Abstract
We demonstrate that carbon, hydrogen and sulphur isotope ratios both, in the total peat (δ13Ctp=−25.52 to −28.27‰, δDtp=−78.67 and −109.24‰, δ34S=4.35 to 19.87‰), and in cellulose from the peat (δ13Cnc=−25.06 to −27.33‰ and δDnc=−92.43 to −118.02‰) are not affected by postdepositional changes. Therefore, the original isotope composition of plants are in general preserved in the peat and represent an archive of the past environmental variations. These can be supported by (i) good correlations between δ13Ctp and δ13Cnc, and between δDtp and δDnc, (ii) high horizontal homogeneity of δ13Ctp and δ13Cnc in the scale of one peat-bog – the same major factor(s) control(s) C isotopic ratios, (iii) no correlation between organic sulphur concentrations and δ34S value −δ34S results from variations in the water level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental variations during vegetation results in various isotope fractionation in various plants. Therefore, peat profiles are a potentially abundant archive of past climates especially when the peat is composed of a single species. The dominant organic mass of raised peat bogs in a temperate climate zone is Sphagnum sp. However, it is still not clear to what extent, stable isotope composition of carbon, sulphur and hydrogen in the primary organic mater in peat, is determined by (i) environmental conditions, (ii) physiology of the living Sphagnum sp., (3) isotopic composition of assimilated CO2, SO x and H2O, and (iv) isotopic fractionation during assimilation and diagenesis. Its seems that in contrast to trees, the relatively simple physiology of Sphagnum may help to calibrate isotope ratios of peat and potentially provide a valuable tool for paleoenvironmental reconstruction.
The main source of atmospheric sulphur delivered to peat bogs is anthropogenic origin compounds, especially: SO2, particulate SO4 2− and aerosols, and sulphuric acid. The δ34S of anthropogenic sulphate ranges from about −3 to 10‰ and δ34S of marine sulphur is about 21‰ (mainly dimethylsulphide and sulphates). Since the 19th century anthropogenic impact has resulted in lower δ34S values of atmospheric sulphur but local effects are strongly controlled by regional variations in atmospheric circulation. At the peat-bog scale it was found that 15–30% of the aerosol sulphur is of biogenic origin. Sulphur is mostly assimilated by plants in the form of sulphate from the soil (water) through the root system or from the atmosphere in the form of SO2. The SO2 uptake may occur in three ways: dry deposition on cuticule, wet deposition on cuticule, and the most important, diffusion through stomata. Assimilation of sulphur by lower plants, such as Sphagnum species, is less complicated and faster due to lack of any root system or stomata sensu stricte S-compounds are assimilated directly to cells. The cysteine and methionine (amino acids) are the main sulphur-bearing compounds in peat and some H2S can be directly incorporated into cysteine. This can be important because the H2S is strongly depleted in 34S as compared to other S-compounds.
In general, two factors controlling trends in isotopic variations should be considered: primary factors, especially climatic variations and anthropogenic impact; and secondary factors, especially the methanogenic processes that constrain speculations on δ13C and δ34S isotope signature in organic matter (Jedrysek et al. 1999; Bottrel and Coulson 2003; Skrzypek and Jedrysek 2004). Novak et al. (2000) suggest that the diagenetic processes are the most important to the δ34S value in the peat as a certain portion of the sulphur in peat bogs does not survive early diagenetic processes, thus S-isotope analysis is not useful to the study of past climates. In this paper authors make attempts to verify the present knowledge and calibrate hydrogen and carbon isotope fractionation in peat cellulose and carbon, hydrogen and sulphur isotope fractionation of the total peat. This was carried out to find a new tool for paleoenvironmental reconstructions which are less precise, with respect to time resolution, but offers longer periods (thousands years) of uniform records and probably reflects different environmental parameters, as compared to isotope analysis of tree ring cellulose (see citations in Skrzypek and Jedrysek 2004). Moreover, peat-bogs frequently occur in areas above timberlines and at high longitudes, where trees are not available, thus tree ring based paleoenvironmental reconstruction is not possible.
Experimental
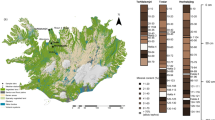
The hydrogen, carbon (in cellulose and in total organic mater) and organic sulphur stable isotope analyses have been carried out in Polish peat bog Suche Bagno profiles. This peat bog is situated in a wooded lowland at about 140 m asl in the Wigry Lake District (NE Poland, Wigry National Park, the GPS coordinates of the centre of the peatbog are 54°01′22″N and 23°09′10″E WGS84). It is a raised type peat bog with domination of Sphagnum species (Ombro-Sphagnioni type). Three peat cores, c.a. 160 to 120 cm long, have been drilled (drill Eijkelkamp Agr. Eq.) along a transect which was perpendicular to the peat bog bank (the cores S2, S1 and S3 were located about 48, 90 and 127.5 m away from the peat-bog bank respectively, Fig 1). The sampled cores were divided into 3–4 cm thick intervals and 14C dating was used to correlate corresponding profiles. The average rate of accumulation of the peat ranges form 0.02 to 0.24 cm/year. The oldest samples dated in each profile S2, S1, S3 were 4,700±120, 2,210±160, 1,170±130 BP, respectively. Thus, the lowermost sample in the core S3 limited our comparative analysis (2 older, not dated samples, moves the lowermost limit to about 1,400 years).
