Abstract
We investigated soil net nitrogen mineralization rate, above- and belowground biomass allocation, and nitrogen use in a Cryptomeria japonica plantation chronosequence. Total biomass accumulation showed an asymptotic accretion pattern, and the peak total biomass accumulation rate occurred approximately 30 years after afforestation. Soil net nitrogen mineralization rate was lowest 30 years after afforestation. Between years 30 and 88, net nitrogen mineralization increased again. These results indicate that an imbalance in soil nitrogen supply and plant nitrogen demand occurred approximately 30 years after afforestation. Furthermore, leaf nitrogen concentration, which was used as an index of plant nitrogen status, was lower in mature forest than in young forest, suggesting that mature stands did not take up nitrogen as successfully. If soil resources such as nitrogen limit plant growth, plants may increase biomass allocation to fine root structure; however, fine root biomass was not higher in 30- and 88-year-old stands than in younger stands, suggesting that changes in biomass allocation may not be effective against nitrogen deficiency in a C. japonica plantation chronosequence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest biomass tends to increase throughout stand development until reaching an upper limit (Kira and Shidei 1967; Odum 1969). Age-related decline in net primary production and biomass accumulation rate have been explained by the increasing ratio of respiratory tissue to gross primary production (GPP) with forest age (Kira and Shidei 1967). This hypothesis suggests that carbon limitation is the primary factor affecting age-related decline. However, Ryan and Waring (1992) demonstrated that increasing sapwood respiration accounts for only a small portion of the decline in net primary production. Other possible explanations include decreased nutrient supply and decreased canopy photosynthesis (Binkley et al. 1995; Ryan et al. 1997). Photosynthesis may decrease as a result of increased hydraulic resistance, lower leaf area due to abrasion or nutrient limitation, genetic programming, or reduced foliar growth because of reduced turgor pressure with tree height. Among these possible causes, several mechanisms are closely related to the availability of soil resources, such as nutrients or water.
For individual trees, Martinez-Vilalta et al. (2007) recently reported that older trees could increase their productivity after suppression of competing trees by thinning. However, whether stand-level age-related decline in forest productivity is inevitable remains unclear. Resource availability fluctuates throughout forest development, and previous chronosequence studies have not attempted to manipulate such factors (Ryan et al. 2004). For example, soil nitrogen mineralization increases after clear-cutting, peaks quickly, and then decreases over time (Borman and Likens 1979; White et al. 2004). Soil water content also increases after clear-cutting due to decreased evapotranspiration and canopy interception (Adams et al. 1991). Such temporal changes in soil nitrogen and water availability appear to regulate the rate of biomass accumulation throughout forest development in a complex manner.
Plants modulate their efficiency of resource acquisition through carbon allocation in above- versus belowground components (Shaver and Melillo 1984; Tilman 1988). Limited soil resources result in high biomass allocation to belowground parts to increase nutrient uptake (Tilman 1988). Among nutrients, decreased nitrogen availability often limits the productivity of forest ecosystems (Pastor et al. 1984; Reich et al. 1997) and results in higher allocation to fine root production (Keyes and Grier 1981; Tateno et al. 2004; but see Hendricks et al. 2006). Low soil water availability also results in a shift to belowground production (Gower et al. 1992). If age-related decline in net primary production is due to limited soil resources, mature forests may avoid or delay onset of age-related decline by increasing fine root production. However, because the respiration rate in fine roots tends to be higher than that in other organs (i.e., the stem), increasing fine root allocation may cause an overall carbon imbalance (Ryan et al. 1996). Limited carbon availability may prevent allocation to fine root production, despite limited soil resource availability. Thus, mature forests may reach a certain size beyond which carbon is insufficient to maintain aboveground parts and to increase fine root allocation for resources.
Despite the importance of allocation patterns in forest productivity and nutrient cycling, very little information is available regarding the pattern of above- versus belowground biomass allocation throughout forest development. Vanninen et al. (1996) and Vanninen and Mäkelä (1999) reported that the fine root to needle biomass ratio increased between years 12 and 178 in a forest composed of Pinus sylvestris L. In contrast, Helmisaari et al. (2002) reported a declining trend in fine root to needle biomass ratio throughout forest development. Here, we examined soil net nitrogen mineralization rate, above- and belowground biomass accumulation, and nitrogen use in a Cryptomeria japonica plantation chronosequence. The objectives of our study were to determine the patterns of biomass accumulation in this ecosystem and to explore the relationship among soil nitrogen, plant nitrogen status, and biomass accumulation in above- versus belowground components.
