Abstract
Natural attenuation of MTBE and BTEX compounds in a petroleum contaminated coastal aquifer in the Tel-Aviv area was investigated. Significant decrease in MTBE concentration and complete disappearance of BTEX compounds occur within 100 m groundwater flow. Highly anaerobic conditions were determined in the close vicinity to the spill source. In order to examine the contribution of microbial degradation to the attenuation process at anaerobic conditions, compound-specific carbon isotope ratio analysis was employed. Carbon isotope enrichment of toluene up to 2.4% along with the drop in its concentration up to 80% was observed within 20 cm below the water table. The lack of isotope fractionation despite the significant concentration decrease for other studied compounds indicates significant contribution of abiotic processes to the natural attenuation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum hydrocarbons are among the most common groundwater contaminants. They enter the subsurface as a result of leaking underground storage tanks, improper disposal, or accidental spills. Water soluble fuel components such as benzene, toluene, ethyl benzene, xylenes (BTEX) and methyl t-butyl ether (MTBE) are of utmost concern from the perspective of human health (Mehlman 1992). In addition to remediation strategies such as pump-and-treat, excavation, and air sparging, widely initiated by man, there is increasing interest in natural attenuation as a promising alternative (Kostecki and Nascarella 2003). Natural attenuation includes both non-destructive abiotic and destructive biotic processes. Undoubtedly, understanding these processes is of great importance because this may, in due course, lead to ability to stimulate them and enhance their efficiency.
Although, initially, biodegradation of petroleum hydrocarbons was believed to proceed under aerobic conditions (Jamison et al. 1975; Smith 1990), anaerobic degradation utilizing electron acceptors (e.g., nitrate, iron or sulfate) or via methanogenesis was demonstrated (Barker and Wilson 1997; Cozzarelli et al. 1990). Several approaches are usually employed to prove the biodegradation of the contaminants in groundwater. One of them is the simultaneous measurement of contaminant concentrations versus depletion of electron acceptors. However, the depletion of the electron acceptors not always correlates well to the degradation of specific contaminants, but reflects the biodegradation of all organic matter in aquifer including natural one. On the other hand, monitoring the fate of the contaminants is often complicated due to aquifer heterogeneity. Another approach, namely detection of metabolites, may be a useful indicator of the degradation. However, in situ concentrations of metabolites are low and some specific metabolites are not detected in every case when biodegradation occurs. One of the most promising developments suggested in recent years is the use of the compound-specific isotope ratio analysis (CSIA) to detect and quantify the extent of biodegradation in contaminated aquifers (Ahad et al. 2000; Anderson et al. 2001). This technique relies on the fractionation of stable isotopes during microbial degradation, leading to enrichment of the residual fraction of a contaminant by the heavier isotopes. In contrast, abiotic non-destructive processes do not change the isotope ratios significantly (Slater et al. 2000; Huang et al. 1999).
The aim of the present study was to investigate bio-geochemical processes occurring in a shallow coastal aquifer contaminated by petroleum fuel hydrocarbons. Additional objective was to estimate the contribution of biodegradation of BTEX compounds and MTBE to natural attenuation.
Experimental
Site overview
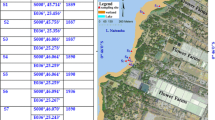
The studied site is a gasoline station in the Tel-Aviv area, about 2 km off the Mediterranean Sea shore. The gasoline releases on the site were detected in 2000. Figure 1 is a site map showing the locations of the monitoring wells used for this investigation.
These are 2 in. outer diameter, PVC wells screened from 5.3 to 11 m. The aquifer consists mainly of poorly graded sand and gravel. The groundwater table is generally between 8 and 9 m below the ground surface and dissolved Fe and Mn. Sampling of the groundwater was performed using disposal bailers. For the depth profile of BTEX and MTBE, passive multi-level sampler with dialysis cells were inserted into the well for 21 days.
Sampling and chemical analysis
All groundwater samples were transferred directly to appropriate sample bottles, which were filled completely with no headspace. All the samples were stored at 4°C until analyzed. Analyses of nitrate and sulfate were performed by ion chromatography. Mn and Fe were analyzed by means of ICP-AES; Arsenic was determined by ICP-MS. The concentrations of the BTEX compounds and MTBE were determined by P&T-GCMS using EPA method 624/8260. Dissolved oxygen (DO) was determined on site using “dissolved oxygen” kit.