For C and H isotope analysis, about 3–6 mg of dry peat was combusted with CuO wire, under vacuum at 900°C. Nitration and Parr bomb techniques were used for cellulose and sulphur isotope preparation, respectively. The δD, δ13C, δ34S values (measured with Finnigan-Mat CH7 mass spectrometer) are quoted relative to the international standards V-SMOW, PDB and CDT, respectively and expressed in permil. The analytical error is lower than ±0,10, ±0,15 and 1.0‰ for δ13C, δ34S and δD, respectively.
Results and discussion
Homogeneity of δD, δ13C, δ34S in peat horizons local and regional factors
From a thermodynamic point of view, temperature is the most important factor controlling carbon isotope fractionation in the plant tissue CO2 system. However, the carbon isotopic composition of the plant tissue is the effect of complex factors, particularly of environmental conditions and metabolism. The role of these factors is referred to in the equation proposed by Farquhar et al. (1989). The isotopic fractionation proceeds in two stages: (1) adsorption and diffusion of CO2 into the plant tissue, and (2) the initial carboxylation. The first stage fractionation during the diffusion of carbon dioxide into the internal part of a leaf is about −4.4‰ (for vascular plants). The second stage: dissolution of CO2, transportation into the plant cells and equilibration in CO2-HCO3 2− system, results in 12C-enrichment of the plant tissue, as compared to the atmospheric CO2 (−7.9‰). Consequently, the cumulated 12C-enrichment of the plant tissue with respect to the atmospheric carbon dioxide is from about 18 to 27‰ (C3 plants) or from 4 to 6‰ (C4 plant), (e.g. Lajtha and Marshal 1994).
A useful unit to calibrate isotope variation versus temperature we define here as the Fq value and express as the rate between the isotope ratio in the total carbon (‰) and average daytime temperature during vegetation season (°C). Generally, the Fq value for various plants varies from −2.4‰/°C to 0.33‰/°C (see Skrzypek and Jedrysek 2004). In the peat-forming Sphagnum, Fq varies from −0.2 do −0.4‰/°C (Menot and Burns 2001) or −0.6‰/°C for peat (Skrzypek and Jedrysek 2004). Much more reliable are our recent calibrations of δ13C in leaving peatforming plants (Sphagnum S. and Politrychum sp. separately) versus temperature of the vegetation period, measured (monitored) at exactly the same areas where the sampled plants have been growing, which show Fq=−1.7‰/°C (Skrzypek and Jedrysek in preparation).
In this work, the δ13Ctp value (total organic matter of the peat) ranges from –25.52 to –28.27‰ (Fig. 1). Profiles have been compared with each other in pairs (S1-S2, S2-S3 and S3-S1) of samples representing the same age. In general, the same peat levels at different cores (S1, S2, S3) show concordant carbon isotope variations. The correlation of the δ13C value, between corresponding samples from different profiles, is remarkable. The R 2 values for the respective pairs are: \(R_{\text{S1 - S2}}^2 = 0.67\), \(R_{\text{S3 - S1}}^\text{2} = 0.61,\) \(R_{\text{S2 - S3}}^\text{2} \text{ = 0}\text{.46}\) (or \(R_{\text{S2 - S3}}^\text{2} = 0.87\) when two samples of AD 1770 and 1820 are excluded from the statistics). This may suggest that the local (metres scale) environmental variations had little importance as compared to the other factors of regional scale, and that, in contrast to tree rings, the temperature of the vegetation period is the major factor controlling the isotopic composition of the peat.
The δDtp value ranges between −78.67 and −109.24‰. In contrast to carbon isotopes, correlation factors between selected paired profiles are relatively low and R 2vary from 0.00 to 0.30. However, when we exclude the sample AD 737 from the statistics, the \(R_{\text{S1 - S2}}^\text{2} = 0.46,\) \(R_{\text{S2 - S3}}^\text{2} = 0.56\) and \(R_{\text{S1 - S3}}^\text{2} = 0.04.\) According to Edwards and Fritz (1986) D/H ratios in plants reflects that in the water used by these plants. However, our data contradicts this statement. Because the δDtp values measured are substantially lower as compared to that in precipitation (δD in water from the peatbog during vegetation period is about −75‰). Therefore, it may be suggested that the peat-forming plants assimilate the water largely from (or equilibrate with) the atmospheric vapour which in turn, under isotope equilibrium, is substantially depleted in deuterium as compared to the liquid phase of the water. In fact, it is well known from botanical observations that peatforming plants may assimilate water vapour directly from the atmosphere.
