) and Fe(IV). However, no F− ion was observed in any of the tested samples. Based on the most recent results we can say that stoichiometric amounts of fluoride were formed indicating that the PFC is completely mineralized by the Fe(IV) and Fe(V). This indicates incomplete mineralization of studied fluoro compounds by Fe(V) and Fe(IV). Another possibility is the adsorption/co-precipitation of F− ion by Fe(III) oxides/hydroxides, formed from reduction of ferrates. This removal of F− ion would be similar to removal of anions (e.g., arsenic, phosphate, and chromate) and metal ions by Fe(VI) (Filip et al. 2011; Lee et al. 2009; Sharma et al. 2007; Sylvester et al. 2001).
Conclusion
Fe(IV) and Fe(V) were able to oxidize perfluorooctansulfonate and perfluorooctanoic acid under neutral and alkaline pH conditions. However, both compounds were not completely removed by ferrates under the studied conditions. The pH effect on removing the compounds indicates performing the studies over a wide pH range to determine the optimum pH and dosages of ferrates. Products of oxidative transformations of perfluorooctansulfonate and perfluorooctanoic acid also need to be conducted to establish the formation of benign by-products by ferrates. The ability of ferrates to oxidize perfluorooctansulfonate and perfluorooctanoic acid would provide a less energy intensive and more sustainable method for treating PFC-contaminated water both in situ and ex situ.
References
Appleman TD, Higgins CP, Quiñones O, Vanderford BJ, Kolstad C, Zeigler-Holady JC, Dickenson ERV (2014) Treatment of poly- and perfluoroalkyl substances in U.S. full-scale water treatment systems. Water Res 51:246–255
Butler EC, Chen L, Darlington R (2013) Transformation of trichloroethylene to predominantly non-regulated products under stimulated sulfate reducing conditions. Groundw Monit Remediat 33(3):52–60
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211–212:112–115
De la Cruz N, Giménez J, Esplugas S, Grandjean D, De Alencastro LF, PulgarÃn C (2012) Degradation of 32 emergent contaminants by UV and neutral photo-Fenton in domestic wastewater effluent previously treated by activated sludge. Water Res 46(6):1947–1957
Dedushenko SK, Perfilev YD, Tcheboukov DE, Pankratov DA, Kiselev YM (1999) A Mossbauer study of pentavalent iron in a vanadium(V) oxide matrix. Mendeleev Commun 5:171–172
Dedushenko SK, Perfil’ev YD, Chuevb MA, Afanasev AM (2010) Identification of iron oxidation states in the products of interaction of Na2O2 and Fe2O3 by Mossbauer absorption spectroscopy. Russ J Inorg Chem 55(6):942–949
Dhakshinamoorthy A, Navalon S, Alvaro M, Garcia H (2012) Metal nanoparticles as heterogeneous Fenton catalysts. ChemSusChem 5(1):46–64
Filip J, Yngard RA, Siskova K, Marusak Z, Ettler V, Sajdl P, Sharma VK, Zboril R (2011) Mechanisms and efficiency of the simultaneous removal of metals and cyanides by using ferrate(VI): crucial roles of nanocrystalline iron(III) oxyhydroxides and metal carbonates. Chem Eur J 17(36):10097–10105
Horst C, Sharma VK, Clayton Baum J, Sohn M (2013) Organic matter source discrimination by humic acid characterization: synchronous scan fluorescence spectroscopy and Ferrate(VI). Chemosphere 90(6):2013–2019
Jeannot C, Malaman B, Gerardin R, Oulladiaf B (2002) Synthesis, crystal, and magnetic structures of the sodium ferrate(IV) Na4FeO4 studied by neutron diffraction and Mossbauer techniques. J Solid State Chem 165:266–277
Jiang JQ (2007) Research progress in the use of ferrate(VI) for the environmental remediation. J Hazard Mater 146:617–623
Jiang JQ (2014) Advances in the development and application of ferrate(VI) for water and wastewater treatment. J Chem Technol Biotechnol 89:165–177
Knepper TP, Lange FT (2012) Polyfluorinated chemicals and transformation products
Lee Y, von Gunten U (2012) Quantitative structure-activity relationships (QSARs) for the transformation of organic micropollutants during oxidative water treatment. Water Res 46(19):6177–6195