Materials and methods
Study site
This study was carried out in the Gomadan-san Experimental Forest (GEF) in Totsukawa, Nara Prefecture, central Japan (34°04′N, 135°35′E), which is the location of a series of commercial C. japonica plantations with a 90-year harvest cycle. The forest is located in a mountainous area at elevations of 860–1,370 m above sea level, and consists of 1- to 41-year-old plantations and an 88-year-old plantation. Stand structure in the study sites is summarized in Table 1.
Rotational clear-cutting of the entire watershed as a management unit (about 2–24 ha) has been conducted in the GEF. There, within a few months after clear-cutting, stems are transported to logging yards using a skyline system. The slash and logging debris are gathered and arranged into windrows. For 5–6 years after afforestation, weeds and understory vegetation are removed every summer. Thinning (approximately 30% of the stand) is performed once after approximately 30 years and thinned trees are not harvested. Canopy closure occurs approximately 15 years after afforestation. Before canopy closure, understory vegetation at this site is dominated by Japanese pampas grass and pioneer deciduous broad-leaved tree species. After canopy closure, understory vegetation at this site consists mainly of dwarf bamboo and deciduous shrub species. In young stands, large slash and logging debris can be found on the forest floor. Amounts of L and F layers tend to increase with stand age, but H layers are not well developed throughout stand age. Thin H layers (about 1–2 cm) are observed only in the mature stand.
The mean annual temperature and precipitation in the study region are 12.3°C and 2,650 mm, respectively, with snow cover from December to March. The underlying geology is sandy sedimentary rock, and the watershed encompasses the headwaters of the Totsukawa River. Nitrogen deposition in the GEF is about 12 kgN ha−1 year−1 (2002–2007 annual mean; Fukushima et al. 2009). Nitrogen loss in stream water is estimated to be about 3 to 16 kgN ha−1 year−1 (Fukushima et al. 2009). Fertilization is not usually conducted in the GEF. Further details regarding the GEF are available elsewhere (Fujimaki et al. 2007; Fukushima and Tokuchi 2008, 2009; Fukushima et al. 2009).
Vegetation census
We established two 20 × 20-m plots on the lower slopes on both sides of streams in the 4-, 15-, 30-, 41-, and 88-year-old stands. For the 30-year-old stands, thinning had not yet been conducted at the time of the census. Diameter at breast height (DBH) was measured for all trees. We then selected 10–25 trees and measured tree height (H) to estimate DBH–H relationships for each study plot.
Soil analysis
We collected three mineral soil samples (about 50 g in weight) using a trowel from one 20 × 20-m plot in each age at depths of 0–10, 10–30, and 30–50 cm after gently removing the litter layers. The soil samples were sieved and divided into a fine soil texture fraction (<2 mm size class) and a coarse fraction (>2 mm size fraction). Net nitrogen mineralization rate was measured in soil samples incubated in polyethylene bags at varying depths (0–10, 10–30, and 30–50 cm) in each stand. This “buried bag method” estimates net ammonification and nitrification as indicated by changes in soil NH4 +-N and NO3 −-N, respectively (Eno 1960; Pastor et al. 1984; Binkley and Hart 1989). Field incubations were performed four times: from mid-April to late June 2003, from late June to late September 2003, from late September to early December 2003, and from early December 2003 to mid-April 2004. Soil samples were collected from each soil layer, placed unsieved in polyethylene bags, sealed, and returned to their original locations in the soil. After incubation, the soil samples were brought to the laboratory and refrigerated for a maximum of 24 h before analysis. The samples were passed through a 2-mm sieve, and roots and other organic debris were removed by hand. A 5-g subsample was extracted in 50 ml 2 N KCl and filtered to determine the NH4 +-N and NO3 −-N concentrations. Net ammonification and nitrification were calculated from differences in the NH4 +-N and NO3 −-N pool sizes, respectively. Annual net nitrogen mineralization and nitrification rates (at 0–50 cm) were summed for all three layers in each period. Percent nitrification was calculated by dividing the net nitrification rate by the net mineralization rate. Where the calculated percent nitrification exceeded 100%, this was probably due to immobilization or denitrification.
