Abstract
The reduction of Cr(VI) by humic substances from leonardite and peat was investigated by capillary zone electrophoresis at various pHs. Both humic materials reduced Cr(VI) at pH 5.4, but not at basic pH. The capacity of leonardite humic substances to reduce Cr(VI) was lower than that of peat humic substances. Fe(III) accelerated the reduction of Cr(VI) by peat humic substances, but not by leonardite humic substances. Cr(VI) reduction mechanisms are proposed. The coal humic substances seem more suitable for remediation of Cr(VI)-contaminated sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is released into the environment as a result of various industrial activities. It is known to be stable in trivalent [Cr(III)] and hexavalent [Cr(VI)] states in aqueous and in soil environments. Whereas Cr(III) is essential for maintenance of normal physiological functions of living organisms, Cr(VI) is more soluble, toxic and mutagenic (Palmer and Puls 1994). Understanding the reactions that govern redox speciation of chromium is essential for predicting its fate, mobility, and toxicity in the environment.
Humic substances are natural macroligands that are among the main factors governing redox behavior of chromium in the environment. Humic substances were reported to reduce Cr(VI) to Cr(III) (Gu and Chen 2003; Fukushima et al. 1997; Wittbrodt and Palmer 1995, 1996a, 1996b). The reduction is slow with a half-life in the range of days or weeks. It is strongly pH-dependent, with the rate increasing with decreasing pH. For this process, Fukushima et al (1997) developed the first-order rate equation with respect to Cr(VI) with rate constants k equaling 1,990×10−6 h−1 (pH 5.1) and 2,710×10−6 h−1 (pH 3.2) at a humic acids (HA) concentration of 40 mg/L. In contrast, Wittbrodt and Palmer (1995, 1996a, 1996b), for reduction of Cr(VI) by soil humic acids and fulvic acids (FA), derived the complicated forms of the corresponding rate equations with rate constants of non-integer orders. These authors also reported a catalytic effect of Fe(III) on the reduction of Cr(VI) in the presence of humic and fulvic acids. This provides an experimental proof that the presence of iron and humic substances strongly influence the behaviour of Cr(VI) in the environment.
The ability of humic substances to reduce Cr(VI) is not only of geochemical, but of substantial practical importance. Given the vast resources of raw humic materials, e.g., low-rank coals and peat, humic preparations can be used as reactive materials for remediation of Cr(VI)-contaminated sites. To facilitate the use of humic substances for decontamination, their reducing power in relation to Cr(VI) has to be evaluated. To reach this goal, particular attention should be given to adequate determination of redox speciation of chromium in the presence of humic substances. In previous studies, Cr(VI) was determined spectrophotometrically. This technique requires a complete removal of humic substances prior to analysis. In this study, capillary zone electrophoresis (CZE) was used for this purpose as suggested by Kaniansky et al. (1999) and Baraj et al. (2000). The aim of this study was (1) to assess the reduction rates of Cr(VI) by humic substances from the sources of industrial value, peat and leonardite, using CZE; and (2) to investigate the influence of Fe(II) and Fe(III) on the reduction of Cr(VI) by humic substances under environmentally relevant conditions.
Experimental
Humic materials used
Two humic substances samples were used: a commercial preparation of potassium humate obtained from leonardite (Powhumus) kindly provided by Humintech (Germany), and peat humic substances extracted from highland peat (T7) using 0.1 M NaOH. The samples were characterized using elemental analysis and 13C NMR spectroscopy. The peat and leonardite humic substances, respectively, had the following elemental compositions, on an ash-free basis (wt%): 51.9 and 62.7 for C, 4.72 and 4.43 for H, 1.1 and 1.0 for N, and 42.28 and 31.9 for O. The quantitative solution state 13C nuclear magnetic resonance (NMR) spectra were acquired as described in our previous studies (Hertkorn et al. 2002) using a Bruker DMS 400 NMR spectrometer operating at 100 MHz 13C frequency. Inverse gated decoupling and a relaxation delay of 8 s were used to provide quantitative conditions. The samples of humic materials were dissolved in 0.1 M NaOD at 100 g/L. The assignments were made after Hertkorn et al. (2002). The contents of carbon in the main structural fragments for peat and leonardite humic substances, respectively, were as follows (percentage of total carbon): carbonylic C (220–187 ppm), 3.6 and 5.7%; carboxylic C (187–165 ppm), 12.0 and 19.0%; aromatic C (165–108 ppm), 35.5 and 63.8%; carbohydratic C (108–48 ppm), 23.2 and 0.9%; and alkylic C (48–5 ppm), 25.6 and 10.5%.
