Abstract
Although two billion people in the world are suffering from iodine deficiency there is little information on the chemical fate of iodine in the terrestrial environment. Here we show that peatlands play a major role in terrestrial iodine cycling. Chemical data from two peat profiles from Patagonia, Chile imply that transformation of iodine from its inorganic form to organoiodine compounds during early humification in peatlands is a key process in storage of iodine in the terrestrial environment. Once bound in peat, iodine remains stable for thousands of years. In the earth’s peatlands, net accumulation of iodine since the last glacial period is estimated to be 12–36 teragrams (1 Tg=1012 g). These data suggest that peatlands are a major reservoir of iodine in terrestrial ecosystems. Our novel model of iodine distribution in the terrestrial environment demonstrates the key role of peatlands in burial and reemission of organically bound iodine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iodine is an essential nutrient for animals and humans. For instance, the lack of iodine-containing hormones results in iodine deficiency disorders such as goiter. Nowadays one third of the world’s population, especially children, is at risk from iodine deficiency disorders. In addition to its role in human nutrition, iodine is involved in tropospheric chemistry in processes such as ozone depletion (Vogt 1999) and aerosol formation (O’Dowd 2002). Furthermore, radioactive iodine isotopes released from nuclear plants can strongly affect human health as they can be concentrated in the human thyroid gland.
Many studies have been conducted to explore the geochemistry and distribution of iodine in the environment (e.g. Whitehead 1984; Fuge and Johnson 1986; Wong 1991; Muramatsu and Wedepohl 1998; Moran et al. 2002). However, the biogeochemical cycles of iodine are still not sufficiently known; thus fate and fluxes of iodine for specific terrestrial ecosystems remain largely unknown.
Field measurements and laboratory studies on iodine speciation using the long-lived radioactive 129I isotope have contributed to our knowledge of the behaviour of iodine in the environment (Fabryka-Martin et al. 1985; Behrens 1988; Michel 1999; Rädlinger and Heumann 2000, Moran et al. 2002). In this context, it has been suggested that humic substances play a major role in converting and binding iodine in soils. The importance of these findings for the availability of iodine for humans becomes clear in the light of the observation that iodine bound to soluble natural organic complexes such as fulvic acids is removed during drinking water processes. The inorganic form of another halogen, chlorine, is known to be converted to organic chlorine compounds by a variety of organisms (Gribble 1996) and can easily be incorporated into organic matter in soil (Öberg 2002). Very recent work suggested that organochlorine compounds are formed during weathering of plant material (Myneni 2002). In contrast, little is known about organoiodine compounds and the biogeochemical processes that lead to their formation in natural terrestrial systems.
Rainfed (ombrotrophic) peat bogs are often used as natural archives of historic deposition of atmospheric derived elements (Shotyk et al. 1998; Martínez-Cortizas et al. 1999). Here we use peat bogs as a model ecosystem to study the fate of iodine in terrestrial ecosystems. These natural archives provide evidence that the formation of organoiodine compounds is a key biogeochemical process in iodine retention in terrestrial soils.
Experimental
Sample collection, preparation and dating
Peat cores were collected from two solely ombrotrophic peat bogs (“GC”, S52°47.443’/W 72°56.616’, “SKY, S52°30.668’/W 72°07.505’) located in different climatic zones of the Magellanic Moorlands, Southern Chile. The GC bog is a cushion plant bog typical of the super-humid zone of the southern Andes, which is characterized by high precipitation rates of about 6,000 mm yr-1. The SKY bog is located in the drier transition zone located between the super-humid zone and the grassland zone (Pampa), where precipitation rates of about 1,500 mm yr-1 are distinctively lower. Both cores were sectioned in the field and packed into polyethylene bags. The GC core was sectioned into 2 cm slices, whereas the Sky core was sliced into 4 cm sections. A detailed description of the sampling sites, sample preparation and the ombrotrophic nature of these bogs is given elsewhere (Biester et al. 2003).
The past 100 years could be only dated in the GC core using 210Pb technique. A detailed description of the dating techniques used for this bog is given elsewhere (Biester et al. 2002). All other ages were obtained by accelerator mass spectrometry (AMS) 14C dating. The activity of 14C was determined in humic acid extracts and in the humic acid extraction-residues. Conventional 14C-ages were calibrated using CALIB rev4.0, test version 6 (data set 1). All ages are given as the means of the one-sigma values.
