Abstract
Bacillus velezensis B006 is a biocontrol agent which functions through effective colonization and surfactin production. To reveal the surfactin-producing mechanism, gas chromatography–mass spectrometry based untargeted metabolomics was performed to compare the metabolite profiles of strain B006 grown in industrial media M3 and M4. Based on the statistical and pathway topology analyses, a total of 31 metabolites with a fold change of less than − 1.0 were screened as the significantly altered metabolites, which distributed in 15 metabolic pathways. Fourteen amino acids involving in the metabolisms of alanine/aspartate/glutamate, glycine/serine/threonine, arginine/proline, glutathione/cysteine/methionine and valine/leucine/isoleucine as well as succinic acid in TCA cycle were identified to be the hub metabolites. Aminoacyl-tRNA biosynthesis, glycerolipid metabolism, and pantothenate/CoA biosynthesis also contributed to surfactin production. To the best of our knowledge, this study is the first to investigate the metabolic pathways of B. velezensis on surfactin production, and will benefit the optimization of commercial fermentation for higher surfactin yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful colonization in rhizosphere and production of lipopeptides are two important mechanisms of Bacillus spp. to suppress soil-borne plant pathogens [46]. Surfactin is one of the three most important lipopeptides detected in the rhizosphere and plantlets [15, 21, 28], and consists of seven amino acids (l-leucine-d-leucine-l-aspartic acid-l-valine-d-leucine-l-leucine-l-glutamate acid) bonded to the carboxyl and hydroxyl groups of a 12-16-carbon fatty acid. Surfactin has been extensively studied due to the strong biosurfactant activity, great capability of suppressing plant diseases, and low toxicity to human and environment [5]. Different from other fungicides that suppress fungal growth through targeting the protein synthesis or DNA replication, surfactin inhibits bacterial growth through membrane disruption or disintegration via physicochemical interaction [8] or suppresses fungi by promoting the beneficial bacteria colonization [17] and inducing systemic resistance [5, 20, 31]. However, the high production cost of surfactin has limited its commercial application in agriculture.
Many strategies have been adopted to improve the surfactin yield as well as to reduce the production cost, such as engineering Bacillus spp. for commercial purposes [30], using agro-residues as the carbon source [12, 38, 53], optimizing the fermentation medium [10, 22, 43, 50] and conditions [35], designing specific bioreactor and employing foam-free techniques [26, 42, 49], and so on. Lately, Coutte et al. [7] improved the surfactin production level up to 20.9-fold and optimized the surfactin composition by optimizing the medium composition. Dahli et al. [9] increased the surfactin yield as well as the proportion of active surfactin isoforms. However, the physiological mechanism underlying the surfactin production in industrial medium has not been revealed yet [14].
Metabolomics, as a bio-prospecting tool [6], has attracted much attention in fermentation industry for bottleneck identification or production improvement of secondary metabolites, especially some active chemicals [3, 33, 45]. Using metabolomics, Lu et al. [23] analyzed the effects of dissolved oxygen on the metabolite production of Saccharopolyspora spinosa and found the enhanced level of primary metabolism promoted the cell growth and synthesis of precursors and cofactors; Wu [44] revealed the roles of 3,4-dihydroxy-2-butanone-4-phosphate in riboflavin synthesis; and Cao et al. [4] and Xia et al. [45] improved the production levels of several target secondary metabolites such as avermectin and FK506.
Bacillus velezensis strain B006 is an excellent biocontrol agent that has great efficacy in suppressing cucumber wilt and pepper root rot diseases caused by Fusarium oxysporum f. sp. cucumerinum and Phytophthora capsici [47]. Effective colonization of the Bacillus cells in rhizosphere as well as surfactin production is crucial to suppress the pathogen growth. In addition to its suppressive function, surfactin also facilitates the Bacillus cells to form biofilm on the roots during the initial colonization process [34]. Our previous studies have shown that industrial medium M3 is favorable for the spore formation of strain B006 [11], while medium M4 is favorable for surfactin production [40]. How these media affect the metabolism of strain B006 is much intriguing. In the present study, an untargeted metabolomics study was performed via gas chromatography (GC)-mass spectrometry (MS) technology to undermine the mechanisms of surfactin production of B. velezensis B006. Potential key metabolites and related biological pathways were systematically revealed, and putative metabolic pathways were proposed.