To estimate bulk density, we collected three mineral soil blocks (400 cm2 × 10 cm for the 0–10 cm layer and 400 cm2 × 20 cm for the 10–30 and 30–50 cm layers) in each age class. The soil samples were sieved and divided into a fine soil texture fraction (<2 mm size class) and a coarse fraction (>2 mm size fraction). Fine soils were dried to a constant weight at 105°C and then weighed. Total carbon and nitrogen concentration of fine soils were determined using an NC analyzer (NC-900; Shimadzu, Kyoto, Japan).
Root census
We collected three 25-cm2 mineral soil blocks from three soil profiles at depths of 0–10, 10–30, and 30–50 cm from one 20 × 20-m plot in each age class. The sampling sites for root census located near mineral soil sampling profiles. Fine roots (<2 mm in diameter) and small roots (2–5 mm in diameter) were wet-sieved and then hand-sorted to separate living from dead material, based on morphology and condition. After sorting, the roots were dried at 70°C for 72 h and weighed. The details of this root census are reported elsewhere (Fujimaki et al. 2007).
Nitrogen and carbon content in plant organs and isotope analysis
In August 2003, five sunny branches from the crowns of five trees in each stand were harvested using a pole pruner. The samples were taken to the laboratory and separated into leaves and other parts. We could not strictly divide leaf age classes because this species has one or two flushes of leaves per year, depending on the circumstances. So we compounded all age classes together. Leaf samples were dried at 40°C and milled. Total nitrogen and carbon content was determined using an NC analyzer (NC-900; Shimadzu).
The composition of stable nitrogen isotopes in leaves was measured using a continuous-flow system (Finnigan MAT Delta-S; Finnigan, Bremen, Germany). The stable isotope ratios used were the standard delta (δ) values expressed in per mil (‰) according to the following equation:
where R reference = 15N/14N using atmospheric N2 as references for δ 15N.
Estimation of above- and belowground biomass
The relationship between DBH (D) and tree height (H) for each stand was estimated using the following equation (Thomas 1996):
where H max is the asymptote for maximum tree height.
The biomass of stems, branches, leaves, and coarse roots (>5 mm in diameter) were estimated from D and H using the allometric relationships derived from a C. japonica plantation forest in Japan (Karizumi 1974). The allometric equations used were
where W s, W b, W l, and W rc are the biomass of stems, branches, leaves, and coarse roots, respectively. These allometric equations were obtained from 10- to 50-year-old plantations across Japan. This is much lower than the age of the mature stands used for this study. However, the maximum DBH in the allometric studies of Karizumi (1974) was 38 cm, which is almost the same as the 41.7 cm of mature stands in our study, suggesting that these relationships can be used for our purposes. We estimated biomass of stems, branches, leaves, and coarse roots for individual trees and calculated area-based biomass by dividing the sum of tree biomass of each study plot by plot area. Fine root biomass (kg ha−1) and small root biomass (kg ha−1) were based on living fine root biomass determined from soil blocks. The total aboveground biomass (kg ha−1) was calculated as the sum of stem, branch, and leaf biomass. Total belowground biomass (kg ha−1) was calculated as the sum of coarse, small, and fine root biomass.
We used a nonlinear least squares regression to fit the following logistic equation:
where W and t are the biomass of each organ (in kg ha−1) and forest age (years), respectively, and a, b, and c are constants.
Statistical analysis
Vegetation data, such as tree density, mean DBH, and biomass of each organ, were averaged for two study plots for each age. To analyze the effects of forest age on nitrogen concentration and δ 15N of leaves, we used 10 trees from both plots (5 trees from each side of the stream) of the same age as replicates. The effects of forest age on fine and small root biomass, nitrogen concentration, δ 15N of leaves, soil carbon content, soil nitrogen content, and carbon to nitrogen ratio of soil were tested using ANOVAs. If significant effects of age were detected, we conducted a Tukey’s HSD test for multiple comparisons. If the results of Levene’s test were significant (P < 0.05), the data were log-transformed before the ANOVA and Tukey’s HSD tests. Because δ 15N of leaves had negative values, we multiplied the data by −1 before log transformation. Because data were lacking for small roots in some cases, we added 1 to all the data before log transformation. We conducted an ANOVA on data for soil net nitrogen mineralization rate, net nitrification rate, and percent nitrification after summing the data to average values for each season and each soil layer using the three soil columns as replicates. Seasonal patterns of soil mineral nitrogen dynamics were beyond the scope of this study and will be reported elsewhere (Fukushima et al., unpublished manuscript). To fit the data, logistic regression equations were used with the least-squares method. All statistical analyses were performed using SPSS for Windows (SPSS, Chicago, IL, USA).