Lee Y, Zimmermann SG, Kieu AT, Gunten GV (2009) Ferrate (Fe(VI)) application for municipal wastewater treatment: a novel process for simultaneous micropollutant oxidation and phosphate removal. Environ Sci Technol 43:3831–3838
Lee YC, Lo SL, Kuo J, Huang C- (2013) Promoted degradation of perfluorooctanic acid by persulfate when adding activated carbon. J Hazard Mater 261:463–469
Luo Z, Strouse M, Jiang JQ, Sharma VK (2011) Methodologies for the analytical determination of ferrate(VI): a review. J Environ Sci Health, Part A Toxic/Hazard Subst Environ Eng 46(5):453–460
Mitchell S, Mushtaque A, Teel AL, Watts RJ (2014) Degradation of perfluorooctanoic acid by reactive species generated through catalyzed H2O2 propagation reactions. Enviorn Sci Technol Lett 1:117–121
Noorhasan N, Patel B, Sharma VK (2010) Ferrate(VI) oxidation of glycine and glycylglycine: kinetics and products. Water Res 44:927–937
Prucek R, Tuček J, Kolařík J, Filip J, Marušák Z, Sharma VK, Zbořil R (2013) Ferrate(VI)-induced arsenite and arsenate removal by in situ structural incorporation into magnetic iron(III) oxide nanoparticles. Environ Sci Technol 43(7):3283–3292
Rahman MF, Peldszus S, Anderson WB (2014) Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res 50:18–340
Rush JD, Bielski BHJ (1989) Kinetics of ferrate(V) decay in aqueous solution. A pulse-radiolysis study. Inorg Chem 28:3947–3951
Sharma VK (2007) Disinfection performance of Fe(VI) in water and wastewater: a review. Water Sci Technol 55(1–2):225–232
Sharma VK (2011) Oxidation of inorganic contaminants by ferrates(Fe(VI), Fe(V), and Fe(IV))—kinetics and mechanisms—a review. J Environ Manage 92:1051–1073
Sharma VK, O’Connor DB, Cabelli DE (2001) Sequential one-electron reductions of Fe(V) to Fe(III) in alkaline solution. J Phys Chem B 105:11529–11532
Sharma VK, Dutta PK, Ray AK (2007) Review of kinetics of chemical and photocatalytical oxidation of arsenic(III) as influenced by pH. J Environ Sci Health Part A Toxic/Hazard Subst Environ Eng 42(7):997–1004
Sharma VK, Sohn M, Anquandah G, Nesnas N (2012) Kinetics of the oxidation of sucralose and related carbohydrates by ferrate(VI). Chemosphere 87:644–648
Sharma VK, Liu F, Tolan S, Sohn M, Kim H, Oturan MA (2013) Oxidation of β-lactam antibiotics by ferrate(VI). Chem Eng J 221:446–451
Sylvester P, Rutherford LA Jr, Gonzalez-Martin A, Kim J, Rapko BM, Lumetta GJ (2001) Ferrate treatment for removing chromium from high-level radioactive tank waste. Environ Sci Technol 35(1):216–221
United Nations Educational, Scientific, and Cultural Organization (2003) Water for people, water for life—the United Nations World Water Development Report, Edition 1 World Water Assessment Programme (WWAP)
Yang B, Ying GG, Zhao J-, Liu S, Zhou LJ, Chen F (2012) Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate(VI) treatment of secondary wastewater effluents. Water Res 46(7):2194–2204
Zboril R, Andrle M, Oplustil F, Machala L, Tucek J, Filip J, Marusak Z, Sharma VK (2012) Treatment of chemical warfare agents by zero-valent iron nanoparticles and ferrate(VI)/(III) composite. J Hazard Mater 211–212:126–130
Acknowledgments
B.J. Yates and R. Darlington acknowledge support from Battelle’s Internal Research and Development funds. V. K. Sharma thanks United States National Science Foundation (CBET 1236331) for supporting ferrate research. The authors also acknowledge the support by the Operational Program Research and Development for Innovations–European Regional Development Fund (CZ.1.05/2.1.00/03.0058) and by Technological Agency of the Czech Republic—the project Environmental Friendly Nanotechnologies and Biotechnologies in Water and Soil Treatment (TE01020218).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yates, B.J., Darlington, R., Zboril, R. et al. High-valent iron-based oxidants to treat perfluorooctanesulfonate and perfluorooctanoic acid in water. Environ Chem Lett 12, 413–417 (2014). https://doi.org/10.1007/s10311-014-0463-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-014-0463-5