Materials and methods
Bacterial strain and media
Bacillus velezensis B006 was conserved in the Agricultural Culture Collection of China (ACCC; Beijing, China) under registration number 60130. The seed inoculum was prepared by growing the cells in nutrient broth (10 g L−1 glucose, 10 g L−1 peptone, 3 g L−1 yeast extract, and 3 g L−1 beef extract, pH 7.0) at 30 °C with an agitation of 180 rpm for 16–18 h. Two media were used for fermentation: M3 contained 17.5 g L−1 corn starch, 5.25 g L−1 sucrose, 14.0 g L−1 soybean cake powder, and 5.25 g L−1 CaCO3 [11], and M4 contained 10.0 g L−1 beef extract, 15.0 g L−1 corn flour, 3.0 g L−1 NaNO3, and 3.0 g L−1 NaCl [40]. The soybean cake powder and corn flour were pre-sieved through 150 µm stainless steel mesh, and the pH of both media was adjusted to pH 7.0 before autoclave. Six milliliter of the seed inoculum was transferred into 500 mL Erlenmeyer flasks containing 60 mL of medium M3 or M4 and incubated at 30 °C with an agitation of 180 rpm for 56 h. Each medium had triplicate for sporulation observation and surfactin yield measurement, and seven replicates for metabolomics analysis.
Sample collection and analysis
Aliquots of 1 mL culture broth were collected from each flask from 8 to 56 h at the interval of 4 h. The amounts of bacterial cells and sporulation rate were enumerated under microscope, and the surfactin yields were assessed using the oil-dispersing method [18]. The final surfactin yield was determined using the HPLC-electrospray ionization tandem (ESI)-MS technique as described by Jia et al. [17, 18]. By comparing the retention times and mass spectra with reference standard surfactin, the total amounts and compositions of surfactin were determined by calculating the relative peak areas in the chromatograms using MassLynx software (waters).
GC–MS sample preparation
Aliquots of 20 mL culture broth of each medium were collected with seven replicates after 16-h growth and transferred into 50 mL centrifuge tubes. The tubes were immersed in liquid nitrogen for 1 s followed by immediately shaking to avoid freezing the samples. This procedure was repeated for ten times to cool the tube down to 9 ± 2 °C. The samples were then centrifuged at 3380 g for 2 min, washed by 0.9% NaCl (w/v) for three times, frozen in liquid nitrogen, and stored at − 80 °C until use.
The frozen cells of B. velezensis B006 were mixed with 5 mL of cold 60% ethanol including 5 μg mL−1 l-norvaline (as the internal standard), and sonicated by five cycles of 10 min with intervals of 10 min. The lysates were kept at − 40 °C for 2 h, followed by centrifugation at 16, 000 g and 4 °C for 15 min. One hundred microliters of the mixtures were evaporated to dryness under nitrogen stream, dissolved in 30 μL of pyridine containing 20 mg mL−1 methoxyamine hydrochloride, and incubated at 37 °C for 90 min. The mixtures were added with 30 μL of BSTFA containing 1% TMCS (v/v), and was subjected to derive at 70 °C for 60 min prior to GC–MS analysis [25].
GC–MS analysis
GC–MS analysis was performed on an Agilent 7890A/5975C system equipped with a HP-5 ms fused-silica capillary column (30 m × 0.25 mm × 0.25 μm). Helium (> 99.99%) was used as the carrier gas at a constant flow rate of 1 mL min−1. The injection volume was 1 mL in splitless mode, and the solvent delay time was 6 min. The initial oven temperature was held at 70 °C for 2 min, ramped to 160 °C at a rate of 6 °C min−1, to 240 °C at a rate of 10 °C min−1, to 300 °C at a rate of 20 °C min−1, and finally held at 300 °C for 6 min. The temperatures of injector, transfer line, and electron impact ion source were set to 250, 250 , and 230 °C, respectively. The impact energy was 70 eV, and the data were collected in a full scan mode (m/z 50–600).
Data processing
The total ion current chromatograms (TIC) were created by summing up intensities of all mass spectral peaks of the same scan. The peak picking, alignment, deconvolution, and further processing of raw GC–MS data were carried out according to the previously published protocols [19]. The retention time and mass spectra of each metabolite were recorded, and the abundance of each metabolite was normalized against the total peak abundance before performing univariate and multivariate statistic analysis.
Statistical analysis and identification of differential metabolites
For multivariate statistical analysis, the normalized data were imported to SIMCA 13.0 (Umetrics), in which the data were preprocessed by unit variance (UV) scaling and mean centering before performing the principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA). The model quality was described by the R2X (PCA) or R2Y (OPLS-DA) and Q2 values. R2X and R2Y, the proportions of variance in the data explained by the models, indicated the goodness of fit. Q2, the proportion of variance in the data predicted by the model, was calculated by the cross-validation procedure and functioned as an indicator of the predictability of current model. To avoid model over-fitting, a default 7-round cross-validation in SIMCA 13.0 was performed to determine the optimal number of principal components.