Results
Biomass accumulation and allocation pattern
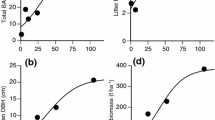
The biomass accumulation patterns of plant organs, other than fine and small roots, fit the logistic equation, with r 2 values ranging from 0.992 to 0.999 (Fig. 1; Table 2). However, fine root biomass showed a markedly different pattern in relation to stand age (Fig. 1c). Fine root biomass first increased sharply from year 4 to 15, then decreased from year 15 to 88; however, fine root biomass from year 15 to 88 did not significantly differ. Small root biomass also showed the same pattern, but only small root biomass for year 15 was significantly higher than that of other ages.
The biomass accumulation of a total, aboveground, and belowground (n = 2 plots), b stem, branch, leaf, and large root (n = 2 plots), and c fine and small roots (n = 3 soil blocks) along the stand age chronosequence. Error bars represent the SD. The regression curves shown for each organ are least-squares fits of the logistic equation: W(t) = a/{1 + b × [exp(–c × t)]}. Regression statistics are summarized in Table 2 (n = 5 stand ages per compartment). For fine and small roots (c), different letters indicate significant differences between stands of different ages (P < 0.05; ANOVA with Tukey’s HSD test). The fine and small root data are from Fujimaki et al. (2007)
According to logistic equations fitted to the biomass accumulation pattern, the peak accumulation (kg ha−1 year−1) for total, above-, and belowground biomass occurred at years 30, 30, and 28, respectively (Fig. 1a). Peak biomass accumulation for leaves, stems, branches, and coarse roots occurred at years 21, 32, 28, and 30, respectively (Fig. 1b). Peak biomass accumulation for fine roots occurred at 15 years (Fig. 1c). The ratio of fine root to leaf biomass increased sharply from year 4 to 15 and then decreased sharply from year 15 to 30. From year 30 to 88, the ratio of fine root to leaf biomass gradually decreased with stand age (Fig. 2). The change in the ratio of fine root to leaf biomass along the age gradient was not statistically tested due to the lack of replication.
Soil nitrogen and leaf characteristics in relation to stand development
The concentration and amount of soil total nitrogen and carbon were significantly lower in year 30 than at other stand ages (Fig. 3a, b). The soil carbon to nitrogen ratio was not significantly different from year 4 to 41, but was significantly higher in 88-year-old trees than in trees of other ages (Fig. 3c). The net soil nitrogen mineralization rate showed an increase between years 4 and 15, a decrease at year 30, and then another increase between years 30 and 88, but no significant differences were observed among ages except those between age 30 and 88 (Fig. 4a). The net nitrification rate was not significantly different among stand ages (Fig. 4b). However, the percent nitrification to mineralization rates almost always exceeded 100% from year 4 to 41, followed by a decrease in 88-year-old trees (Fig. 4c); however, no significant differences were found among ages except for trees between age 41 and 88 (Fig. 4c).
a Carbon content, b nitrogen content, and c C/N ratio in the sums of mineral soil (0–50 cm) along the stand age chronosequence. Different letters indicate significant differences between stands of different ages (P < 0.05; ANOVA with Tukey’s HSD test). Error bars represent the SD (n = 3 soil blocks)
Foliar nitrogen concentration in C. japonica decreased from years 4 to 88 (Fig. 5a). Foliar δ 15N decreased from year 4 to 41 (Fig. 5b).
a Leaf nitrogen concentration and b foliar δ15N value of Cryptomeria japonica along the stand age chronosequence. Different letters indicate significant differences between stands of different age (P < 0.05; ANOVA with Tukey’s HSD test). Error bars represent the SD (n = 10 branches from two plots in each stand)
Discussion
Biomass accumulation in a chronosequence
Biomass accumulation showed an asymptotic accretion pattern, which is consistent with previous studies reporting on chronosequence patterns in plantation forests all over the world (Kira and Shidei 1967; Ryan et al. 2004). The peak biomass accumulation rate occurred approximately 30 years after afforestation. All woody components, such as stems, branches, and coarse roots, showed similar accumulation patterns. In contrast, leaves and fine roots showed slightly different patterns. Leaf biomass accumulation peaked earlier than other organs, at approximately 20 years, and fine root biomass peaked at approximately 15 years and then decreased with forest age. These results are consistent with previous studies reporting that fine root biomass peaked almost simultaneously with canopy closure (Helmisaari et al. 2002; Law et al. 2003).