Iodine speciation
We used a recently developed combustion-ion chromatography method (TX/TOX-IC) (Putschew et al. 2003) to speciate, in peat, between inorganic iodine and iodine that is chemically bound to organic matter. Between 10–50 mg dw of peat sample was used for each measurement. The amount of total iodine (TI) and total organic iodine (TOI) were measured directly whereas the fraction of inorganic iodine was calculated by the difference between TI and TOI. Mean standard deviations of the determination of TOI was 10% (n=2–5). A detailed description of the sample preparation and the analysis is given by Putschew et al. (2003). Total iodine in rainwater was determined by ion chromatography (IC)/inductively coupled plasma mass spectrometry (ICP-MS) (n=3–5).
Carbon/Nitrogen ratio (C/N)
Ratios of carbon and nitrogen (C/N) were used as an indicator of changes in peat humification; where low C/N ratios indicate high peat humification and vice versa (Kury and Vitt 1996). Carbon and nitrogen concentrations were determined by means of a C/N-Analyzer (ELEMENTAR) burning 10–20 mg sample aliquots in a tin capsule. Mean standard deviations of the determination of C was 2.2% and 2.1% for N (n=3).
Results and discussion
Organoiodine formation during humification processes
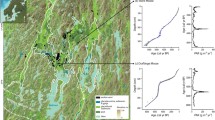
To study the relationship between humification of plant material and the conversion of inorganic iodine to organic iodine, two peat cores dating back to 2,500, named “GC”, and 6,000 years BP, named “SKY”, were investigated. Average iodine concentrations in rain were 0.43 µg l-1 at the GC site and 0.6 µg l-1 at the SKY site. Based on precipitation rates of 6,000 mm at GC and 1,500 mm at SKY, mean annual wet iodine deposition amounts to 2.58 and 0.84 mg m-2 yr-1, respectively. In the peat cores, total iodine concentrations range between 5 and 36 mg kg-1 and average at 18.4 ± 5.9 mg kg-1, in the GC core and between 6.2 and 17.6 mg kg-1 and average 9.8 ± 7.7 mg kg-1, in the SKY core, which corresponds to mean iodine net accumulation rates of 0.9 and 0.37 mg m-2 yr-1, respectively. Iodine accumulation rates were normalized to carbon losses occurring during peat decomposition (Biester et al. 2003). Accordingly, 46% of the wet deposited iodine was retained by the GC peat and about 37% by the SKY peat.
The iodine speciation measurements indicate that most iodine in peat exists in organically bound form. Indeed, 73% of total iodine in the GC peat core and 83% in the SKY peat core was organoiodine. Comparing the records of organic iodine of both cores with those of their corresponding C/N ratios clearly indicates that both concentrations of organically bound iodine and total iodine are directly related to the degree of peat humification (Fig. 1 and 2). Direct positive correlations with coefficients up to r=0.95 were found between the degree of peat decomposition and total organic iodine (TOI) in most sections of both cores. The good correlations were disturbed in parts with the tephra layers from volcanic eruptions of Mount Burney and close to the basement. Decreasing C/N ratios related to increasing peat decomposition lead to an increase in total organic iodine and total iodine concentrations and vice versa. In case of the GC core the highest concentration of organic iodine of 30 mg kg-1 at a depth of 40 cm was found to be associated with lowest C/N ratio (Fig. 1). The high C/N ratios in the uppermost peat sections indicate that the organic material here consists of fresh or low degraded plant material. In the same section, concentrations of organic iodine are low, about 1 mg kg-1, but increase dramatically with increasing humification of the peat shown by decreasing C/N ratios.This finding is consistent with the theory that the iodination of organic matter takes place during an early stage of humification of fresh plant material. This is also reflected by the increasing ratio of organically bound iodine to inorganic iodine (TOI/Iinorg), which increases from 0.2 in the uppermost peat sample to 10 at a depth of 40 cm.
As shown on figure 2, the SKY core generally shows greater variation of C/N ratios, which is attributed to the more intense climatic variations in the transition zone. Peat decomposition will dramatically increase when the bog falls dry, which results in strongly decreasing C/N ratio. Due to the larger variations in peat decomposition, the relation between peat decomposition and the formation and enrichment of organo-iodine is very pronounced in this core. As both cores show only moderate variations in iodine accumulation rates within the peat profiles, we assume that the iodine compounds in the catotelm, the permanent anoxic section of the bogs, are mostly immobile and not released during further humification of the peat. Changes in iodine concentrations in peat are therefore a result of changes in peat humification rather than changes in atmospheric iodine deposition.