The differential metabolites were identified according to the variables with VIP values of OPLS-DA model bigger than one and P values of univariate statistical analysis lower than 0.05. Fold change was calculated as the binary logarithm of average normalized peak intensity ratio between groups M3 and M4, in which positive values meant that the average mass response of group M3 are higher than that of group M4.
Structural identification of the differential metabolites
The raw GC–MS data were first subjected to deconvolution by the AMDIS 14.1 (NIST), and the purified mass spectra were automatically matched with an in-house standard library of Shanghai Profleader Biotech (China) including retention time and mass spectra, GolmMetabolome Database, and Agilent Fiehn GC/MS Metabolomics RTL Library. Metabolic pathways of the differential metabolites were constructed according to their pathways in KEGG. The differential metabolites in the cells of B. velezensis B006 grown in media M3 and M4 were analyzed by a heatmap built by the R 3.2.4 package. The pathway enrichment analysis of differential metabolites was performed on the on-line software MetaboAnalyst 4.0 (http://www.metaboanalyst.ca/). By matching against the metabolite library of Bacillus subtilis that contains 80 pathways, unmatched metabolites were filtered, and the objective metabolites and their pathways were searched and plotted against the databases HMDB, Pubchem, and KEGG.
Results
Physiological characteristics of B. velezensis B006
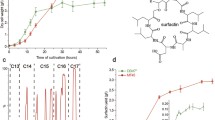
Bacillus velezensis B006 showed similar cell densities, 7.75 ± 0.35 × 108 mL−1 and 6.95 ± 0.28 × 108 mL−1, respectively, after 16 h-growth in the media M3 and M4. However, some cells in the medium M3 began to form endospores while not in medium M4 (Fig. 1a, b). It suggested that different medium compositions have distinct effects on the physiological characters of strain B006. The surfactin-producing capabilities of strain B006 grown in different media were preliminarily assessed using the oil-dispersing method. As shown in Fig. 1c, the oil-dispersing diameters of both culture broth kept increasing after 16-h incubation, but slowed down after 40 h. Thus, the samples were collected at 16 h for metabolomics analysis. In comparison to medium M3, medium M4 was more favorable for the surfactin production, in which the surfactin yields were more than twofold over the duration from 24 to 56 h.
The surfactin amounts produced in media M3 and M4 were further analyzed with the reference standard surfactin. As shown in Fig. S1, the retention time of standard surfactin was between 9.19 and 13.97 min, and with the m/z at 994.6, 1008.6, 1022.6, 1036.6, and 1050.6, which corresponded to the [M + H]+ of surfactin isoforms C12, C13, C14, C15 and C16, respectively. Surfactin in the cell extracts of strain B006 grown in media M3 and M4 showed similar retention times and m/z values (Fig. S1), but varied a lot in the surfactin yields (9.3 and 283.8 mg L−1 for groups M3 and M4, respectively). Further composition analysis demonstrated that the ratios of surfactin isoforms C12:C13:C14:C15:C16 in media M3 and M4 were 6:33:22:27:12 and 0:18:33:39:10, respectively, and isoforms C14 and C15 having higher antagonistic activities were more abundant (72 vs. 49) in group M4. It indicated that medium M4 is not only beneficial for the surfactin production by B. velezensis B006, but also promotes the great biocontrol efficiency against plant pathogens.
Difference analysis using the PCA and OPLS-DA score plots
TIC chromatograms of the cell extracts indicated the different media had significant effects on the composition and abundance of intracellular metabolites (Fig. S2). Reliable PCA and OPLS-DA models were then constructed, with the R2X of 0.72 for the model PCA, and R2X of 0.7, R2Y of 0.996, and Q2 of 0.981 for the model OPLS-DA. The score plots in Fig. 2 demonstrated that group M3 was obviously separated from group M4. It indicated that B. velezensis B006 grown in media M3 and M4 produced different metabolites.