Soil and plant nitrogen throughout stand development
Several previous studies showed that nitrogen availability increased immediately after disturbance as a result of increased decomposition and decreased biological demand (Bormann and Likens 1979; Vitousek et al. 1989; White et al. 2004). After peaking relatively early in stand development, soil nitrogen mineralization decreased continuously or showed smaller increases in the mature phase (Bormann and Likens 1979; Vitousek et al. 1989; White et al. 2004). Also, in this study, the net soil nitrogen mineralization rate increased between years 4 and 15, decreased at year 30, and then increased again between years 30 and 88; however, no significant differences were observed among ages except for the differences found between ages 30 and 88 (Fig. 4a). In contrast to soil nitrogen mineralization patterns, peak plant growth occurred approximately 30 years after afforestation, suggesting an imbalance in soil nitrogen supply versus plant nitrogen.
If net nitrogen mineralization rate is an accurate indicator of soil nitrogen supply, soil nitrogen does not appear to be a limiting factor in mature forest compared to plant nitrogen demand. However, leaf nitrogen concentration, as an index of plant nitrogen status, was lower in mature forest than in young forest (Fig. 5a). Canopy nitrogen, which is calculated by multiplying the concentration of nitrogen in foliage by its biomass, also decreased in 41-year-old forest compared to 88-year-old forest, which may result in decreased GPP in mature forests. Recently, Shigenaga et al. (2008) reported the average leaf nitrogen content of C. japonica for 531 sites across Japan of 1.4%, with half the sites ranging from 1.25 to 1.55%. Compared to these values, leaf nitrogen content of our study sites tended to be low, especially in mature stands. Thus, mature stands in this study site are thought to be nitrogen-limited. However, the discrepancy in soil net nitrogen mineralization versus leaf nitrogen content remains unclear.
Net nitrogen mineralization rate is often correlated with forest production and nitrogen uptake and is thought to reflect soil nitrogen availability (Pastor et al. 1984; Reich et al. 1997). However, the net rate of mineralization is not always indicative of the gross rate (Davidson et al. 1992; Hart et al. 1994). Most inorganic nitrogen is readily immobilized by soil microbes (Jackson et al. 1989; Hodge et al. 2000). In this study site, the percent net nitrification to mineralization exceeded 100% except in the mature stand, probably due to immobilization of inorganic nitrogen. Even under such conditions, trees may successfully take up soil nitrogen because they could use inorganic nitrogen, even though the net rate of mineralization was low (Tateno et al. 2005). The most likely explanation for the discrepancy between soil nitrogen supply and plant demand observed in this study is that net nitrogen mineralization rate as determined by the buried bag method was not a good index of soil nitrogen supply at this study site.
The form of inorganic nitrogen (i.e., nitrate and ammonium) in soil also affects soil nitrogen availability (Binkley and Hart 1989). Net nitrification rate showed a pattern very similar to that of net nitrogen mineralization rate throughout stand development, but the percent nitrification to mineralization did not exceed 100% in mature forests (Fig. 4b, c). Such a decrease may indicate a decrease in nitrogen uptake in mature trees and may be analogous to the pattern observed along slopes in temperate Japanese forests (Hirobe et al. 1998; Tateno and Takeda 2003). In these forests, the percent net nitrification to mineralization was lower on upper slopes, and thus soil nitrogen availability was lower on these upper slopes. The soil carbon to nitrogen ratio, which is often correlated with net nitrification rate, was higher in sites with low soil nitrogen availability (Hirobe et al. 1998; Tateno and Takeda 2003). In the present study, the soil carbon to nitrogen ratio was higher in mature stands than in younger forest (Fig. 3c).
Leaf δ 15N is often used as an index of plant nitrogen utilization (Garten 1993; Nadelhoffer et al. 1996; Högberg 1997; Koba et al. 2003; Tateno et al. 2003, 2005). The δ 15N of NO3 − is lower than that of NH4 + (Nadelhoffer et al. 1996; Koba et al. 1998), so that a lower δ 15N value indicates greater reliance on NO3 −. In our study, the δ 15N decreased with stand age until year 41, and then showed an increase at year 88. We noted that the percent nitrification was highest in the 41-year-old stand, probably leading to the lowest δ 15N value because of soil nitrogen dynamics. Although the mechanism underlying this shift was beyond the scope of our study, our results may indicate that 88-year-old trees are less dependent on NO3 − as a nitrogen source. At this study site, infection by mycorrhizae in mature stands was greater in number per root tip than in younger stands (Hishi et al., unpublished data). However, the effects of mycorrhizal uptake of organic nitrogen on leaf δ 15N values in this study site still remain unclear.