Extrapolations on a global scale
Based on our results and data derived from peat bogs in other continents, we conclude that the effective retention of iodine in peatlands through the formation of organic iodine compounds is a global process. To estimate a global model of iodine fluxes and reservoirs, we suggest an average concentration of total iodine in peatlands of 10 to 30 mg kg-1. On the basis of total carbon stored in peatlands globally (Gorham 1991), we calculated that 12–36 Tg of organically-bound iodine has been accumulated in these terrestrial ecosystems during the Holocene (~11,000 years).
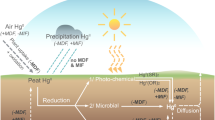
The fate and dynamics of iodine in peatlands are characterized by two major transformation processes, which are believed to mainly take place in the temporarily oxic parts of the bog, named “acrotelm”. One process is the volatilisation of iodine by formation of short chain alkyl iodides. Emission of methyl iodide from the terrestrial environment has indeed been considered to be a significant source of reactive iodine in the atmosphere (Dimmer et al. 2001; Keppler et al. 2000; Redeker et al. 2000). Assuming that methyl iodide is the major volatile iodinated compound released from peat bogs, the global flux of iodine to the atmosphere is estimated at 1.4–4.2 Gg I yr-1 for peatland ecosystems This would mean that a significant fraction of the atmospheric iodine flux to the world’s peatlands is reemitted to the atmosphere.
The second major biogeochemical process is the formation of non-volatile organic iodine compounds during humification of plant material and their long-term storage in the permanent anoxic parts of the bogs, the “catotelm”. Taking net accumulation rates of 0.36–1.1 mg I m-2 yr-1, and a total area of peatlands of 3×1012 m2, the global sequestration of iodine is then estimated at 1.1–3.3 Gg I yr-1. Both processes, deposition and volatilisation of iodine in peat bogs, are characterized by the conversion of iodine from its inorganic form to covalently bound organic iodine. Sequestration and reemission of organically bound iodine could account for the total wet deposition of inorganic iodine, mainly as I- and IO3 -, of about 2.3-10.6 Gg I yr-1. This calculation is in good accordance with the data derived from the Patagonian bog sites where between 37 and 46% of wet deposited iodine was retained by the peat. However, there is some uncertainty about the extent of iodine losses from peat bogs through leaching or surface runoff.
Figure3 illustrates major fluxes and pools of iodine in the terrestrial environment based on our results and data from the literature. For our model, we have considered only those systems which contribute significantly to the active biogeochemical cycle of iodine within time spans of hundred or perhaps thousands of years. Iodine fluxes to and from the earth’s crust and deep groundwater aquifers were not considered. The world’s oceans provide the largest source of atmospheric iodine transported to the terrestrial ecosystems. We suggest that soils, including peatlands, are in general the largest terrestrial iodine pool amounting from 21 to 63 Tg, but within the group of soils, peatlands, which cover only 2% of the earth’s continental surface, provide the largest reservoir amounting from 12 to 36 Tg. The major role of peatlands in the biogeochemical cycle of iodine results from their high storage capacity and the permanent anoxic conditions that predominate in peat bogs. In aerobic soils the residence time of organoiodine compounds is assumed to be much shorter than in peat as the turnover of the organic matter is much faster.
Conclusion
Organic-rich soils such as peatlands have a high capacity to retain and store iodine through the formation of organoiodine compounds. This finding gives strong indications that this process mainly controls the bioavailability of iodine in terrestrial ecosystems. Our calculations add emphasis to the major role of peat bogs as an iodine sink during climatic periods where peat formation takes place in large areas. At this time, it is not clear what happens to the sequestered iodine in peatlands during changes in climate. The present warming of the earth’s climate which would also involve the thawing of large areas of peaty permafrost soils (tundra and alpine meadows 8×1012 m2), could have a major effect on the distribution of iodine. Global warming, therefore, could drastically change the balance between storage and reemission of iodine, favouring the formation of volatile compounds. As a consequence this would cause local increases in the emission rates of alkyl iodides to the lower atmosphere over land, which could affect the photooxidant budget, accelerate the aerosol formation (O’Dowd 2002) and therefore, markedly change the regional tropospheric chemistry.