Reagents
The chemicals were of analytical grade. The humic substances solutions were prepared by dissolution in the minimal amount of KOH and further dilution with distilled water. The humic substances stock solutions were prepared at concentrations of 3.7 and 1 g C/L for leonardite and peat humic substances respectively. Concentration of humic substances on the organic carbon basis was calculated from the carbon content in the humic substances sample. K2Cr2O7 was used for preparing 20 mM Cr(VI) stock solution. The solution of humic substances with Fe(II) was prepared by adding FeSO4×7H2O in the humic substances solution and adjusting the pH to 9 with 0.1 M NaOH. The solution obtained contained 1 g C/L of humic substances and 2 mM of Fe(II) and was prepared immediately prior to its use. In the experiments with Fe(III), 10 mM FeCl3 in 0.05 M HCl was used.
CZE analysis
A Beckman P/ACE 2050 Series CE capillary electrophoresis system with an ultraviolet (UV) detector was used. The separation capillary was an unmodified fused silica 57 cm-long (50 cm to UV-detector)×75 μm i.d.; 25 kV separation voltage, 22±2 °C. Carbonate buffer (5 mM) at pH 9.1±0.1 was used for separation. Before each measurement, the capillary was rinsed at 20 psi with 0.1 M NaOH (2 min), distilled water (2 min), 0.1 M HCl (5 min), water again (2 min), and carrier solution (2 min). The sample was injected in the low pressure mode (0.5 psi) for 10 s. Cr(VI) was detected at 280 nm (absorption peak of CrO42−, the predominant species at pH 9.2). The raw electrophoretic data were treated using Gel-Treat software (Kudryavtsev et al., 2000).
Kinetic experiments on Cr(VI) reduction by humic substances
The rate of Cr(VI) reduction by humic substances was determined by measuring Cr(VI) concentration versus time. The corresponding batch experiments were performed at 22±2 °C under oxic conditions. Cr(VI) was determined using CZE. The ratios of peak area to migration time were calculated for the peaks migrating in the range 11–14 min. The experiments with leonardite humic substances were conducted at pH 5.4 and 9.2. The concentrations of Cr(VI) were 0.2 and 0.8 mM, and of humic substances, 0.5 g C/L. The experiments with peat humic substances were conducted at pH 5.4. The concentration of Cr(VI) was 0.2 mM, and of humic substances, 0.5 g C/L. To adjust the pH to 5.4, 0.05 M CH3COONa and 0.02 M CH3COOH were used. To adjust the pH to 9.2, 0.05 M NaHCO3 and 0.005 M Na2CO3 were used.
All the solutions were prepared in Eppendorf microtubes (total volume 1 mL). The reagents were added as follows: humic substances solution, stock buffer, water, Cr(VI).
Kinetic experiments on Cr(VI) reduction by humic substances in the presence of Fe(III) and Fe(II)
The influence of iron (III) on the reduction of Cr(VI) was investigated both for leonardite and peat humic substances. The following concentrations were used: humic substances, 0.5 g C/L; Cr(VI), 0.2 mM; Fe(III), 0.02 and 0.2 mM; pH 5.4. For making up the desired iron concentration, 10 mM solution of FeCl3 in 0.05 M HCl was added to the stock solution of humic substances. The reagents were added as follows: solution of humic substances, buffer, water, iron, Cr(VI). The use of buffer was to prevent a decrease in pH after addition of Fe(III). The influence of Fe(II) on the reduction of Cr(VI) was studied for leonardite humic substances. The same conditions as described above for Fe(III) were used; Fe(II) concentrations were 0.2, 0.5, 1, and 2 mM. The required amount of Fe(II) was introduced into humic substances solution in the form of its humic complex (2 mM Fe, 1 g−1 C/L of humic substances). Cr(VI) was then added.