Another possible explanation for the decrease in leaf and canopy nitrogen may be the lack of some other nutrient. The ratio of one nutrient to another falls within a certain range for each nutrient pair. For example, the leaf nitrogen to phosphorus ratio varies between 10 and 20 among a wide range of species in various ecosystems (Güsewell 2004). In the absence of some other key nutrient required for production, trees may not take up nitrogen even when it is readily available. Further studies are required to determine whether the availability of other nutrients, such as phosphorus, decreases with forest age. However, the observed decrease in leaf nitrogen suggests that soil nutrient availability, either nitrogen and/or other nutrients, limited production in mature forests. Such increasing soil nutrient limitation in older stands was also reported in a lodgepole pine plantation chronosequence (Binkley et al. 1995).
Biomass allocation pattern throughout stand development
Biomass allocation to fine roots varies in relation to soil water and nitrogen (Keyes and Grier 1981; Gower et al. 1992; Tateno et al. 2004), and the fine root to leaf ratio is useful for understanding how plants adapt to fluctuations in these parameters. The ratio of fine root to foliage biomass is typically higher in nutrient-poor sites compared to fertile sites (Keyes and Grier 1981; Vogt et al. 1986, 1996; Gower et al. 1992). However, the general pattern of fine root to foliage biomass over time remains somewhat unclear (Vanninen et al. 1996; Vanninen and Mäkelä 1999; Helmisaari et al. 2002; Law et al. 2003). At the young stage, nitrogen deficiency may not occur, which may be partly due to the high root to leaf ratio. In stands exhibiting peak biomass accumulation at approximately 30 years, the soil net nitrogen mineralization rate was low and the demand for production was high. However, the fine root to leaf ratio was not higher than at other stand ages. Thus, the peak fine root to leaf ratio did not coincide with peak deficiency of soil nitrogen supply.
In mature stages, the lack of soil nitrogen or other nutrients may impose a stricter limit on production. However, the ratio of fine root to foliage biomass was lowest in mature forest. Although lower leaf nitrogen in mature stands may indicate nitrogen deficiency, the trees could not increase biomass allocation to fine roots. Under such conditions, plants must maintain leaf production at the expense of leaf nitrogen concentration, and lower canopy nitrogen may result in lower carbon acquisition because of nitrogen-related decreases in photosynthesis. Thus, the mature forest may have been experiencing a carbon as well as soil resource limitation. Because the trees did not shift allocation to fine roots, our results suggest that mature forests rely on a conservative strategy for soil resource acquisition and production. Our results are consistent with those of Helmisaari et al. (2002) and Law et al. (2003), who reported that fine root to leaf biomass ratio decreased with stand age (cf. Vanninen et al. 1996; Vanninen and Mäkelä 1999).
Factors that limit production, such as water, nutrients, and carbon availability, may fluctuate throughout stand development. The degree of limitation, relatively and absolutely, changes with environmental conditions. Although environmental conditions surrounding even-aged forests fluctuate widely, it is almost universally observed that leaf biomass reaches an asymptote, sooner or later, in both even- and uneven-aged forests. After attaining maximum leaf biomass, canopy photosynthesis tends to decrease as a result of hydraulic and/or nutrient limitations. Increased fine root biomass throughout stand development may moderate soil nutrient limitation and delay the decline in GPP; however, Ryan et al. (2004) reported that GPP and wood increment declined even under high nutrient availability. Thus, it appears that additional carbon investment in belowground components is too costly and creates an overall negative carbon balance under severe carbon limitation.
This mechanism may partly explain the diverging results obtained for age-related root to leaf ratios obtained for different series of developing stands. However, the primary factors affecting age-related decline in biomass accumulation remain unclear. Further studies are required to examine environmental conditions, such as: soil nitrogen and the availability of other nutrients and water; belowground processes, such as root turnover, root respiration, exudates, and mycorrhizae; and physiological processes, such as photosynthesis, respiration, transpiration, and retranslocation.