Stable isotope analyses
MTBE and BTEX compounds were extracted from the water samples using Tekmar purge and trap (P&T) apparatus interfaced to GC-IRMS. P&T was used with the following program: purge time 12 min at 40°C, desorbtion 4 min at 180°C, and bake 20 min at 200°C. Analytes were transferred into GC without cryfocusing via heated transfer line. GC column was HS-5 MS, 30 m, 0.25 mm i.d., film thickness 0.25 μm. Flow rate was 1 ml/min (constant pressure); temperature program: 40°C for 2 min, 4°C/min to 110°C, 10°C/min to 220°C. The combustion oven was set at 940°C.
Isotope ratio analysis of nitrate (δ15N and δ18O) was performed by GB-IRMS after its conversion to the N2O (McIlvin and Altabet 2005).
Results and discussion
Four monitoring wells located on different distances from the spill source were examined (Table 1).
Comparison of data obtained from the monitoring wells shows that BTEX compounds have a restricted distribution. The high concentrations of the BTEX and MTBE were detected in the MW-1. In MW-2, MTBE was detected as the dominant contaminant (Ca: 3 mg/l), whereas the total concentration of the BTEX compounds was about 12 μg/l (Table 1). The concentration of MTBE detected in MW-3 was 33 μg/l and only trace amounts were present in the MW-4. This distribution of contaminants suggests that most hydrocarbons are attenuated within 100 m off the source, while only MTBE migrates further down the flow path.
Distribution of electron acceptors (O2, NO3 −, SO4 2−) and metabolic by-products (dissolved Fe and Mn) varied with the groundwater flow path (Table 1). On the other hand, pH values of water remains almost constant for the all studied wells (6.3–6.7), as well as δ18O (about 5.2%). We found that in the close proximity to the contaminant source (MW-1, Fig. 1) conditions were highly reducing: dissolved oxygen and nitrate were almost consumed and dissolved manganese and iron were present in the water, whereas Eh value was 238 mV. Extremely low nitrate concentration with δ15N of 39% and δ18O of 19% (Fig. 2a, b) points out microbial denitrification process.
Significant concentration of the dissolved manganese and iron up to 0.57 and 2.4 mg/l, respectively were observed at this well. In addition, elevated level of the dissolved arsenic (up to 37 μg/l) was detected at MW-1. Dissolution of arsenic in groundwater obviously coupled to Fe (III) reduction (Cummings et al. 1999). Further downstream, the concentrations of the electron acceptors increase, and dissolved Fe and As are no longer present. Nitrate at MW-2 and MW-3 present at higher concentrations (6 and 9 mg/l, respectively) and less 15N and 18O enriched (δ15N in the range of 22–28%, δ18O in the range of 10–13%, Fig. 2a), indicate less denitrification. High nitrate concentration accompanied by δ15N of 15% and δ18O of 6% at MW-4 reflects the lighter background values in the aquifer.
MTBE and BTEX concentration lowered drastically along with groundwater flow path. To investigate the processes occurring in close vicinity to the spill source, depth distribution of the hydrocarbons was examined at the MW-1 using passive multi-level sampler. Depth profiles showed significant decrease in contaminants concentrations within 106 cm of the water table (Table 2). The most pronounced decline was observed for toluene (up to 90% within the depth of 106 cm).
Since, biodegradation of the BTEX compounds and MTBE under anaerobic conditions is accompanied by carbon isotope enrichment (Meckenstock et al. 2004), we used compound-specific carbon isotope ratio analysis to investigate biodegradation. Table 2 shows the δ13C values of the dissolved hydrocarbons with respect to the source. Small but resolvable isotope 13C enrichment of 2.4% for toluene, along with drastic lowering in its concentration (from 6,300 to 1,540 μg/l) in a small interval below the water table should be attributed to biodegradation. Further decrease of toluene concentration up to 510 μg/l was not accompanied by δ13C change, and, therefore, resulted from the non-destructive abiotic processes, such as dilution or sorption. The significant concentration decrease of MTBE, benzene, ethyl benzene and o-xylene without any carbon isotope fractionation observed at MW-1, obviously indicates the minor or no contribution of biodegradation to their attenuation. Microbial degradation of these compounds could be restricted by competition for electron acceptors from other hydrocarbons and toluene. Thus, for example, the lack of biodegradation of MTBE at anaerobic conditions in the presence of BTEX compounds was demonstrated earlier (Spence et al. 2005). Significant decrease of the MTBE and BTEX concentrations in the interval 94–106 cm below the water table, possibly, originates from the presence of some barrier (for example, clay layer) in the water flow path.