Results and discussion
Determination of Cr(VI) by capillary zone electrophoresis
The first task was to develop experimental setup for CZE determination of Cr(VI) in the presence of humic substances. It was reported that CZE determination of Cr(VI) can be efficiently performed both in alkaline and acidic media (Baraj et al. 2000, Kaniansky et al. 1999). An alkaline medium is preferable for humic substances studies, as it prevents precipitation of humic substances. Hence, a carbonate buffer at pH 9.1±0.1 was used for CZE separation. The negative power supply was used in the separation setup that allows turning up the chromate ion in the electropherograms (Baraj et al. 2000). Under the above conditions the negatively charged Cr(VI) ions were eluted at a migration time of 11–14 min; far behind an electroosmotic flow (EOF) (neutrals) and positively charged Cr(III) ions. In all cases, the EOF and Cr(VI) migrated in opposite directions. Only one peak of chromate ion, at a migration time of 11–14 min, was observed both in the presence and absence of humic substances. No peak of humic substances was observed, which shows that a good separation of Cr(VI) and humic substances under the conditions used was obtained.
To make sure that the described experimental approach allows separation of Cr(VI) and Cr(III) ions, an excess of ascorbic acid was added to the solution of Cr(VI), and then the reaction mixture obtained was analysed. Ascorbic acid was added to reduce Cr(VI) to Cr(III). It was assumed that if the adopted technique determines both Cr(III) and Cr(VI), the peak of Cr(III) would appear on the electropherogram. In practice, there was no peak registered after addition of ascorbic acid. Hence, the conclusion can be drawn that Cr(III) does not interfere with determination of Cr(VI) under the conditions employed.
For quantitative measurements of Cr(VI), calibration plots were constructed. The peak area and its ratio to migration time were used as analytical signals. Both relationships were linear up to a concentration of Cr(VI) of 4 mM, but the linear regression was better for the ratio of peak area to migration time. The relative standard deviation found for this parameter at 0.1 mM Cr(VI) was 4% (n=5), and the detection limit obtained (according to the 3σrule) was 0.01 mM Cr(VI). CZE is thus a suitable technique for determination of Cr(VI) in the presence of humic substances. The technique developed was applied for kinetic studies on reduction of Cr(VI) by humic substances as described below.
Reduction of Cr(VI) by humic substances.
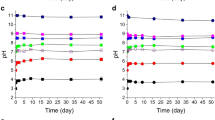
At pH 5.4, both humic substances samples from leonardite and peat reduced Cr(VI) (Fig. 1a). It was observed that the reduction of Cr(VI) by peat humic substances occurred much faster than that by leonardite humic substances (Fig. 1a). In the initial time period, the plots of Cr(VI) concentration versus time for both humic substances samples dropped abruptly, and then became linear. The obtained data show that the rate equation of Cr(VI) reduction by humic substances is rather complex, corroborating the findings of Wittbrodt and Palmer (1996b). For the sake of simplicity, to estimate the difference in the Cr(VI) reduction rate produced by leonardite and peat humic substances, the obtained kinetic curves were approximated using the model of the first order reaction rate. The estimated value of the reaction rate lay in the range of 0.003–0.01 h−1 for leonardite humic substances, whereas for peat humic substances it was a factor of 2–6 higher, and accounted for 0.02 h−1. These estimates are on the same order of magnitude as the value of 1,910×10−6 h−1 reported by Fukushima et al. (1997) for pH 5.1 at a ten times higher concentration of humic substances sample.