References
Adams PW, Flint AL, Fredriksen RL (1991) Long-term patterns in soil moisture and revegetation after a clearcut of a Douglas-Fir Forest in Oregon. For Ecol Manag 41:249–263
Binkley D, Hart SC (1989) The components of nitrogen availability assessments in forest soils. Adv Soil Sci 10:57–112
Binkley D, Smith FW, Son Y (1995) Nutrient supply and declines in leaf area and production in lodgepole pine. Can J For Res 25:621–628
Bormann FH, Likens GE (1979) Pattern and process in a forested ecosystem. Springer, New York
Davidson EA, Hart SC, Firestone MK (1992) Internal cycling of nitrte in soils of a mature coniferous forest. Ecology 73:1148–1156
Eno CF (1960) Nitrate production in the field by incubating the soil in polyethylene bags. Soil Sci Soc Am J 24:277–279
Fujimaki R, Tateno R, Tokuchi N (2007) Root development across a chronosequence in a Japanese cedar (Cryptomeria japonica D. Don) plantation. J For Res 12(9):6–102
Fukushima K, Tokuchi N (2008) Effects of forest clearcut and afforestation on streamwater chemistry in Japanese cedar (Cryptomeria japonica) forests: comparison among watersheds of various stand ages. J Jpn For Soc 90:6–16 (in Japanese with English summary)
Fukushima K, Tokuchi N (2009) Factors controlling the acid-neutralizing capacity of Japanese cedar forest watersheds in stands of various ages and topographic characteristics. Hydrol Process 23:259–271
Fukushima K, Tokuchi N, Tateno R, Katsuyama M (2009) Water yield and nitrogen loss during regrowth of Japanese cedar forests after clearcutting. In: Taniguchi M, Fukushima Y, Burnett WC, Haigh M, Umezawa Y (eds) From headwaters to the ocean: hydrological change and watershed management. Taylor & Francis, London, pp. 97–104
Garten CT (1993) Variation in foliar 15N abundance and the availability of soil nitrogen on Walker Branch Watershed. Ecology 74:2098–2113
Gower ST, Vogt KA, Grier CC (1992) Carbon dynamics of Rocky Mountain Douglas-fir: influence of water and nutrient availability. Ecol Monogr 62:43–65
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Hart SC, Nason GE, Myrold DD, Perry DA (1994) Dynamics of gross nitrogen mineralizations in an old-growth forest: the carbon connection. Ecology 75:880–891
Helmisaari HS, Makkonen K, Kellomaki S, Valtonen E, Malkonen E (2002) Below- and above-ground biomass, production and nitrogen use in Scots pine stands in eastern Finland. For Ecol Manag 165:317–326
Hendricks JJ, Hendrick RL, Wilson CA, Mitchell RJ, Pecot SD, Guo D (2006) Assessing the patterns and controls of fine root dynamics: an empirical test and methodological review. J Ecol 94:40–57
Hirobe M, Tokuchi N, Iwatsubo G (1998) Spatial variability of soil nitrogen transformation patterns along a forest slope in a Cryptomeria japonica D. Don plantation. Eur J Soil Biol 34:123–131
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Ecol Evol 5:304–308
Högberg P (1997) Tansley review No. 95 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Jackson LE, Schimel JP, Firestone MK (1989) Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol Biochem 21:409–415
Karizumi N (1974) The mechanism and function of tree root in the process of forest production. I: method of investigation and estimation of the root biomass. Bull Gov For Exp Stn 259:1–99
Keyes MR, Grier CC (1981) Above- and below-ground net production in 40-year-old Douglas-fir stands on low and high productivity sites. Can J For Res 11:599–605
Kira T, Shidei T (1967) Primary production and turnover of organic matter in different forest ecosystems of the western pacific. Jpn J Ecol 17:70–87
Koba K, Tokuchi N, Yoshioka T, Hobbie EA, Iwatsubo G (1998) Natural abundance of nitrogen-15 in a forest soil. Soil Sci Soc Am J 62:778–781
Koba K, Hirobe M, Koyama L, Kohzu A, Tokuchi N, Nadelhoffer KJ, Wada E, Takeda H (2003) Natural 15N abundance of plants and soil N in a temperate coniferous forest. Ecosystems 6:457–469
Law BE, Sun OJ, Campbell J, Van Tuyl S, Thornton PE (2003) Changes in carbon storage and fluxes in a chronosequence of ponderosa pine. Glob Chang Biol 9:510–524
Martinez-Vilalta J, Vanderklein D, Mencuccin M (2007) Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.). Oecologia 150:529–544