The δ34S value ranges between 4.34 and 19.87‰. Correlation of the δ34S values between peat profiles is very variable (\(R_{\text{S2 - S3}}^\text{2} \text{ = 0}\text{.59}\) or \(R_{\text{S2 - S3}}^\text{2} \text{ = 0}\text{.79}\) when we exclude AD 1354 the; \(R_{\text{S2 - S1}}^\text{2} \text{ = 0}\text{.00;}\) and \(R_{\text{S1 - S3}}^\text{2} \text{ = 0}\text{.01}\) or \(R_{\text{S1 - S3}}^\text{2} \text{ = 0}\text{.68}\) for the period AD 1900–1350). No correlation in the δ13−δ34S, δ34−δD and δ13−δD system has been observed (R 2 below 0.2).
The variability of δD and δ13C in cellulose from the peat – primary record or postdiagenetic signals?
In order to remove isotopically exchangeable hydrogen from the peat analysed, the nitration technique has been applied (in S2 and S3 profiles). The δ13Cnc and δDnc values in nitrocellulose (nc), extracted form the peat, ranges from −25.1 to –27.3‰ and from –92.4 to –118.0‰, respectively. In general, the δ13Cnc are on average 0.61‰ (in S2) and 0.86‰ (S3) higher values as compared to δ13Ctp values, whereas δDnc is on average 13‰ (in S2) and 2‰ (S3) lower as compared to the δDtp (Figs. 2 and 3). Positive correlations in the δ13Ctp–δ13Cnc system, with \(R_{\text{S}3}^2 = 0.64\) and \(R_{\text{S3}}^2 = 0.43\), have been observed.
The concordant variations of the total peat and nitrocellulose profiles (Figs. 2 and 3), together with the above mentioned correlation factors, may suggest that conservation of the total organic matter of peat is relatively good, i.e. the total peat C isotope ratios are comparable to those in the not exchangeable cellulose from the same samples.
Despite concordant variations in δDtp and δDnc profiles (Figs. 2 and 3) the correlation in these systems are very low (R 2<0.2). Although, it can be proposed that a selective decomposition of organic group compounds (the yield of nitrocellulose show no correlation with δDtp nor δDnc) was responsible for that, we believe that the rather variable ratio between peat-forming species (individual isotopic fractionation and content of the cellulose) contained at respective levels (Figs. 2 and 3) of the peat makes the δDtp–δDnc correlation poor.
The variability of δ34S primary record or postdiagenetic signals?
The main sources of sulphur in peat bog ecosystems are usually atmosphere and decomposed organic matter. The isotopic picture can be complicated when various portions of isotopically depleted H2S and SO2 are assimilated. Low temperature can induce different kinds of stress to plant tissues: (i) photo-chilling - Calvin cycle is slowed down (often below at 5°C), (ii) frost is detrimental to tissues mainly due the formation of ice crystals, which removes water from the liquid phase and causes damage to proteins and ultrastructure cells. Therefore, stress conditions may block or modify biochemical reactions, hence isotope effects resulting from assimilation may deviate from its regular value. However, it seems that at a temperature above 5°C, two main factors control the stable isotope composition of sulphur in peat profiles: (i) primary factors: δ34S is controlled by isotopic composition of primary organic matter or (ii) secondary factors: δ34S is affected by diagenetic and postdiagenetic isotope fractionation. The secondary factors seem to be negligible as maritime versus continental records are well preserved (Jedrysek et al. 1999, and see citations in: Skrzypek and Jedrysek 2004). This is concordant to a preliminary study by Price and Casagrande (1991) who proposed that the organic sulphur isotope ratio in freshwater peat is close to that of sulphate in the peat-forming waters. Further studies by Bottrel and Coulson (2003) also evidence that the sulphur isotopic composition of peat does not vary significantly due to diagenetic alterations, and in pure peat, the organic sulphur isotope signal may reflect that in the water sulphate with potential 34S depletion up to 9.0‰ of the organic S (Δ34Ssulphate-org is about 0 to 9.0‰). It can be expected that release of substantial amounts of sulphur due to decomposition of organic matter during digenetic processes has to be reflected in a downward decrease in the concentration of sulphur in each peat profile and older peat has to show a lower concentration of sulphur (SC). No such trends has been observed in our study (Fig. 1). Moreover, we have not observed any correlation in the SC – δ34S system except one selected short portion of the S2 profile (AD 1990–1820, n=9) where R 2=0.68. So, we do not have evidence that any potentially selective decomposition of sulphur-bearing compounds results in a measurable variation in δ34S value.