References
Behrens H (1988) Speciation of radioiodine in aquatic and terrestrial systems under the influence of biogeochemical processes. In: Bulman RA, Cooper JR (eds) Speciation of fission and activation products in the environment. Elsevier, London, pp 223–230
Biester H, Kilian R, Hertel C, Woda C, Mangini A, Schöler, HF (2002) Elevated mercury concentrations in peat bogs of South Patagonia, Chile–an anthropogenic signal. Earth & Plan Sci Letters 201:609–620
Biester H, Martinez-Cortizas A, Birkenstock S, Kilian R (2003) Historic mercury records in peat bogs. The role of peat decomposition, and mass losses. Environ Sci Technol 37:32–39
Dimmer CH, Simmonds PG, Nickless G, Bassford MR (2001) Biogenic fluxes of halomethanes from Irish peatland ecosystems. Atmos Environ 35:321–330
Fabryka-Martin J, Bentley H, Elmore D, Airley PL (1985) Natural iodine-129 as an environmental tracer. Geochim Cosmochim Acta 49:337–347
Fuge R, Johnson CC (1986) The geochemistry of iodine–A review. Environ Geochem Health 8:31–54
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Application 1:182–195
Gribble GW (1996) Naturally occurring organohalogen compounds–a survey. J Nat Prod 55:1353–1395
Keppler F, Eiden R, Niedan V, Pracht J, Schöler HF (2000) Halocarbons produced by natural oxidation processes during degradation of organic matter. Nature 403:298–301
Kuhry P, Vitt DH (1996) Spatial variation in rates of carbon and nitrogen accumulation in a boreal bog. Ecology 77:271–275
Martínez-Cortizas A, Pontvedra-Pombal X, García-Rodeja E, Nóvoa-Muñoz JC, Shotyk W. (1999) Mercury in a Spanish peat bog: archive of climate change and atmospheric metal deposition. Science 284:939–942
Michel R (1999) Long-lived radionuclides as tracers in terrestrial and extraterrestrial matter. Radiochim Acta 87:47–73
Moran JE, Oktay SD, Santschi PH (2002) Sources of iodine and 129iodine in rivers. Water Res 38:1149 10.1029/2001WR000622
Muramatsu Y, Wedepohl KH (1998) The distribution of iodine in the earths crust. Chem Geol 147:201–216
Myneni SCB (2002) Formation of stable chlorinated hydrocarbons in weathering plant material. Science 295:1039–1041
Öberg G (2002) The natural chlorine cycle–fitting the scattered pieces. Appl Microbiol Biotechnol 58:565–581
O’Dowd CD, Jimenez JL, Bahreini RA, Flagan RC, Seinfeld JH, Hämeri K, Pirjola L, Kulmala M, Jennings SC, Hoffmann T (2002) Marine aerosol formation from biogenic iodine emissions. Nature 417:632–636
Putschew A, Keppler F, Jekel M (2003) Differentiation of the halogen content of peat samples using ion chromatography after combustion (TX/TOX-IC). Anal Bioanal Chem 375:781–785
Rädlinger G, Heumann KG (2000) Transformation of iodide in natural and wastewater systems by fixation on humic substances. Environ Sci Technol 34:3932–3936
Redeker KR, Wang N-Y, Low J C, McMillan A, Tyler SC, Cicerone RJ (2000) Emissions of methyl halides and methane from rice paddies. Science 290:966–969
Shotyk W, Weiss D, Appleby PG, Cheburkin AK, Frei K, Gloor M, Kramers JD, Reese S, Knaap van der WO (1998) History of atmospheric lead deposition since 12,370 14C yr BP from a peat bog, Jura Mountains, Switzerland. Science 281:1635–1640
Vogt R (1999) Iodine compounds in the atmosphere. In: Fabian EP, Singh ON (eds) The Handbook of Environmental Chemistry Vol 4 Part. Springer, Berlin Heidelberg NewYork pp 113–128
Whitehead DC (1984) The distributions and transformations of iodine in the environment. Environ Intl 10:321–339
Wong GTF (1991) The marine geochemistry of iodine. Rev Aquatic Sci 4:45–73
Acknowledgements
The authors express their gratitude to D.B. Harper and J.T.G. Hamilton for helpful advice regarding the manuscript. We thank W. Shotyk for providing the iodine data from the Shetland Islands. Thanks to M. Petri for measuring the rainwater samples. This work was supported by grants from the Deutsche Forschungsgemeinschaft (BI734/1–1/2 and GRK 273) and through a European Community Marie Curie Fellowship (MCFI-2002–00022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keppler, F., Biester, H., Putschew, A. et al. Organoiodine formation during humification in peatlands. Environ Chem Lett 1, 219–223 (2003). https://doi.org/10.1007/s10311-003-0044-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-003-0044-5