Identification of intracellular differential metabolites
By matching the retention time and mass spectra with an in-house standard library, GolmMetabolome Database, and Agilent Fiehn GC/MS Metabolomics RTL Library, a total of 78 differential metabolites were identified. Twenty-six metabolites were then filtered out after matching with the B. subtilis metabolite pathway. Of the 44 up-regulated metabolites observed in group M4, 39 metabolites with a fold change of less than − 1.0, including 14 amino acids (glutamine, glutamic acid, aspartic acid, alanine, glycine, cysteine, serine, threonine, proline, tyrosine, isoleucine, lysine, phenylalanine, and valine) and derivatives, aminoacyl-tRNA, four nucleotides (adenine, inosine, guanosine, and xanthosine), two organic acids (fumaric acid and succinic acid), and 1,3-dihydroxyacetone, might be closely related to the surfactin production and were selected for further pathway analysis (Table S1, Fig. 3). In contrast, seven of eight differential metabolites in group M3 were up-regulated, with a fold change of more than 1.0 (Table S1, Fig. 3). These metabolites might involve lysine degradation (glutaric acid), phenylalanine metabolism (benzoic acid), glyoxylate and dicarboxylate metabolism (glycolic acid, glyoxylic acid, oxalic acid, and citric acid), fatty acid metabolism (palmitic acid), and TCA cycle (citric acid), and probably promoted the endospore formation of strain B006.
Distinguished differential metabolites and closely related pathways
Pathway enrichment analysis using the MetaboAnalyst 4.0 demonstrated that the metabolisms of alanine, aspartate, glutamate, glycine, serine, threonine, arginine, proline, glutathione, cysteine, methionine, glycerolipid, purine, sulfur, glyoxylate and dicarboxylate, biosynthesis of aminoacyl-tRNA, pantothenate/CoA and valine/leucine/isoleucine, and TCA cycle (Table S2, Fig. 4) probably accounted for the metabolite profile differences. Of the 39 differential metabolites with an impact value of over zero, 31 metabolites with a fold change of less than − 1.0 were determined to be closely related to surfactin production, involving in 15 pathways (Table 1).
Pathway view of the differential metabolites in the cells of B. velezensis B006 grown in media M3 and M4. Pathway enrichment was performed on the MetaboAnalyst 4.0. -log(p) and pathway impact represent the probability and contribution of the pathway to the group difference, respectively. The circle area is positively correlated to the pathway contribution
Five amino acid metabolic pathways (alanine/aspartate/glutamate metabolism, glycine/serine/threonine metabolism, arginine/proline metabolism, glutathione/cysteine/methionine metabolism and valine/leucine/isoleucine biosynthesis) were found to be crucial for the surfactin production (Table 1). Some amino acids even involved in several metabolite pathways, such as glutamic acid participated in alanine/aspartate/glutamate metabolism, glutathione metabolism, aminoacyl-tRNA biosynthesis, and d-glutamine/d-glutamate metabolism. As the structure components of surfactin, aspartate, valine, leucine, isoleucine, and glutamate were detected in group M4. Moreover, alanine, serline, glycine, cysteine, threonine, proline and 4-hydroxyproline were also important metabolites for surfactin production. Alanine, cysteine and threonine are downstream metabolites of aspartate (Fig. S3) and upstream metabolites of valine, leucine and isoleucine biosynthesis (Fig. S4), while proline and 4-hydroxyproline are downstream metabolites of glutamate and upstream metabolites of glycine, serine and threonine metabolism (Fig. S5). These amino acids may function as the hub metabolites and play key roles in the surfactin production.
Of the three detected metabolites of TCA cycle, fumaric acid and succinic acid were found to involve in surfactin production (Fig. S6). Other pathways including aminoacyl-tRNA biosynthesis, glycerolipid metabolism, pantothenate and CoA biosynthesis, purine metabolism, sulfur metabolism and glutamine/glutamate metabolism also contributed to the sufactin production. Moreover, it is the first time to find metabolites ornithine, cadaverine, four nucleotides (adenine, inosine, guanosine, and xanthosine), 1,3-dihydroxyacetone, and homocysteine are related to the surfactin production.
Discussion
Surfactin is an important lipopeptide with great application potential in the medical, industrial and agricultural fields. For industrial purposes, a large amount of research work has been conducted to improve the surfactin yield. One common and traditional practice is to optimize the nutrient supply and culture conditions of the microbial source. As results, the surfactin yield, composition and antagonistic activity have been greatly enhanced [1, 28, 41]. Latest progress in molecular biology and genomics provides some new ideas. The biosynthesis elements of surfactin, i.e., non-ribosome peptide synthases (NRPSs), have been revealed, which is encoded by the srfA operon (srfAA, srfAB, srfAC, and srfAD) [27] and regulated by the pheromone ComX and global regulators DegU, AbrB, and CodY [9, 29, 36, 39]. By genetic engineering of the branched fatty acid metabolic pathway, Dahli et al. [9] improved the production level of surfactin, especially the isoform C14 with linear fatty acid chain, and found acetoin was the key metabolite associated with the overproduction of this linear fatty acid-surfactin. These traditional and modern studies cast an insight into the biological and molecular mechanism of surfactin biosynthesis. However, limited by the scarce metabolic information [14], the underlying mechanism of surfactin production is still one-sides and pending for further studies and excavation.