Reduction of Cr(VI) by humic substances at pH 5.4. Initial concentration of Cr(VI): 0.2 mM (a) and 0.8 mM (b); humic substances concentration, 0.5 g C/L. It can be seen that the both humic substances used—from peat and leonardite—reduce Cr(VI), while the reduction rate by peat humic substances is faster
Different reducing properties of the two humic materials used in relation to Cr(VI) can be explained by the differences in the structure intrinsic to coal (leonardite) and peat humic substances. The particular feature of coal humic substances is the higher contribution of the aromatic backbone and a lack of carbohydrate periphery resulting from the higher humification degree of the coal humic material. In contrast to coal, peat humic substances are highly enriched with oligosaccharidic units. As follows from the 13C NMR data, the content of carbohydratic carbon in the peat humic substances sample used in this study accounted for 23.2% from the total carbon, compared to 0.9% in the sample of leonardite humic substances. Given the above differences in the structure of coal against peat humic substances, it can be suggested that the reductive properties of coal humic substances are provided mostly by the reversible reactions of quinone–hydroquinone moieties, whereas for peat humic substances, the irreversible oxidation of oligosaccharidic fragments can be a dominating process. The reversibility of the redox reactions of leonardite humic substances makes this material more attractive for use for remedial purposes. Of particular interest was estimating the reducing capacity of leonardite humic substances in relation to Cr(VI).
The experiments at higher Cr(VI) concentration were used for this purpose. The obtained kinetic curve is shown in Fig. 1b. As can be seen, the concentration of Cr(VI) dropped from 0.8 down to 0.2 mM in the course of reduction. Normalizing the loss in Cr(VI) content to the humic substances concentration of 0.5 g C/L, a value of 1.2 mmol/g C was obtained. At pH 9.2, there was no reduction of Cr(VI) by leonardite humic substances observed during 400 h exposure time at both the humic substances concentrations used, 0.5 and 1 g C/L. The results obtained corroborate well the findings of Wittbrodt and Palmer (1995), who reported that the rates of Cr(VI) reduction were strongly pH dependent, the rate increasing with decreasing pH.
Influence of Fe(III) on Cr(VI) reduction by humic substances
For the studies on Fe(III) influence on Cr(VI) reduction by the target humic materials, two concentrations of Fe(III), 0.02 and 0.2 mM, were used. We observed that an addition of Fe(III) exhibited totally different effects on the redox reactions of peat and leonardite humic substances with Cr(VI) (Fig. 2). So, in the case of leonardite humic substances there were no changes detected in the reduction rate of Cr(VI), whereas in the case of peat humic substances a significant acceleration of the reduction rate was observed. The concentration of Fe(III) of 0.02 mM used in our experiments was a factor of 30 less than that of Cr(VI), suggesting a catalytic effect of Fe(III) on oxidation of peat humic substances through partial reduction to Fe(II). The obtained results corroborate the findings of Wittbrodt and Palmer (1996b), who observed an increase in the reduction rate of Cr(VI) by soil humic acids and fulvic acids upon addition of Fe(III) at pH 2.
Influence of Fe(II) on Cr(VI) reduction by humic substances.
To explain the mechanism of catalytic action of Fe(III), Wittbrodt and Palmer (1996b) surmised a reduction of Fe(III) to Fe(II) with follow up reduction of Cr(VI) by Fe(II). If this mechanism is valid, then Fe(II) should rapidly reduce Cr(VI) in the presence of humic substances. The corresponding experiments were conducted under conditions of both an excess and a lack of Fe(II) with respect to Cr(VI). In the absence of humic substances, the excess of Fe(II) rapidly reduced the whole pool of Cr(VI) [Cr(VI) could not be detected 5 min after Fe(II) addition; data not shown]. However, in the presence of leonardite humic substances, the full reduction of Cr(VI) did not take place (Fig. 3).
At the initial reaction time, an abrupt reduction of Cr(VI) was observed, the rate increasing with increasing concentration of Fe(II). At the second stage, reduction rates became almost constant,which was assigned to a partial oxidation of Fe(II) to Fe(III) by air. The residual Fe(II) rapidly reduced Cr(VI), and then was slowly reduced by humic substances. The results obtained suggest that humic substances can stabilize the Fe(III)/Fe(II) ratio, acting as redox-buffer.
Implications for remediation technologies.