Nadelhoffer K, Shaver G, Fry B, Giblin A, Johnson L, McKane R (1996) 15N natural abundances and N use by tundra plants. Oecologia 107:386–394
Odum EP (1969) The strategy of ecosystem development. Science 164:262–270
Pastor J, Aber JD, McClaugherty CA (1984) Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65:256–268
Reich PB, Grigal DF, Aber JD, Gower ST (1997) Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 78:335–347
Ryan MG, Waring RH (1992) Maintenance respiration and stand development in a subalpine lodgepole pine forest. Ecology 73:2100–2108
Ryan MG, Hubbard RM, Pongracic S, Raison RJ, McMurtrie RE (1996) Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol 16:333–343
Ryan MG, Binkley D, Fownes JH (1997) Age-related decline in forest productivity: pattern and process. Adv Ecol Res 27:213–262
Ryan MG, Binkley D, Fownes JH, Giardina CP, Senock RS (2004) An experimental test of the causes of forest growth decline with stand age. Ecol Monogr 74:393–414
Shaver GR, Melillo JM (1984) Nutrient budgets of marsh plants: efficiency concepts and relation to availability. Ecology 65:1491–1510
Shigenaga H, Takahashi M, Nagakura J, Akama A (2008) Spatial variations in needle nitrogen content in sugi (Cryptomeria japonica D. Don) plantations across Japan. J Jpn For Soc 90:182–189 (in Japanese with English summary)
Tateno R, Takeda H (2003) Forest structure and tree species distribution in relation to topography-mediated heterogeneity of soil nitrogen and light at forest floor. Ecol Res 18:559–571
Tateno R, Katagiri S, Kawaguchi H, Nagayama Y, Li C, Sugimoto A, Koba K (2003) The use of foliar 15N and 13C abundance to evaluate effects of microbiotic crust on nitrogen and water utilization of Pinus massoniana in deteriorated pine stands of south China. Ecol Res 18:279–286
Tateno R, Hishi T, Takeda H (2004) Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. For Ecol Manag 193:297–306
Tateno R, Osada N, Terai M, Tokuchi N, Takeda H (2005) Inorganic nitrogen source utilization by Fagus crenata on different soil types. Trees 19:477–481
Thomas SC (1996) Asymptotic height as a predictor of growth and allometric characteristics in Malaysian rain forest trees. Am J Bot 83:556–566
Tilman D (1988) Plant strategies and the dynamics and structure of plant communities. Princeton University Press, Princeton
Vanninen P, Mäkelä A (1999) Fine root biomass of scots pine stands differing in age and soil fertility in southern Finland. Tree Physiol 19:823–830
Vanninen P, Ylitalo H, Sievanen R, Mäkelä A (1996) Effects of age and site quality on the distribution of biomass in Scots pine (Pinus sylvestris L.). Trees 10:231–238
Vitousek PM, Matson PA, Van Cleve K (1989) Nitrogen availability and nitrification during succession: primary, secondary, and old-field seres. Plant Soil 115:229–239
Vogt KA, Grier CC, Vogt DJ (1986) Production, turnover, and nutrient dynamics of above- and belowground detritus of world forests. Adv Ecol Res 15:303–377
Vogt KA, Vogt DJ, Palmiotto PA, Boon P, O’Hara J, Asbjornsen H (1996) Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 187:159–219
White LL, Zak DR, Barnes BV (2004) Biomass accumulation and soil nitrogen availability in an 87-year-old Populus grandidentata chronosequence. For Ecol Manag 191:121–127
Acknowledgments
We would like to thank technical staffs of Wakayama Forest Research Station, Field Science Education and Research Center, Kyoto University for support of fieldworks. We owe thanks to Sei-ichi Oohata for arranging for us to participate in this study. We wish to thank Muneto Hirobe and Keisuke Koba, Takuo Hishi, Miki Ueda, and Ken-ichiro Osaki for suggestions and for assistance with field and laboratory work. This study was financially supported by Research Program (No. 5-2) of Research Institute for Humanity and Nature (RIHN). This study was also partly supported by a grant of (17710019 and 20780120) from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tateno, R., Fukushima, K., Fujimaki, R. et al. Biomass allocation and nitrogen limitation in a Cryptomeria japonica plantation chronosequence. J For Res 14, 276–285 (2009). https://doi.org/10.1007/s10310-009-0135-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10310-009-0135-7