Metabolomics is a practical technique to provide in-depth information of complex physiological mechanisms, and to explore the relationship of intracellular metabolites [23, 37, 44, 45]. In the present study, a total of 31 metabolites involved in five amino acid metabolism pathways, aminoacyl-tRNA biosynthesis, TCA cycle, glycerolipid metabolism, and pantothenate/CoA biosynthesis were found to be, directly or indirectly, related to the surfactin production of B. velezensis B006. These results provide us the clues to improve surfactin yield through further commercial fermentation.
Of the differential metabolite pathways, we found amino acid metabolic pathways and aminoacyl-tRNA synthesis have significantly impact on surfactin synthesis (Fig. 4). It is known that aminoacyl-tRNA is responsible for the transport of amino acid to ribosome for incorporation into a polypeptide chain [2], as well as the surprising function associated with amino acid biosynthesis [16], thus its up-regulation in group M4 is a key factor for the improved production of surfactin. In previous studies, three structural amino acids (leucine, valine, and isoleucine) [7, 32, 48] and three non-structural amino acids (lysine, threonine, and serine) [22, 47, 52] of surfactin have been used to improve the surfactin yield, which function as precursors or intermediates. In this study, besides the amino acids described above, nine other amino acids (glutamine, glutamic acid, aspartic acid, alanine, glycine, cysteine, proline, tyrosine and phenylalanine) and their relatives, as well as aminoacyl-tRNA were up-regulated in group M4 (Fig. 3) and might be closely associated with the surfactin synthesis (Fig. 4). Further pathway analysis (Figs. S3–6) suggested that aspartate, alanine, cysteine, threonine, proline, and 4-hydroxyproline as the upstream or downstream metabolites of structural amino acids of surfactin might function as precursors or intermediates to improve the surfactin yields.
Surfactin produced by Bacillus spp. have several isoforms up to eight [13]. Of them, C14 and C15 with the greatest disease-suppressing capability [24] have attracted much attention. Coutte et al. [7] improved the surfactin yield as well as the proportion of surfactin isoform C14 by supplementing leucine, while Liu et al. [22] and Zhu et al. [52] increased the proportions of surfactin isoforms with odd β-hydroxy fatty acids by supplementing cysteine, histidine, isoleucine, leucine, methionine, serine, and threonine, and surfactin isoforms with even β-hydroxy fatty acids by supplementing arginine, glutamine or valine. These different findings might be ascribed to the different cultures and Bacillus strains. In this study, five main isoforms (C12–16) of surfactin were produced by B. velezensis B006, and their proportions vary a lot in groups M3 and M4. The proportions of surfactin isoforms C14 and C15 in medium M4 were higher than that in M3 medium. In combination with the analysis of differential metabolites, the up-regulation of arginine, glutamine, valine, isoleucine, serine, threonine, and cysteine might contribute to the synthesis of surfactin isoforms C14 and C15. Further targeted metabolomics will reveal the roles of each amino acid in the production of surfactin isoforms C14 or C15 by B. velezensis B006.
It has been reported that glycolysis/gluconeogenesis and pentose phosphate pathways can provide crucial precursors for the surfactin production in B. amyloliquefaciens MT45, and the genes involved in the utilization of sucrose, glycolysis and the TCA cycle are also up-regulated [51]. However, our study had no similar findings. We only found fumaric acid and succinic acid in the TCA cycle of B. velezensis B006 were up-regulated, and might improve the surfactin production through alanine, aspartate and glutamate metabolism (Fig. S3). Different microbial sources might be the cause. Moreover, not all differential metabolites identified in B. velezensis B006 can match to the reference metabolites of B. subtilis (with 80 metabolic pathways), such as glucose-6-phosphate, sucrose-6-phosphate, and some fatty acid metabolites. Although these metabolites were filtered for further analysis, their importance for cell growth and precursors as carbon skeleton and energy is still in our consideration. The metabolisms of glyoxylate, dicarboxylate, and butanoate in B. velezensis B006 had an impact value of over zero, but the related metabolites were up-regulated in group M3 other than in group M4. We thus infer that these metabolites might be responsible for the endospore formation other than surfactin production.