With respect to the remedial application of humic substances for Cr(VI)-contaminated sites, the results obtained allow the following conclusion. Peat humic preparations can be applied alone or in combination with Fe(III) salts if the Cr(VI) contamination source is already eliminated and a single application of the excessive amount of reducing agent could solve the problem. An excessive amount is necessary because decomposition of oligosaccharidic units is irreversible, and once they are degraded, the redox activity of the residual peat humic substances will be very low. Humic preparations from coal work slower, but their advantage is reversibility of the quinoic–hydroquinoic transformations. It means that on being introduced into the contaminated system, they will be active for a much longer period of time. In other words, if the contaminated site has a constant source of Cr(VI), the use of coal humates would be more beneficial in the long-term.
Conclusions
Both peat and leonardite humic substances were able to reduce Cr(VI) at close to neutral pH (5.4), the rate of reduction for peat humic substances being much higher than that for the leonardite humic substances. There was no Cr(VI) reduction observed at pH 9.2. An addition of Fe(III) accelerated the reduction of Cr(VI) by peat humic substances, but did not influence that by coal humic substances. The reducing ability of Fe(II) towards Cr(VI) was decreased drastically by leonardite humic substances. The different mechanisms of reduction of Cr(VI) by peat and coal humic substances were surmised: peat humic substances reduce Cr(VI) on account of the irreversible oxidation of carbohydrate units, whereas coal humic substances reduce Cr(VI) owing to reversible transformations of quinoic-hydroquinoic units. The coal humic preparations were concluded to be advantageous for an application of Cr(VI)-contaminated sites due to their long-lasting capabilities for reducing Cr(VI).
References
Baraj B, Niencheski LFH, Soares JA, Martinez M, Merkoci A (2000) Comparison of chromium speciation by CZE and ion exchange followed by AAS. Fresenius J Anal Chem 367:12–16
Fukushima M, Nakayasu K, Tanaka Sh, Nakamara H (1997) Speciation analysis of chromium after reduction of chromium (VI) by humic acid. Toxicol Environ Chem 62:207–215
Gu B, Chen J (2003) Enhanced microbial reduction of Cr(VI) and U(VI) by different natural organic matter fractions. Geochim Cosmochim Acta 67:3575–3582
Hertkorn N, Permin A, Perminova I, Kovalevskii D, Yudov M, Petrosyan V, and Kettrup A (2002) Comparative analysis of partial structures of a peat humic and fulvic acid using one- and two-dimensional nuclear magnetic resonance spectroscopy. J Environ Qual 31:375–387
Kaniansky D, Masar M, Marak J, Bodor R. (1999) Capillary electrophoresis of inorganic anions. J Chromatogr A 834:133–178
Kudryavtsev A.V., Perminova I.V., Petrosyan V.S. (2000). Size-exclusion chromatographic descriptors of humic substances. Anal Chim Acta 407:193–202
Palmer CD, Puls RW (1994) Natural attenuation of hexavalent chromium in groundwater and soils. EPA Ground Water Issue EPA/540/5–94/505 October 1994
Wittbrodt PR, Palmer CD (1995) Reduction of Cr(VI) in the presence of excess soil fulvic acid. Environ Sci Technol 29:255–263
Wittbrodt PR, Palmer CD (1996a) Reduction of Cr(VI) by soil humic acids. Eur J Soil Sci 47:151–162
Wittbrodt PR, Palmer CD (1996b) Effect of temperature, ionic strength, background electrolytes and Fe(III) on the reduction of hexavalent chromium by soil humic substances. Environ Sci Technol 30:2470–2477
Acknowledgements
The young-scientist fellowship provided to D.M. Zhilin from INTAS (YSF2001/2–126) is deeply appreciated. The particular thanks are to INTAS officer Ms. Gyll-Murray for friendly collaboration. The research was supported by the GSF funding (Neuherberg, Germany) – FE 75184, BA 31/139166/02/U.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhilin, D.M., Schmitt-Kopplin, P. & Perminova, I.V. Reduction of Cr(VI) by peat and coal humic substances. Environ Chem Lett 2, 141–145 (2004). https://doi.org/10.1007/s10311-004-0085-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-004-0085-4