Higher concentrations of dissolved oxygen were measured in the wells located further down-gradient. In addition, higher concentrations of nitrate were observed at these wells. As could be seen (Table 1), BTEX concentration dropped to 12 μg/l when they reached the monitoring well MW-2 located within 50 m from the spill. These concentrations were too low to allow accurate compound-specific carbon isotope ratio analysis. Isotopic analysis of MTBE from this well yielded a δ13C of −30.1. No significant isotopic variation in the MTBE compared to the value obtained for MW-1, indicates that either the magnitude of any biodegradation-induced isotopic fractionation is small, or that relatively little degradation has occurred under these conditions. Due to the fact that any isotope ratio analysis of BTEX compounds for the wells with sufficiently high concentrations of dissolved oxygen has not been performed, we could not assess a contribution of the aerobic biodegradation to the attenuation process.
Conclusions
Groundwater contamination by gasoline leads to oxygen depletion and development of highly anaerobic conditions in the close vicinity of the contamination source. Under iron reducing conditions, toluene was degraded more rapidly than other studied compounds. In this zone, significant contribution of abiotic processes to natural attenuation was observed. Significant lowering of MTBE concentration was accompanied by an increase of oxygen concentration and a disappearance of BTEX compounds, but was not accompanied by any valuable carbon isotope enrichment. The lack of the MTBE isotope enrichment implies that either the magnitude of biodegradation induced isotope fractionation is small, or that the contribution of biodegradation to the attenuation process is not meaningful.
References
Ahad ME, Lollar BS, Edwards EA, Slater GF, Sleep BE (2000) Carbon isotope fractionation during anaerobic biodegradation of toluene: implications for intrinsic bioremediation. Environ Sci Technol 34:892–896
Anderson RT, Hunkeler D, Anderson N, Aravena R, Bernasconi SM, Butler BJ (2001) Hydrogen and carbon isotope fractionation during aerobic biodegradation of benzene. Environ Sci Technol 35:3462–3467
Barker JF, Wilson JT (1997) Natural biological attenuation of aromatic hydrocarbons under anaerobic conditions. In: Ward CH, Cherry JA, Scalf MR (eds) Subsurface restoration ann arbor press. Chelsea, MI, pp 289–300
Cozzarelli IM, Eganhouse RP, Baedecker MJ (1990) Transformation of monoaromatic hydrocarbons to organic acids in anoxic groundwater environment. Environ Geol Water Sci 16:135–141
Cummings DE, Caccavo F, Fendorf S, Rosenzweig RF (1999) Arsenic mobilization by dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ Sci Technol 33:723–729
Huang L, Sturchio NC, Abrajano T., Heraty LJ, Holt BD (1999) Carbon and chlorine isotope fractionation of chlorinated aliphatic hydrocarbons by evaporation. Org Geochem 30:777–785
Jamison VW, Raymond R L, Hudson JOJ (1975) Biodegradation of high-octane gasoline in groundwater. Dev Ind Microbiol 16:305–312
Kostecki P, Nascarella M (2003) LUST Cleanup landscape changing: landfilling still in, pump and treat on the way out. Contam Soil Sedim Water 9–11
Meckenstock RU, Morasch B, Griebler C, Richnow HH (2004) Stable isotope fractionation analysis as a tool to monitor biodegradation in contaminated aquifers. J Contam Hydrol 75:215–255
Mehlman MA (1992) Dangerous and cancer-causing properties of products and chemicals in the oil refining and petrochemical industry VIII health effects of motor fuels: carcinogenicity of gasoline—scientific update. Environ Res 59:238–249
McIlvin MR, Altabet MA (2005) Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal Chem 77:5589–5595
Slater GF, Ahad ME, Lollar SB, Allen-King R, Sleep B (2000) Carbon isotope effects resulting from equilibrium sorption of dissolved VOCs. Anal Chem 72:5669–5672
Spence MJ, Bottrell SH, Thornton SF, Richnow HH, Spence KH (2005) Hydrochemical and isotopic effects associated with petroleum fuel biodegradation pathways in a chalk aquifer. J Contam Hydrol 79:67–88
Smith MR (1990) The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation 1:191–206
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gelman, F., Binstock, R. Natural attenuation of MTBE and BTEX compounds in a petroleum contaminated shallow coastal aquifer. Environ Chem Lett 6, 259–262 (2008). https://doi.org/10.1007/s10311-007-0125-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-007-0125-y