In summary, we studied the metabolomics of B. velezensis strain B006 grown in two industrial media, which are distinct in surfactin yields. It was found that the metabolisms of alanine/aspartate/glutamate, glycine/serine/threonine, arginine/proline and glutathione, and the biosynthesis of valine/leucine/isoleucine, are closely related to surfactin production and responsible for the high proportions of surfactin isoforms C14 and C15. These amino acids and derivatives might function as precursors or intermediates. In TCA cycle, succinic acid is the important metabolite related to surfactin production. To the best of our knowledge, it is the first report to reveal the metabolic pathways of B. velezensis on surfactin production, and explore the candidate metabolites closely related to surfactin biosynthesis. This study will benefit our understanding of the metabolic mechanisms of strain B006 and its fermentation optimization for commercial surfactin production.
References
Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NA (2008) Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl Biochem Biotechnol 150:289–303. https://doi.org/10.1007/s12010-008-8153-z
Berg J, Tymoczko JL, Stryer L (2006) Biochemistry, 6th edn. W. H Freeman, New York ISBN 0-7167-8724-5
Buchholz A, Hurlebaus J, Wandrey C, Takors R (2002) Metabolomics: quantification of intracellular metabolite dynamics. Biomol Eng 19:5–15. https://doi.org/10.1016/S1389-0344(02)00003-5
Cao P, Hu D, Zhang J, Zhang BQ, Gao Q (2017) Enhanced avermectin production by rational feeding strategies based on comparative metabolomics. Acta Microbiol Sin. 57:281–292 10.13343/j.cnki.wsxb.20160290
Cawoy H, Mariutto M, Henry G, Fisher C, Vasilyeva N, Thonart P (2014) Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol Plant Microb Interat 27:87–100. https://doi.org/10.1094/MPMI-09-13-0262-R
Cevallos-Cevallos JM (2013) Microbial metabolomics: towards pathogen detection and biological prospecting. Metabolomics 3:1. https://doi.org/10.4172/2153-0769.1000e125
Coutte F, Niehren J, Dhali D, John M, Versari C, Jacques P (2015) Modeling leucine’s metabolic pathway and knockout prediction improving the production of surfactin, a biosurfactant from Bacillus subtilis. Biotechnol J 10:1216–1234. https://doi.org/10.1002/biot.201400541
Deleu M, Lorent J, Lins L, Brasseur R, Braun N, EI Kirat K, Nylander T, Dufrêne YF, Mingeot-Leclercq MP (2013) Effects of surfactin on membrane models displaying lipid phase separation. Biochem Biophys Acta Biomembr 1828:801–815. https://doi.org/10.1016/j.bbamem.2012.11.007
Dhali D, Coutte F, Arias AA, Auger S, Bidnenko V, Chataigné G, Lalk M, Niehren J, de Sousa J, Versari C, Jacques P (2017) Genetic engineering of the branched fatty acid metabolic pathway of Bacillus subtilis for the overproduction of surfactin C14 isoform. Biotechnol J 12:1600574. https://doi.org/10.1002/biot.201600574
Ghribi D, Ellouze-Chaabouni S (2011) Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol Res Int 2011:653–654. https://doi.org/10.4061/2011/653654
Guo RJ, Wang JQ, Li SD, Jing YL, Gao YH, Sun RL (2018) A Bacillus velezensis strain and its application with mutifunctions in suppressing disease occurrence, promoting plant growth and drought resistance. Patent application number CH201810017581.8 (In chinese)
Gurjar J, Sengupta B (2015) Production of surfactin from rice mill polishing residue by submerged fermentation using Bacillus subtilis MTCC 2423. Bioresour Technol 189:243–249. https://doi.org/10.1016/j.biortech.2015.04.013
Haddad NI, Liu X, Yang S, Mu B (2008) Surfactin isoforms from Bacillus subtilis HSO121: separation and characterization. Protein Pept Lett 25:265–269. https://doi.org/10.2174/092986608783744225
Henkel M, Geissler M, Weggenmann F, Hausmann R (2017) Production of microbial biosurfactants: status quo of rhamnolipid and surfactin towards large-scale production. Biotechnol J 12:1600561. https://doi.org/10.1002/biot.201600561
Henry G, Deleu M, Jourdan E, Thonart P, Ongena M (2011) The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell Microbiol 13:1824–1837. https://doi.org/10.1111/j.1462-5822.2011.01664.x
Ibba M, Francklyn C, Cusack S (2005) The aminoacyl-tRNA synthetases. Landes Bioscience, Georgetown (ISBN: 1587061899)