An increase in the water level, due to high precipitation, may dilute the sulphate (meteoric water in natural conditions, distanced from the ocean, shows a very low concentration of sulphate) and result in the deficit of sulphate. If the fractionation in the SO4 2− plant system is higher than 0‰, a kinetic isotope effect can be expected and a deficit of sulphur (although not limiting the primary production) may result in a higher δ34S in sulphate, thus consequently in organic sulphur. These mechanisms can be particularly important for raised peat-bogs which show, in general, a strong deficit of bio-elements. However, apparently, there is no isotope fractionation in the sulphate-peatforming plant system, (Δ34Ssulphate-org value is close to zero) but significant assimilation of SO2 (usually 34S-depleted) may decrease δ34S value in the peat-forming plants. Therefore, the δ34S value in peat can be water level sensitive (the higher water level, the limited SO2 and elevated SO4 2− assimilations, the higher δ34S in peat). Likewise, decrease in the water level at peat-bogs may result in a sudden release of significant amounts of dissolved H2S (strongly depleted in 34S isotope), from the bacterial SO4 2− reduction zone (BSR). Some of that H2S can be directly incorporated to the leaving plant tissue (see Introduction) and some of the H2S can be partly oxidized to sulphate. This in turn will decrease the δ34S value in peat and the δ34S value in the dissolved sulphate which is the source of sulphur for peat-forming plants. Consequently, in all cases, a decreasing water level may result in a decrease in δ34S in peat. Therefore, we postulate that the sulphur isotope ratio can be considered an archive of past environmental variations and maintain our thesis that δ34S in peat profiles may reflect variations in the water level on the peat-bog.
Conclusions
Concordant variations and relatively good correlations in the δ13Cnc and δ13Ctc values prove that the carbon stable isotope records are not modified due to diagenetic processes and may record past environmental conditions. High horizontal homogeneity of the δ13Ctc value suggest that the regional factor controlling carbon isotope variations in peat is temperature. The δ34S record in peat profiles reflects environmental variations, especially variations in the water level. The role of diagenetic processes are probably negligible.
References
Bottrel S, Coulson J (2003) Preservation of environmental sulphur isotope records in maritime peats: a test of baseline pre-anthropogenic signal and diagenetic effects in a mid-Pleistocene peat. Chem Geol 201:185–190
Edwards TWD, Fritz P (1986) Assessing meteoric water composition and relative humidity from 18O and 2H in wood cellulose: paleoclimatic implications from southern Ontario, Canada. Appl Geochem 1:715–723
Farquhar GD, Ehleringer JR, Hubic KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Jedrysek MO, Skrzypek G, Halas S, Kral T, Pazdur A, Wada E, Takai Y, Vijarnsorn P, Doroszko B, Kaluzny A, Weber-Weller A, Wójcik A (1999) Seawater/freshwater records in stable isotope composition of sediments: marine muds from Baltic’s Gotland deep and mangrove peat profile from Thailand. Quaternary Studies in Poland. Special Issue:127–133
Lajtha K, Marshal JD (1994) Sources of variation in the stable isotopic composition of plants. In: Lajtha K, Michener RH (eds) Stable isotopes in ecology and environmental sciences. Blackwell Scientific Publication, Oxford.
Leavitt SW, Long A (1986) Stable-carbon isotope variability in tree foliage and wood. Ecology 67:1002–1010
Menot G, Burns SJ (2001) Carbon isotopes in ombrogenic peat bog plants as climatic indicators: calibration from an altitudinal transect in Switzerland. Org Geochem 32:233–245
Novak M, Kirchner JW, Groscheova H, Havel M, Cerny J, Krejci R, Buzek F (2000) Sulfur isotope dynamics in two Central European watersheds affected by high atmospheric deposition of SO x . Geochim Cosmochim Acta 64(3):367–383
Price T, Casagrande DJ (1991) Sulfur distribution and isotopic composition in peats from the Okefenokee Swamp, Georgia and Everglades, Florida. Int J Coal Geol 17:1–20
Skrzypek G, Jedrysek MO, 2004, 13C/12C ratio in peat cores: record of past climates. In: Lichtfouse E, Schwarzbauer J, Robert D (eds) Environmental chemistry—green chemistry and pollutants in ecosystems. Springer-Verlag, Berlin, pp65–73
Acknowledgements
This study was supported from the 6 PO4D 03011, 2022/W/ING/02-04, 1017/S/ING/04-IX grants
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jędrysek, MO., Skrzypek, G. Hydrogen, carbon and sulphur isotope ratios in peat: the role of diagenessis and water regimes in reconstruction of past climates. Environ Chem Lett 2, 179–183 (2005). https://doi.org/10.1007/s10311-004-0093-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-004-0093-4