Jia K, Gao YH, Huang XQ, Guo RJ, Li SD (2015) Rhizosphere inhibition of cucumber fusarium wilt by different surfactin excreting strains of Bacillus subtilis. Plant Pathol J 31:140–151. https://doi.org/10.5423/PPJ.OA.10.2014.0113
Jia K, Li SD, Liu GJ, Guo RJ (2013) Characteristics of surfactin mutants of Bacillus subtilis B006 and their suppressing ability against cucumber Fusarium wilt. Chin J Biol Control 29:538–546
Jin H, Qiao F, Chen L, Lu C, Xu L, Gao X (2014) Serum metabolomic signatures of lymph node metastasis of esophageal squamous cell carcinoma. J Proteome Res 13:4091–4103. https://doi.org/10.1021/pr500483z
Jourdan E, Henry G, Duby F, Dommes J, Barthelemy JP, Thonart P, Ongena M (2009) Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol Plant Microb Interact 22:456–468. https://doi.org/10.1094/MPMI-22-4-0456
Kinsella K, Schulthess CP, Morris TF, Stuart JD (2009) Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol Biochem 41:374–379. https://doi.org/10.1016/j.soilbio.2008.11.019
Liu JF, Yang J, Yang SZ, Ye RQ, Mu BZ (2012) Effects of different amino acids in culture media on surfactin variants produced by Bacillus subtilis TD7. Appl Biochem Biotechnol 166:2091–2100. https://doi.org/10.1007/s12010-012-9636-5
Lu CZ, Yin J, Zhao FL, Li F, Lu WY (2017) Metabolomics analysis of the effect of dissolved oxygen on spinosad production by Saccharopolyspora spinosa. Antonie Van Leeuwenhoek 110:677–685. https://doi.org/10.1007/s10482-017-0835-5
Malfanova N, Franzil L, Lugtenberg B, Chebotar V, Ongena M (2012) Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch Microbiol 194:893–899. https://doi.org/10.1007/s00203-012-0823-0
Meyer H, Weidmann H, Lalk M (2013) Methodological approaches to help unravel the intracellular metabolome of Bacillus subtilis. Microb Cell Fact 12:69. https://doi.org/10.1186/1475-2859-12-69
Najmi Z, Ebrahimipour G, Franzetti A, Banat IM (2018) In situ downstream strategies for cost-effective bio/surfactant recovery. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1641
Nakano MM, Magnuson R, Myers A, Curry J, Grossman AD, Zuber P (1991) srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol 173:1770–1778
Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M (2012) Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol 29:176–191. https://doi.org/10.1111/j.1574-6941.2011.01208.x
Ogura M, Yamaguchi H, Ki Yoshida, Fujita Y, Tanaka T (2001) DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res 29:3804–3813. https://doi.org/10.1093/nar/29.18.3804
Ohno A, Ano T, Shoda M (1995) Production of a lipopeptide antibiotic, surfactin, by recombinant Bacillus subtilis in solid state fermentation. Biotechnol Bioeng 47:209–214
Ongena M, Jacques P (2008) Bacillus lipopeptides: versa-tile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. https://doi.org/10.1016/j.tim.2007.12.009
Peypoux F, Michel G (1992) Controlled biosynthesis of Val7- and Leu7-surfactins. Appl Microbiol Biotechnol 36:515–517. https://doi.org/10.1007/BF00170194
Peyraud R, Kiefer P, Christen P, Massou S, Portais JC, Vorholt JA (2009) Demonstration of the ethylmalonyl-CoA pathway by using 13C metabolomics. Proc Natl Acad Sci USA 06:4846–4851. https://doi.org/10.1073/pnas.0810932106
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. https://doi.org/10.1111/j.1574-6976.2010.00221.x
Sameh F, Krasimir D, Peggy V, Frédérique G, Guillaume D, Philippe J, Iordan N (2013) Oxygen transfer in three phase inverse fluidized bed bioreactor during biosurfactant production by Bacillus subtilis. Biochem Eng J 76:70–76. https://doi.org/10.1016/j.bej.2013.04.004
Serror P, Sonenshein AL (1996) CodY is required for nutritional repression of Bacillus subtilis genetic competence. J Bacteriol 178:5910–5915
Shi SB, Shen Z, Chen X, Chen T, Zhao XM (2009) Increased production of riboflavin by metabolic engineering of the purine pathway in Bacillus subtilis. Biochem Eng J 46:28–33. https://doi.org/10.1016/j.bej.2009.04.008
Slivinski CT, Mallmann E, de Araújo JM, Mitchell DA, Krieger N (2012) Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem 47:1848–1855
Strauch MA, Bobay BG, Cavanagh J, Yao F, Wilson A, Breton YL (2007) Abh and AbrB control of Bacillus subtilis antimicrobial gene expression. J Bacteriol 189:7720–7732. https://doi.org/10.1128/jb.01081-07
Wang JQ, Wang LG, Guo RJ, Ma GZ, Li SD (2017) Optimization of culture conditions for the enhancement of surfactin production from Bacillus substilis B006. Biotechnol Bull 33:214–221. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2017.04.028
Wei YH, Wang LF, Chang JS (2004) Optimizing iron supplement strategies for enhanced surfactin production with Bacillus subtilis. Biotechnol Prog 20:979–983
Willenbacher J, Rau JT, Rogalla J, Syldatk C, Hausmann R (2015) Foam-free production of surfactin via anaerobic fermentation of Bacillus subtilis DSM 10(T). AMB Express 5:21. https://doi.org/10.1186/s13568-015-0107-6
Willenbacher J, Yeremchuk W, Mohr T, Syldatk C, Hausmann R (2015) Enhancement of surfactin yield by improving the medium composition and fermentation process. AMB Express 5:145. https://doi.org/10.1186/s13568-015-0145-0
Wu QL (2007) Study on riboflavin fermentation optimization and metabolomics of recombinant Bacillus subtilis, Ph. D dissertation, Tianjin University
Xia ML, Huang D, Li SS, Wen JP, Jia XQ, Chen YL (2013) Enhanced FK506 production in Streptomyces tsukubaensis by rational feeding strategies based on comparative metabolic profiling analysis. Biotechnol Bioeng 110:2717–2730. https://doi.org/10.1002/bit.24941
Yang QY, Jia K, Geng WY, Guo RJ, Li SD (2014) Management of cucumber wilt disease by Bacillus subtilis B006 through suppression of Fusarium oxysporum f. sp. cucumerinum in rhizosphere. Plant Pathol J 13:160–166. https://doi.org/10.3923/ppj.2014.160.166
Yang QY, Suo YL, Guo RJ, Li SD, Xu XH (2012) Antifungal activities and principal component analysis of Bacillus subtilis B006 against Fusarium oxysporum f. sp. cucumerinum and Phytophthora capsici. Chin J Biol Control 8:235–242
Yao SL, Lu ZX, Hao TY, Lv FX, Bie XM (2014) Effect of amino acids and carbon backbone precursors on surfactin biosynthesis. J Nanjing Agric Univ 37:139–145. https://doi.org/10.7685/j.issn.1000-2030.2014.02.023
Yeh MS, Wei YH, Chang JS (2006) Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Biotechnol Progr 41:1799–1805. https://doi.org/10.1016/j.procbio.2006.03.027
Zhao PC, Quan CS, Jin LM, Wang LN, Wang JH, Fan SD (2013) Effects of critical medium components on the production of antifungal lipopeptides from Bacillus amyloliquefaciens Q-426 exhibiting excellent biosurfactant properties. World J Microbiol Biotechnol 29:401–409. https://doi.org/10.1007/s11274-012-1180-5
Zhi Y, Wu Q, Xu Y (2017) Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci Rep 7:40976. https://doi.org/10.1038/srep40976
Zhu LY, Liu Q, Liu Y, Li S (2015) Optimization of culture media for high production of surfactin C15 component by Bacillus subtilis. Chin J Bioprocess Eng 13:8–13
Zhu Z, Zhang FG, Wei Z, Ran W, Shen QR (2013) The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J Environ Manag 127:96–102. https://doi.org/10.1016/j.jenvman.2013.04.017
Funding
This work was supported by the Program of China Agriculture Research System (CARS-23-D05), the Modern Agricultural Projects Funded by the Agriculture Department of Jiangsu Province (BE2016335), the Special Fund for Agri-Scientific Research in the Public Interest (201503112-2) and the Funds for Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences (CAAS-XTCX2016015).
Author information
Authors and Affiliations
Contributions
RG and SL as the corresponding authors conceived and supervised the study; JW, RG, and GM designed the experiments; JW and RG performed the experiments, analyzed the data and wrote the manuscript; and WW assisted on metabolomics experiments and data analysis. All authors approved the final article.
Corresponding authors
Ethics declarations
Participants
This article does not contain any studies with human participants.
Conflict of interest
The authors declare that they have no competing interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Guo, R., Wang, W. et al. Insight into the surfactin production of Bacillus velezensis B006 through metabolomics analysis. J Ind Microbiol Biotechnol 45, 1033–1044 (2018). https://doi.org/10.1007/s10295-018-2076-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2076-7